Abstract
Risky decision making can be measured using a probability-discounting procedure, in which animals choose between a small, certain reinforcer and a large, uncertain reinforcer. Recent evidence has identified glutamate as a mediator of risky decision making, as blocking the N-methyl-D-aspartate (NMDA) receptor with MK-801 increases preference for a large, uncertain reinforcer. Because the order in which probabilities associated with the large reinforcer can modulate the effects of drugs on choice, the current study determined if NMDA receptor ligands alter probability discounting using ascending and descending schedules. Sixteen rats were trained in a probability-discounting procedure in which the odds against obtaining the large reinforcer increased (n = 8) or decreased (n = 8) across blocks of trials. Following behavioral training, rats received treatments of the NMDA receptor ligands MK-801 (uncompetitive antagonist; 0, 0.003, 0.01, or 0.03 mg/kg), ketamine (uncompetitive antagonist; 0, 1.0, 5.0, or 10.0 mg/kg), and ifenprodil (NR2B-selective non-competitive antagonist; 0, 1.0, 3.0, or 10.0 mg/kg). Results showed discounting was steeper (indicating increased risk aversion) for rats on an ascending schedule relative to rats on the descending schedule. Furthermore, the effects of MK-801, ketamine, and ifenprodil on discounting were dependent on the schedule used. Specifically, the highest dose of each drug decreased risk taking in rats in the descending schedule, but only MK-801 (0.03 mg/kg) increased risk taking in rats on an ascending schedule. These results show that probability presentation order modulates the effects of NMDA receptor ligands on risky decision making.
Keywords: Risky decision making, probability discounting, glutamate, NMDA receptor, rat
1. Introduction
In probability discounting, animals are allowed to choose between a small, certain reinforcer and a large, uncertain reinforcer. One of the most common tasks used to study probability discounting in animals is an adaptation of a delay-discounting procedure developed by Evenden and Ryan (1996). In this procedure, the probability of obtaining a large, uncertain reinforcer decreases across blocks of trials, whereas the probability of obtaining a small magnitude reinforcer remains constant. Animals will generally discount the large reinforcer as the probability of its delivery decreases (or alternatively, as the odds against its delivery increases; odds against = [1/probability] – 1; Rachlin et al., 1991). Consistently choosing the large, uncertain reinforcer is typically considered to reflect risky decision making, although it is important to note that this behavior is not always maladaptive. For example, consider a hypothetical situation in which an individual has a 100% chance of receiving $20 or a 50% chance of winning $100. According to expected utility theory, the expected outcomes of each choice are $20 and $50, respectively. In this case, choosing the probabilistic reinforcer is advantageous. Regardless, risky decision making is associated with attention deficit/hyperactivity disorder (ADHD; see Dekkers et al., 2016 for a meta-analysis), borderline personality disorder (Schuermann et al., 2011; Svaldi et al., 2012), human immunodeficiency virus (HIV; Fujiwara et al., 2015; Hardy et al., 2006), obsessive-compulsive disorder (OCD; Grassi et al., 2015), psychopathy (Takahashi et al., 2014), sleep deprivation (Killgore et al., 2006), substance abuse (Brevers et al., 2014; Schutter et al., 2011), and pathological gambling (PG; Brand et al., 2005; Madden et al., 2009). Directly related to probability discounting, individuals diagnosed with psychopathy and PG often show shallower discounting of a large, uncertain reinforcer relative to matched controls (Madden et al., 2009; Takahashi et al., 2014).
Recently, the glutamatergic system has been linked to several disorders, including ADHD (see Archer and Garcia, 2016 for a review), OCD (see Grados et al., 2015 for a review), drug addiction (see Kalivas, 2009 for a review), psychopathy (Bortolato et al., 2012), and PG (see Pettorruso et al., 2014 for a review). Glutamate is the major excitatory neurotransmitter in the mammalian brain and binds to metabotropic and ionotropic receptors (see Ozawa et al., 1998 for a review). Currently, only ionotropic glutamate receptors have been examined in a probability-discounting procedure. Although administration of CNQX, an α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptor antagonist, does not affect probability discounting, N-methyl-D-aspartate (NMDA) receptor uncompetitive antagonists produce differential effects. MK-801 increases risky decision making, whereas ketamine decreases sensitivity to reinforcer magnitude (i.e., decreases responding for the large, uncertain reinforcer when its delivery is certain) without altering the rate of discounting (Yates et al., 2015). Because previous work has shown that drug effects in a probability-discounting procedure are dependent on the order in which probabilities are presented (St. Onge et al., 2010), the goal of the present study was to determine if probability presentation order modulates the effects of NMDA receptor ligands on risky decision making. MK-801 and ketamine were tested because they exhibit differential effects in a probability-discounting task (Yates et al., 2015). In addition to testing MK-801 and ketamine, we wanted to include an NMDA receptor ligand that lacks the psychotomimetic effects observed with uncompetitive antagonists (Harder et al., 1998; Krystal et al., 1994). As such, we included ifenprodil, a non-competitive antagonist that targets NR2B-containing NMDA receptors (Perin-Dureau et al., 2002).
2. Methods
2.1. Subjects
A total of 16 experimentally naïve, male Sprague Dawley rats (225–250 g upon arrival to the lab; Harlan Industries, Indianapolis, IN) were used in the current experiment. They were acclimated to an animal housing room and handled for six days before testing began. The housing room was maintained on a 12:12-h cycle (lights on at 0630 h), and rats were tested in the light phase (approximately 1100–1500 h). Rats were individually housed in clear polypropylene cages (51 cm long × 26.5 cm wide × 32 cm high) with metal tops containing food and a water bottle. Rats were fed 10 g of food each day immediately following behavioral testing, but they had ad libitum access to water in their home cage. All experimental procedures were carried out according to the Current Guide for the Care and Use of Laboratory Animals (USPHS) under a protocol approved by the Northern Kentucky University Institutional Animal Care and Use Committee.
2.2. Drugs
All drugs were purchased from Sigma Aldrich (St. Louis, MO). (+)-MK-801 hydrogen maleate and (±)-ketamine hydrochloride were prepared in sterile 0.9% NaCl (saline). Ifenprodil (+)-tartrate salt was prepared in distilled water. The two highest doses of ifenprodil (3.0 and 10.0 mg/kg) were heated and stirred prior to each injection. Each drug was injected at room temperature in a volume of 1 ml/kg. The doses were calculated based on salt weight.
2.3. Apparatus
Eight operant-conditioning chambers (28 × 21 × 21 cm; ENV-008; MED Associates, St. Albans, VT) located inside sound attenuating chambers (ENV-018M; MED Associates) were used. The front and back walls of the chambers were made of aluminum, while the side walls were made of Plexiglas. There was a recessed food tray (5 × 4.2 cm) located two cm above the floor in the bottom-center of the front wall. An infrared photobeam was used to record head entries into the food tray. A 28-V white stimulus light was located six cm above each response lever. A 28-V white house light was mounted in the center of the back wall of the chamber. A nosepoke aperture was located two cm above the floor in the bottom-center of the back wall (the aperture was never used in the current experiment). All responses and scheduled consequences were recorded and controlled by a computer interface. A computer controlled the experimental session using Med-IV software.
2.4. Procedure
2.4.1. Magazine training
Rats were given two days of magazine training, in which 45 mg food pellets (F0021 dustless precision pellet, Bio-Serv, Frenchtown, NJ) were non-contingently delivered into the food tray. A total of 20 pellets was delivered according to a variable-time 30-s schedule of reinforcement. Each session lasted 10 min.
2.4.2. Lever-press training
For two sessions, rats learned to respond on each lever according to a fixed ratio (FR) 1 schedule of reinforcement. Each session began with illumination of the house light. A head entry into the food tray resulted in presentation of one lever; each lever was presented pseudo-randomly, with no more than two consecutive presentations of the same lever. A response on either lever resulted in delivery of one food pellet. Following a response on either lever, the house light was extinguished, and the lever was retracted for five s. After five s, the house light was illuminated. Each session ended after a rat earned 40 reinforcers or after 30 min, whichever came first. Following two sessions of FR 1 training, rats received three additional lever-press training sessions, in which the FR requirement increased between sessions (FR 3, FR 5, and FR 10, respectively). Each rat earned all 40 pellets during each session, with the exception of the first FR 1 training session.
2.4.3. Magnitude discrimination
Rats were given five days of magnitude discrimination training. Each session consisted of 40 trials, and each trial lasted 30 s. Each trial began with illumination of the house light. A head entry into the food tray extended one of the levers (the order of presentation between the two levers was pseudo-random, with no more than two consecutive presentations of the same lever). Responses on one lever (FR 10) resulted in immediate delivery of one pellet, whereas responses on the other lever (FR 10) resulted in immediate delivery of four pellets (the lever associated with the large magnitude reinforcer was counterbalanced across rats). Following completion of the response requirement on either lever, the house light was extinguished, and the lever was retracted for the remainder of the trial. If the response requirement was not completed within 20 s, the trial was scored as an omission, and the house light was extinguished for the remainder of the trial.
2.4.4. Probability discounting
Each session consisted of five blocks of 18 trials. The stimuli used to signal the beginning of each trial differed across blocks of trials (first: house light; second: house light and left stimulus light; third: house light and right stimulus light; fourth: house light and both stimulus lights; fifth: both stimulus lights). The first eight trials in a block were forced-choice trials, in which only one lever was pseudo-randomly presented (no more than two consecutive presentations of the same lever). The remaining trials were free-choice trials, in which both levers were extended. Ten responses on one lever always resulted in immediate delivery of one food pellet, whereas ten responses on the other lever resulted in probabilistic delivery of four pellets. For half of the rats, the odds against delivery of the large magnitude reinforcer increased across blocks of trials (0, 3, 7, 15, 31; corresponding to probabilities of 100%, 25%, 12.5%, 6.25%, 3.13%). For half of the rats, the odds against delivery of the large magnitude reinforcer decreased across blocks of trials (31, 15, 7, 3, 0). Following responses on either lever (FR 10), the stimuli used to signal the beginning of each trial were extinguished, and the levers were retracted for the remainder of the trial. If the response requirement was not completed within 20 s, the trial was scored as an omission, and all stimuli were extinguished for the remainder of the trial. Each trial lasted 30 s, regardless of how the rat responded. For example, if a rat completed the response requirement within 5 s, it would receive the reinforcer, and then would wait 25 s before the start of the next trial. Each session lasted 45 min.
2.4.5. Drug treatments
Following behavioral training (28–30 sessions), rats received treatments of the uncompetitive antagonists MK-801 (0.0, 0.003, 0.01, or 0.03 mg/kg; s.c.) and ketamine (0, 1.0, 5.0, or 10.0 mg/kg; i.p.) 15 min before each session, as well as the NR2B-containing NMDA non-competitive antagonist ifenprodil (0, 1.0, 3.0, or 10.0 mg/kg; i.p.) 30 min before each session. The doses and pretreatment times were chosen based on previous work (Ma et al., 2011; Yates et al., 2015). The injection order was pseudo-randomized, such that the order of each drug and the order of each dose of an individual drug were randomized across rats; however, a rat received all four injections of a particular drug before receiving injections of another drug (see Table 1). Rats received additional discounting sessions (2–4 sessions) between injection days.
Table 1.
Injection schedule for each subject.
| Subject | Group | Injection Order |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 101 | Ascending | Ket 5.0 | Ket 0.0 | Ket 10.0 | Ket 1.0 | MK 0.0 | MK 0.003 | MK 0.01 | MK 0.03 | Ifen 3.0 | Ifen 1.0 | Ifen 0.0 | Ifen 10.0 |
| 102 | Ascending | MK 0.01 | MK 0.0 | MK 0.003 | MK 0.03 | Ket 0.0 | Ket 1.0 | Ket 5.0 | Ket 10.0 | Ifen 0.0 | Ifen 10.0 | Ifen 1.0 | Ifen 3.0 |
| 103 | Ascending | Ket 0.0 | Ket 10.0 | Ket 1.0 | Ket 5.0 | MK 0.003 | MK 0.03 | MK 0.0 | MK 0.01 | Ifen 1.0 | Ifen 3.0 | Ifen 10.0 | Ifen 0.0 |
| 104 | Ascending | Ifen 1.0 | Ifen 3.0 | Ifen 10.0 | Ifen 0.0 | Ket 5.0 | Ket 10.0 | Ket 0.0 | Ket 1.0 | MK 0.003 | MK 0.03 | MK 0.01 | MK 0.0 |
| 105 | Descending | MK 0.03 | MK 0.01 | MK 0.0 | MK 0.003 | Ket 1.0 | Ket 0.0 | Ket 10.0 | Ket 5.0 | Ifen 10.0 | Ifen 0.0 | Ifen 3.0 | Ifen 1.0 |
| 106 | Descending | Ket 1.0 | Ket 5.0 | Ket 0.0 | Ket 10.0 | MK 0.03 | MK 0.01 | MK 0.003 | MK 0.0 | Ifen 3.0 | Ifen 0.0 | Ifen 1.0 | Ifen 10.0 |
| 107 | Descending | MK 0.0 | MK 0.003 | MK 0.03 | MK 0.01 | Ifen 0.0 | Ifen 3.0 | Ifen 10.0 | Ifen 1.0 | Ket 5.0 | Ket 0.0 | Ket 10.0 | Ket 1.0 |
| 108 | Descending | Ket 10.0 | Ket 1.0 | Ket 5.0 | Ket 0.0 | Ifen 10.0 | Ifen 0.0 | Ifen 1.0 | Ifen 3.0 | MK 0.03 | MK 0.003 | MK 0.0 | MK 0.01 |
| 109 | Ascending | Ifen 10.0 | Ifen 0.0 | Ifen 3.0 | Ifen 1.0 | Ket 10.0 | Ket 5.0 | Ket 1.0 | Ket 0.0 | MK 0.0 | MK 0.01 | MK 0.003 | MK 0.03 |
| 110 | Ascending | Ket 5.0 | Ket 0.0 | Ket 1.0 | Ket 10.0 | Ifen 3.0 | Ifen 1.0 | Ifen 0.0 | Ifen 10.0 | MK 0.003 | MK 0.03 | MK 0.0 | MK 0.01 |
| 111 | Ascending | MK 0.003 | MK 0.03 | MK 0.01 | MK 0.0 | Ifen 1.0 | Ifen 10.0 | Ifen 3.0 | Ifen 0.0 | Ket 0.0 | Ket 1.0 | Ket 5.0 | Ket 10.0 |
| 112 | Ascending | Ifen 3.0 | Ifen 1.0 | Ifen 0.0 | Ifen 10.0 | MK 0.01 | MK 0.0 | MK 0.03 | MK 0.003 | Ket 1.0 | Ket 10.0 | Ket 0.0 | Ket 5.0 |
| 113 | Descending | Ifen 0.0 | Ifen 10.0 | Ifen 1.0 | Ifen 3.0 | Ket 0.0 | Ket 5.0 | Ket 1.0 | Ket 10.0 | MK 0.01 | MK 0.0 | MK 0.03 | MK 0.003 |
| 114 | Descending | Ket 0.0 | Ket 10.0 | Ket 5.0 | Ket 1.0 | MK 0.0 | MK 0.003 | MK 0.03 | MK 0.01 | Ifen 1.0 | Ifen 10.0 | Ifen 3.0 | Ifen 0.0 |
| 115 | Descending | MK 0.01 | MK 0.03 | MK 0.0 | MK 0.003 | Ifen 0.0 | Ifen 10.0 | Ifen 3.0 | Ifen 1.0 | Ket 10.0 | Ket 5.0 | Ket 1.0 | Ket 0.0 |
| 116 | Descending | Ifen 1.0 | Ifen 10.0 | Ifen 3.0 | Ifen 0.0 | MK 0.003 | MK 0.03 | MK 0.01 | MK 0.0 | Ket 5.0 | Ket 1.0 | Ket 0.0 | Ket 10.0 |
Note: Ifen = ifenprodil; Ket = ketamine; MK = MK-801
2.4.6. Statistical analyses
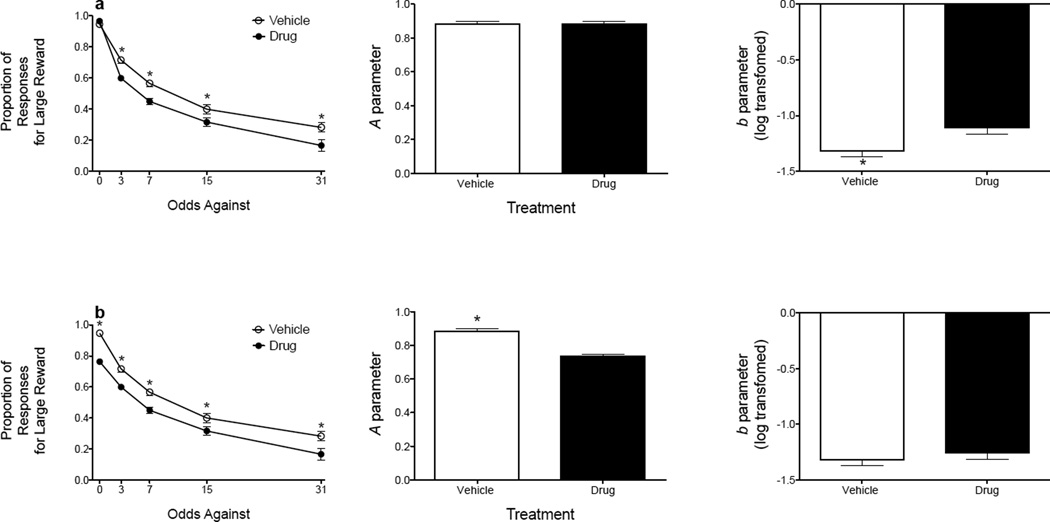
Probability-discounting data are often analyzed by applying a two-way ANOVA to the proportion of responses for the probabilistic reinforcer (Boutros et al., 2014; Ghods-Sharifi et al., 2009; Mendez et al., 2012; Rokosik and Napier, 2012; Schindler et al., 2014; St. Onge and Floresco, 2009, 2010; St. Onge et al., 2010, 2011, 2012; Stopper et al., 2013). However, in the current study the exponential discounting function was fit to each subject’s data using the Solver function in Microsoft Excel and is defined by the equation V = Ae−bΘ, where V is the subjective value of the reinforcer, A is the intercept and refers to sensitivity to reinforcer magnitude (i.e., measure of discriminability between the small and large magnitude reinforcer), e is Euler’s number, b is the rate of discounting (i.e., measure of risky decision making), and Θ is the odds against delivery of the large magnitude reinforcer. This analysis provides a determination of an animal’s sensitivity to reinforcer amount and sensitivity to probabilistic reinforcement, which both independently influence discounting of a reinforcer (Ho et al. 1999). By using a two-way ANOVA on the raw data, one cannot determine if a drug differentially affects sensitivity to reinforcer magnitude and sensitivity to probabilistic reinforcement. Figure 1 presents hypothetical data for 12 subjects and shows how a drug can differentially affect A and b parameter estimates. In Figure 1a, the drug decreases risk taking without altering sensitivity to reinforcer magnitude, as evidenced by a change in the b parameter estimate only. In Figure 1b, the drug decreases sensitivity to reinforcer magnitude without altering risk taking, as evidenced by a change in the A parameter only. When a two-way ANOVA is applied to both datasets, there are main effects of dose and trial block, as well as a dose × trial block interaction, suggesting that the drug decreases risk taking in each scenario1.
Figure 1.
Hypothetical probability-discounting data for 12 rats showing how a drug can differentially alter A and b parameter estimates. (a) The drug decreases responding for the large magnitude reinforcer when the odds against obtaining that reinforcer are set to 3, 7, 15, and 31 (left column), which corresponds to no change in the A parameter estimate (center column) but an increase in the b (log-transformed) parameter estimate (indicating increased risk aversion; right column). (b) The drug decreases responding for the large magnitude reinforcer across each block of trials (left column), which corresponds to a decrease in the A parameter estimate (indicating decreased sensitivity to reinforcer magnitude; center column) but no change in risk taking (right column). The data are presented as mean (± SEM). *p < .05 relative to drug treatment.
Because omissions and A parameter estimates were not normally distributed, these data were analyzed with nonparametric tests. A Mann Whitney test was used to determine if the schedule (ascending vs. descending) affected baseline A parameter estimates. Friedman tests were used to determine if the NMDA receptor ligands significantly altered omissions and/or A parameter estimates. Statistical significance was defined as p < .05 for the Mann-Whitney test. Because separate Friedman tests were conducted for each group of rats, statistical significance was defined as p < .025 to control for Type I error.
Log-transformed b parameter estimates were used, which allowed the use of parametric tests. An independent-samples t test was used to determine if the schedule affected baseline b (log-transformed) parameter estimates. To determine the effects of NMDA receptor ligands on sensitivity to delayed reinforcement, log-transformed b parameter estimates were analyzed using a mixed-factor ANOVA, with schedule as a between-subjects factor and dose as a within-subjects factor. A main effect of dose was probed with Dunnett’s post hoc tests, and significant interactions were probed with one-way repeated-measures ANOVAs, with Dunnett’s post hoc test when appropriate. Statistical significance was defined as p < .05. In one case, a rat did not discount the large magnitude reinforcer, making the b parameter 0; thus, the log-transformed b parameter estimate was arbitrarily set to −2 because this value was smaller than any other log-transformed b parameter observed across all subjects. In all cases, A parameter estimates were constrained, such that the maximum value was 1.
For two rats, the exponential discounting function could not be fit to the data due to a high number of omissions following ketamine (5.0 mg/kg: 22 out of 50 free-choice trials; 10.0 mg/kg; 37 out of 50 free-choice trials) administration. Missing parameter estimates were imputed using the last observation carried forward method.
3. Results
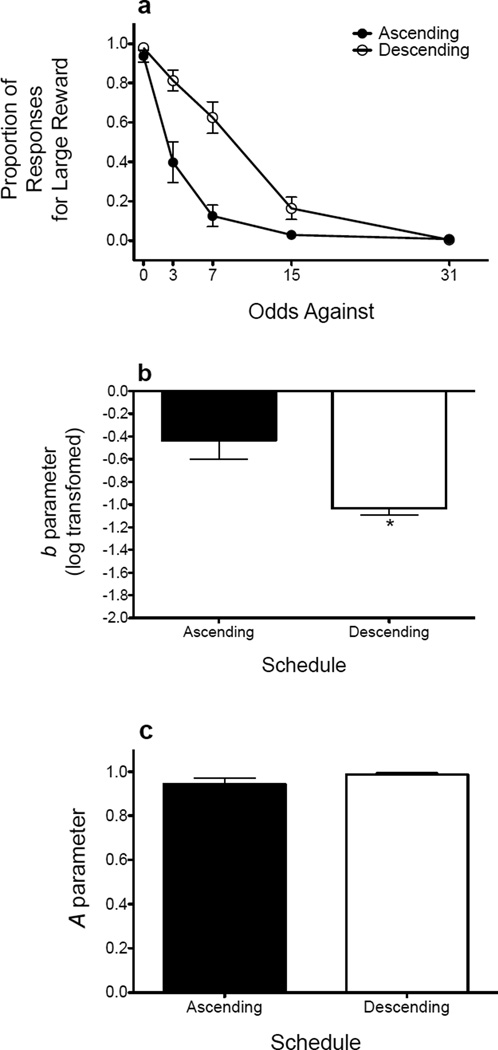
Figure 2 shows baseline performance (average of final 3 sessions) for rats trained in the ascending schedule and for rats trained in the descending schedule. The raw proportion of responses (Fig. 2a) was not analyzed; instead, parameter estimates were derived from the exponential model and were subsequently analyzed. Rats trained in the descending schedule were less sensitive to probabilistic reinforcement (t(14) = 3.441, p = .004; Fig. 2b) relative to rats trained in the ascending schedule. The type of schedule used did not affect sensitivity to reinforcer magnitude (Fig. 2c).
Figure 2.
Baseline performance in rats trained with an ascending (n = 8) and descending (n = 8) schedule. (a) Mean (± SEM) proportion of responses for the large, probabilistic reinforcer as a function of the odds against receiving the reinforcer. (b) Mean (± SEM) log-transformed b parameter estimates. (c) Mean (± SEM) A parameter estimates. *p < .05, relative to rats trained on the ascending schedule.
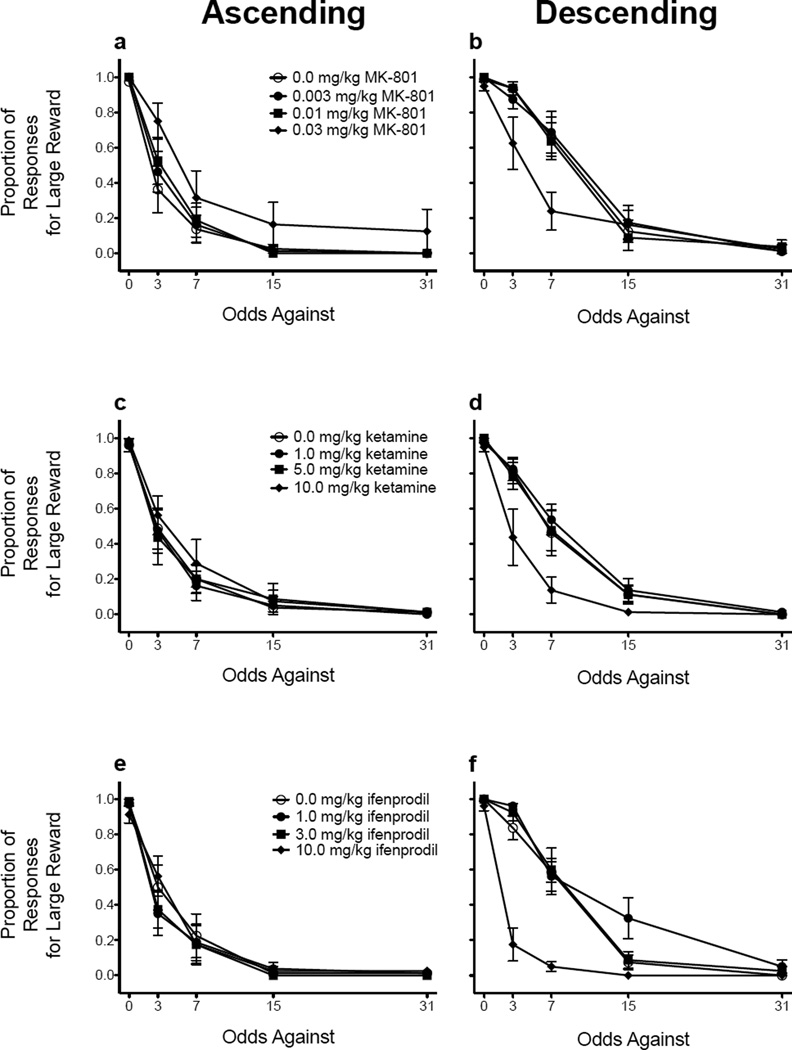
Figure 3 shows the proportion of responses for the large, probabilistic reinforcer following administration of MK-801 (Fig. 3a), ketamine (Fig. 3b), and ifenprodil (Fig. 3c). As stated in the previous paragraph, analyses were not performed on the raw data; instead, parameter estimates were derived from the exponential function, which were then analyzed (see below). Additionally, administration of MK-801, ketamine, or ifenprodil did not significantly increase omissions (data not shown). However, one rat treated with the intermediate dose of ketamine (5.0 mg/kg) and one rat treated with the highest dose of ketamine (10.0 mg/kg) did not respond during two blocks of trials; therefore, exponential functions could not be fit to their data.
Figure 3.
Mean (± SEM) proportion of responses for the large, probabilistic reinforcer following administration of MK-801 (a and b), ketamine (c and d), and ifenprodil (e and f). Figures in the left column correspond to the ascending schedule, whereas figures in the right column correspond to the descending schedule.
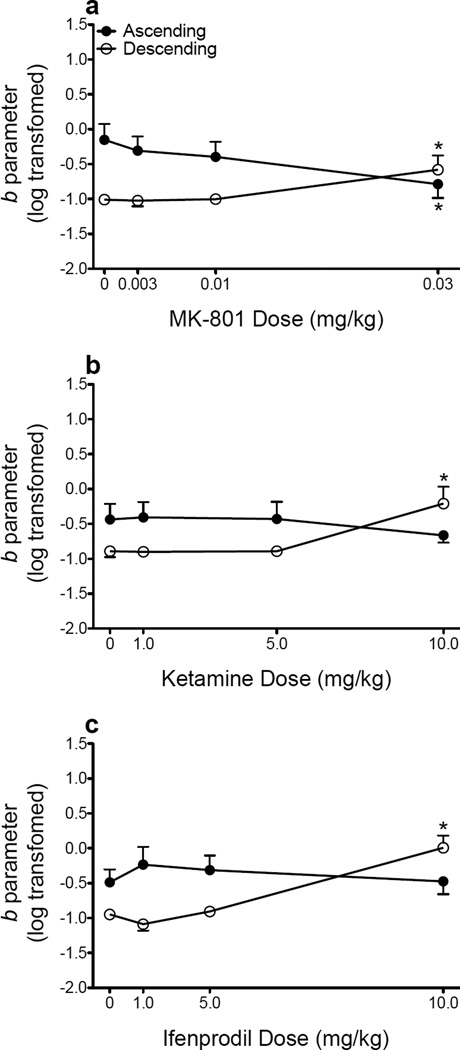
Figure 4 shows log-transformed b parameter estimates (i.e., sensitivity to probabilistic reinforcement) following administration of the NMDA receptor antagonists. Following MK-801 administration, a mixed-factor ANOVA revealed a main effect of schedule (F(1, 14) = 6.988, p = .019) and a dose × schedule interaction (F(3, 42) = 7.017, p = .001). MK-801 significantly decreased sensitivity to probabilistic reinforcement when an ascending schedule was used (F(3, 21) = 3.417, p = .036), and Dunnett’s post hoc tests revealed that this decrease was observed at the highest dose (0.03 mg/kg; Fig. 4a). Conversely, MK-801 significantly increased sensitivity to probabilistic reinforcement when a descending schedule was used (F(3, 21) = 4.200, p = .018), and Dunnett’s post hoc tests revealed that this increase was observed at the highest dose (0.03 mg/kg; Fig. 4a). Following ketamine administration, a mixed-factor ANOVA revealed a significant dose × schedule interaction ((F(3, 42) = 8.812) < .001). Ketamine did not alter probability discounting when an ascending schedule was used, but significantly increased sensitivity to probabilistic reinforcement when a descending schedule was used (F(3, 21) = 8.791, p = .001), and Dunnett’s post hoc tests revealed that this increase was observed at the highest dose (10.0 mg/kg; Fig. 4b). Following ifenprodil administration, a mixed-factor ANOVA revealed a main effect of dose (F(3, 42) = 6.452, p = .001) and a significant dose × schedule interaction (F(3, 42) = 11.358, p < .001). Ifenprodil did not alter probability discounting when an ascending schedule was used, but significantly increased sensitivity to probabilistic reinforcement when a descending schedule was used (F(3, 21) = 17.936, p < .001), and Dunnett’s post hoc tests revealed that this increase was observed at the highest dose (10.0 mg/kg; Fig. 4c). None of the NMDA receptor ligands significantly altered sensitivity to reinforcer magnitude (data not shown).
Figure 4.
Mean (± SEM) log-transformed b parameter estimates following administration of MK-801 (a), ketamine (b), and ifenprodil (c). *p < .05, relative to vehicle.
4. Discussion
In the current experiment, risky decision making is inferred from the rate of probability discounting. Shallow discounting (i.e., more negative log-transformed b parameter estimates) indicates risky decision making, whereas steep discounting (i.e., less negative log-transformed b parameter estimates) indicates risk aversion. The main finding of this experiment is that the order in which probabilities are presented modulates the effects of NMDA receptor antagonists on risky decision making. When a descending schedule is used, blockade of NMDA receptors promotes risk aversion. However, when an ascending schedule is used, MK-801 selectively increases risk taking.
An exponential function was used to derive two parameter estimates in order to characterize discounting in rats. The b parameter estimate measures sensitivity to probabilistic reinforcement. A high b value (corresponding to a less negative log-transformed value) indicates risk aversion, whereas a low b value (corresponding to a more negative log-transformed value) indicates risk taking. The A parameter estimate measures sensitivity to reinforcer magnitude; when this value decreases, rats are less willing to choose the large magnitude reinforcer even when its delivery is certain. This analysis is included because it can help explain why changes in the rate of discounting occur. In the current experiment, analysis of baseline performance showed that rats trained on the ascending schedule were more sensitive to probabilistic reinforcement (i.e., were more risk averse) relative to rats trained on the descending schedule even though they were equally sensitive to reinforcer magnitude. These results are inconsistent with a previous report showing no differences in the rate of probability discounting between rats trained on an ascending and descending schedule (St. Onge et al., 2010). There are procedural differences between the current study and previous work (St. Onge et al., 2010) that may account for the inconsistent findings across studies. The current study used probabilities (100%, 25%, 12.5%, 6.25%, 3.13%) that were smaller relative to the St. Onge et al. (2010) study (100%, 50%, 25%, 12.5%). This discrepancy may account for the steeper discounting observed in the current study, especially for the rats trained on the ascending schedule. Interestingly, inconsistencies have been reported with the delay-discounting task, as some have reported differences in discounting when the order of the delay presentation is manipulated (Fox et al., 2008; Robles and Vargas, 2008; Robles et al., 2009), whereas others have not observed significant differences (Maguire et al., 2014; Robles and Varagas, 2007; Slezak and Anderson, 2009; Tanno et al., 2014). These discrepancies suggest that there are multiple factors, in addition to the order in which probabilities/delays are presented, that affect the rate of discounting in these tasks.
MK-801 has previously been shown to increase risky decision making when an ascending schedule is used (Yates et al., 2015), which was replicated in the current experiment at a dose that did not alter omissions or sensitivity to reinforcer amount. A novel finding is that MK-801 increased risk aversion when the odds against obtaining the probabilistic reinforcer decreased across trials. Considering MK-801 is a known psychotomimetic that increases perseverative responding (Cohn et al. 1992; Davis-MacNevin et al., 2013; Dix et al., 2010; Tuplin et al., 2015), the current results could be interpreted as an impairment in response flexibility, as opposed to an alteration in risky decision making. There is support to this claim, as MK-801 increases dopamine levels in the prefrontal cortex (Tsukada et al., 2005), and amphetamine, which increases forebrain dopamine levels, increases perseverative responding (Todorov et al., 1972) and differentially alters probability and delay discounting when different schedules are used (St. Onge et al., 2010; Tanno et al., 2014). However, St. Onge et al. (2010) argue that the differential findings observed with amphetamine are due to an increase in perseveration on the perceived relative value of the probabilistic reinforcer as the probability changes. For example, when the probability of receiving the large magnitude reinforcer is initially set at 1, rats treat this option as more advantageous even as the probability of earning that reinforcer decreases throughout the session. Because the current results with MK-801 are consistent with those reported with amphetamine (St. Onge et al., 2010), the effects of MK-801 on probability discounting may be mediated, at least in part, by the dopaminergic system. Another consideration is that MK-801 inhibits nicotinic acetylcholine receptors (Amador and Dani, 1991), but it is unlikely that the effects observed following MK-801 administration are mediated by the cholinergic system because blocking these receptors with selective cholinergic receptor antagonists does not alter probability discounting (Mendez et al., 2012).
Unlike MK-801, ketamine and ifenprodil did not alter risk taking when an ascending schedule was used. The finding with ketamine is consistent with previous work (Yates et al., 2015). However, similar to the results obtained with MK-801, ketamine (10.0 mg/kg) and ifenprodil (10.0 mg/kg) increased risk aversion at a dose that did not affect omissions or A parameter estimates. Some caution needs to be taken when interpreting these results, as ketamine and ifenprodil interact with other neurotransmitter systems. Ketamine has actions on sigma receptors (Robson et al., 2012), opioid receptors (Gupta et al., 2011), and 5-HT2 receptors (Kapur and Seeman, 2002), and ifenprodil binds to 5-HT3 receptors (McCool and Lovinger, 1995), sigma receptors (Hashimoto et al., 1994), and adrenergic receptors (Chenard et al., 1991). To our knowledge, the role of sigma receptors in discounting has not been examined; thus, we cannot rule out the possibility that the effects observed by ketamine and ifenprodil are mediated by these receptors. However, it is unlikely that the effects observed in the current experiment are mediated by the serotoninergic or opioid system, as research has shown that depleting serotonin levels (Mobini et al., 2000) or administering the opioid oxycodone do not affect probability discounting (Zacny and de Wit, 2009). Recent evidence has indicated that clonidine, an α2-adrenergic receptor agonist, decreases risky decision making in rats (Montes et al., 2015), indicating that the results obtained with ifenprodil could be mediated by the adrenergic system. There is some argument against this potential alternative explanation. Montes et al. (2015) observed a decrease in the large probabilistic reinforcer when its delivery was certain, suggesting a decrease in sensitivity to reinforcer magnitude, an effect that was not observed with ifenprodil. Considering polymorphisms in the gene that encodes for the NR2B subunit are associated with risky decision making in a gambling task (Ness et al., 2011), future studies should test ligands that are more selective for the NR2B subunit. Indeed, a recent study has shown that Ro 63–1908 decreases impulsive choice in a delay-discounting task, whereas CP-101, 606 (traxoprodil) did not significantly alter discounting (Higgins et al., 2016).
The differential findings observed in rats trained on the ascending and descending schedules could be attributed to baseline differences in discounting. For example, rats trained on the ascending schedule show greater discounting relative to rats trained on the descending schedule. The discounting observed in rats trained on the ascending schedule could reflect a floor effect; therefore, a decrease in responding for the large, probabilistic reinforcer may not be observed following ketamine or ifenprodil administration. Directly related to glutamatergic ligands, such baseline differences have been observed in delay discounting; for example, low impulsive rats (which exhibit shallow discounting) become more sensitive to delayed reinforcement following ketamine administration, whereas high impulsive rats show no change in discounting (Cottone et al., 2013).
One surprising finding is that ketamine (10.0 mg/kg) did not decrease sensitivity to reinforcer magnitude when an ascending schedule was used, which has been previously reported (Yates et al., 2015). One procedural difference that may account for the discrepancy in the current study and previous work (Yates et al., 2015) was that rats in the current procedure responded on an FR 10 schedule of reinforcement, whereas rats in the Yates et al. (2015) study responded on an FR 1 schedule. Altering the response requirement is known to alter the rate of delay discounting. Specifically, increasing the FR requirement increases preference for the large, delayed reinforcer (Huskinson and Anderson, 2013). In the current experiment, the increased response requirement could negate the decreased sensitivity to reinforcer magnitude that was observed following ketamine administration in the Yates et al. (2015) study. This finding is interesting because it suggests that, in addition to the order in which probabilities/delays are presented, the response requirement can modulate drug effects in discounting procedures. Future work is needed to further characterize the interaction between response requirement and drug effects in this task.
In conclusion, this study shows that the order in which probabilities are presented modulates the effects of NMDA receptor non/uncompetitive antagonists in a probability-discounting procedure. Careful consideration needs to be taken into account when testing the effects of drugs on risk taking, as well as on impulsive decision making. For example, an ascending schedule may be a more sensitive measure for detecting decreases in sensitivity to probabilistic/delayed reinforcement, whereas a descending schedule may be a more sensitive measure for detecting increases in probabilistic/delayed reinforcement. Given that rats trained on the descending schedule have higher baseline levels of risky decision making, the current results demonstrate that NMDA receptor blockade decreases risk taking in those prone to engaging in such behavior. Overall, these results further demonstrate the importance of the glutamatergic system in risky decision making.
Research Highlights.
NMDA receptor antagonists decrease risky choice when a descending schedule is used
MK-801 increases risky choice when an ascending schedule is used
A descending schedule increases risky choice relative to an ascending schedule
Acknowledgments
The current study was supported by NIH grant P20GM103436, as well as a Northern Kentucky University Faculty Project Grant. We would like to thank Cliff Brown for providing technical assistance. We would also like to thank Dr. Mark Bardgett for providing feedback on a draft of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The significant interaction observed in Figure 1b is most likely due to the larger percentage decrease in responses for the large magnitude reinforcer between 15 and 31 odds against following drug administration (47.363%) relative to vehicle (29.175%).
References
- Amador M, Dani JA. MK-801 inhibition of nicotinic acetylcholine receptor channels. Synapse. 1991;7:207–215. doi: 10.1002/syn.890070305. [DOI] [PubMed] [Google Scholar]
- Archer T, Garcia D. Attention-deficit/hyperactivity disorder: focus upon aberrant N-methyl-D-aspartate receptors systems. Curr Top Behav Neurosci. 2016;29:295–311. doi: 10.1007/7854_2015_415. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Godar SC, Melis M, Soggiu A, Roncada P, Casu A, Flore G, Chen K, Frau R, Urbani A, Castelli MP, Devoto P, Shih JC. NMDARs mediate the role of monoamine oxidase A in pathological aggression. J Neurosci. 2012;32:8574–8582. doi: 10.1523/JNEUROSCI.0225-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Liu W, Crews FT, Markou A. Adolescent intermittent ethanol exposure is associated with increased risky choice and decreased dopaminergic and cholinergic neuron markers in adult rats. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu003. pii: pyu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Kalbe E, Labudda K, Fujiwara E, Kessler J, Markowitsch HJ. Decision making impairments in patients with pathological gambling. Psychiatry Res. 2005;133:91–99. doi: 10.1016/j.psychres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brevers D, Bechara A, Cleeremsans A, Kornreich C, Verbanck P, Noël X. Impaired decision-making under risk in individuals with alcohol dependence. Alcohol Clin Exp Res. 2014;38:1924–1931. doi: 10.1111/acer.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenard BL, Shalaby IA, Koe BK, Rounau RT, Butler TW, Prochiniak MA, Schmidt AW, Fox CB. Separation of α1 adrenergic and N-methyl-D-aspartate antagonist activity in a series of ifenprodil compounds. J Med Chem. 1991;34:3085–3090. doi: 10.1021/jm00114a018. [DOI] [PubMed] [Google Scholar]
- Cohn J, Ziriax JM, Cox C, Cory-Slechta DA. Comparison of error patterns produced by scopolamine and MK-801 on repeated acquisition and transition baselines. Psychopharmacology. 1992;107:243–254. doi: 10.1007/BF02245144. [DOI] [PubMed] [Google Scholar]
- Cottone P, Iemolo A, Narayan AR, Kwak J, Momaney D, Sabino V. The uncompetitive NMDA receptor antagonists ketamine and memantine preferentially increase the choice for a small, immediate reward in low-impulsive rats. Psychopharmacology. 2013;226:127–138. doi: 10.1007/s00213-012-2898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-MacNevin PL, Dekraker J, LaDouceur L, Holahan MR. Comparison of the MK-801-induced increase in non-rewarded appetitive responding with dopamine agonists and locomotor activity in rats. J Psychopharmacol. 2013;27:854–864. doi: 10.1177/0269881113492029. [DOI] [PubMed] [Google Scholar]
- Dekkers TJ, Popma A, Agelink van Rentergem JA, Bexkens A, Huizenga HM. Risky decision making in Attention-deficit/Hyperactivity Disorder: a meta-regression analysis. Clin Psychol Rev. 2016;45:1–16. doi: 10.1016/j.cpr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Dix S, Gilmour G, Potts S, Smith JW, Tricklebank M. A within-subject cognitive battery in the rat: differential effects of NMDA receptor antagonists. Psychopharmacology. 2010;212:227–242. doi: 10.1007/s00213-010-1945-1. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Fox AT, Hand DJ, Reilly MP. Impulsive choice in a rodent model of attention-deficit/hyperactivity disorder. Behav Brain Res. 2008;187:146–152. doi: 10.1016/j.bbr.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Fujiwara E, Tomlinson SE, Purdon SE, Gill MJ, Power C. Decision making under explicit risk is impaired in individuals with human immunodeficiency virus (HIV) J Clin Exp Neuropsychol. 2015;37:733–750. doi: 10.1080/13803395.2015.1057481. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, St. Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci. 2009;29:5251–5259. doi: 10.1523/JNEUROSCI.0315-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grados MA, Atkins EB, Kovacikova GI, McVicar E. A selective review of glutamate pharmacological therapy in obsessive-compulsive and related disorders. Psychol Res Behav Manag. 2015;28:115–131. doi: 10.2147/PRBM.S58601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Pallanti S, Righi L, Figee M, Mantione M, Denys D, Piccagliani D, Rossi A, Stratta P. Think twice: impulsivity and decision making in obsessive-compulsive disorder. J Behav Addict. 2015;4:263–272. doi: 10.1556/2006.4.2015.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Devi LA, Gomes I. Potentiation of mu-opioid receptor-mediated signaling by ketamine. J Neurochem. 2011;119:294–302. doi: 10.1111/j.1471-4159.2011.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JA, Aboobaker AA, Hodgetts TC, Ridley RM. Learning impairments induced by glutamate blockade using dizocilpine (MK-801) in monkeys. Br J Pharmacol. 1998;125:1013–1018. doi: 10.1038/sj.bjp.0702178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20:355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Mantione CR, Spada MR, Neumeyer JL, London ED. Further characterization of [3H]ifenprodil binding in rat brain. Eur J Pharmacol. 1994;266:67–77. doi: 10.1016/0922-4106(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, MacMillan C, Sevo J, Zeeb FD, Thevarkunnel S. Enhanced attention and impulsive action following NMDA receptor GluN2B–selective antagonist pretreatment. Behav Brain Res. 2016;311:1–14. doi: 10.1016/j.bbr.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Ho M-Y, Mobini S, Chiang T-J, Bradshaw CM, Szabadi E. Theory and method in the quantitative analysis of “impulsive choice” behaviour: implications for psychopharmacology. Psychopharmacology. 1999;146:362–372. doi: 10.1007/pl00005482. [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Anderson KG. Effects of different fixed-ratio requirements on delay discounting in rats. Behav Processes. 2013;100:18–22. doi: 10.1016/j.beproc.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D2 and serotonin 5-HT2 receptors - implications for models of schizophrenia. Mol Psychiatry. 2002;7:837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. J Sleep Res. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Ma YY, Yu P, Gou CY, Cui CL. Effects of ifenprodil on morphine-induced conditioned place preference and spatial learning and memory in rats. Neurochem. Res. 2011;36:383–391. doi: 10.1007/s11064-010-0342-9. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Johnson PS. Pathological gamblers discount probabilistic rewards less steeply than matched controls. Exp Clin Psychopharmacol. 2009;17:283–290. doi: 10.1037/a0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Henson C, France CP. Effects of amphetamine on delay discounting in rats depend on the manner in which delay is varied. Neuropharmacology. 2014;87:173–179. doi: 10.1016/j.neuropharm.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Lovinger DM. Ifenprodil inhibition of the 5-hydroxytryptamine3 receptor. Neuropharmacol. 1995;34:621–629. doi: 10.1016/0028-3908(95)00030-a. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Gilbert RJ, Bizon JL, Setlow B. Effects of acute administration of nicotine and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision making tasks in rats. Psychopharmacology. 2012;224:489–499. doi: 10.1007/s00213-012-2777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2000;152:390–397. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Montes DR, Stopper CM, Floresco SB. Noradrenergic modulation of risk/reward decision making. Psychopharmacology. 2015;232:2681–2696. doi: 10.1007/s00213-015-3904-3. [DOI] [PubMed] [Google Scholar]
- Ness V, Arning L, Niesert HE, Stüttgen MC, Epplen JT, Beste C. Variations in the GRIN2B gene are associated with risky decision-making. Neuropharmacology. 2011;61:950–956. doi: 10.1016/j.neuropharm.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Perin-Dureau F, Rachine J, Neyton J, Paoletti P. Mapping the binding site of the neuroprotectant ifenprodil on NMDA receptors. J Neurosci. 2002;22:5955–5965. doi: 10.1523/JNEUROSCI.22-14-05955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettorruso M, De Risio L, Martinotti G, Di Niccola M, Ruggeri F, Conte G, Di Giannantoni M, Janiri L. Targeting the glutamatergic system to treat pathological gambling: current evidence and future perspectives. Biomed Res Int. 2014;2014:109786. doi: 10.1155/2014/109786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles E, Vargas PA. Functional parameters of delay discounting assessment tasks: order of presentation. Behav Processes. 2007;75:237–241. doi: 10.1016/j.beproc.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Robles E, Vargas PA. Parameters of delay discounting assessment: number of trials, effort, and sequential effects. Behav Processes. 2008;78:285–290. doi: 10.1016/j.beproc.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Robles E, Vargas PA, Bejarano R. Within-subject differences in degree of delay discounting as a function of order of presentation of hypothetical cash rewards. Behav Processes. 2009;81:260–263. doi: 10.1016/j.beproc.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Robson MJ, Elliott M, Seminerio MJ, Matsumoto RR. Evaluation of sigma (σ) receptors in the antidepressant-like effectsof ketamine in vitro and in vivo. Eur Neuropsychopharmacol. 2012;22:308–317. doi: 10.1016/j.euroneuro.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Rokosik SL, Napier TC. Pramipexole-induced increased probability discounting: comparison between a rodent model of Parkinsons’ disease and controls. Neuropsychopharmacology. 2012;37:1397–1408. doi: 10.1038/npp.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Tsutsui KT, Clark JJ. Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcohol Clin Exp Res. 2014;38:1622–1629. doi: 10.1111/acer.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuermann B, Kathmann N, Stiglmayr C, Renneberg B, Endrass T. Impaired decision making and feedback evaluation in borderline personality disorder. Psychol Med. 2011;41:1917–1927. doi: 10.1017/S003329171000262X. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Bokhoven I, Vanderschuren LJ, Lochman JE, Matthys W. Risky decision making in substance dependent adolescents with a disruptive behavior disorder. J Abnorm Child Psychol. 2011;39:333–339. doi: 10.1007/s10802-010-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak JM, Anderson KG. Effects of variable training, signaled and unsignaled delays, and d-amphetamine on delay-discounting functions. Behav Pharmacol. 2009;20:424–436. doi: 10.1097/FBP.0b013e3283305ef9. [DOI] [PubMed] [Google Scholar]
- St. Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci. 2011;31:8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Onge JR, Chiu YC, Florescro SB. Differential effects of dopaminergic manipulations on risky choice. Psychopharmacology. 2010;211:209–221. doi: 10.1007/s00213-010-1883-y. [DOI] [PubMed] [Google Scholar]
- St. Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- St. Onge JR, Floresco SB. Prefrontal cortical contribution to risk-based decision making. Cereb Cortex. 2010;20:1816–1828. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- St. Onge JR, Stopper CM, Zahm DS, Floresco SB. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. J Neurosci. 2012;32:2886–2899. doi: 10.1523/JNEUROSCI.5625-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Khayambashi S, Floresco SB. Receptor-specific modulation of risk-based decision making by nucleus accumbens dopamine. Neuropsychopharmacology. 2013;38:715–728. doi: 10.1038/npp.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaldi J, Phillpsen A, Matthies S. Risky decision-making in borderline personality disorder. Psychiatry Res. 2012;197:112–118. doi: 10.1016/j.psychres.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Takagishi H, Nishinaka H, Makino T, Fukui H. Neuroeconomics of psychopathy: risk taking in probability discounting of gain and loss predicts psychopathy. Neuro Endocrinol Lett. 2014;35:510–517. [PubMed] [Google Scholar]
- Tanno T, Maguire DR, Hensen C, France CP. Effects of amphetamine and methylphenidate on delay discounting in rats: interactions with order of delay presentation. Psychopharmacology. 2014;231:85–95. doi: 10.1007/s00213-013-3209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov JC, Gorayeb SRP, Correa DL, Graeff FG. Effects of amphetamine on choice behavior of pigeons. Psychopharmacologia. 1972;26:395–400. doi: 10.1007/BF00421905. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Nishiyama S, Fukomoto D, Sato K, Kakiuchi T, Domimo E. Chronic NMDA antagonism impairs working memory, decreases extracellular dopamine, and increases D1 receptor binding in the prefrontal cortex of conscious monkeys. Neuropharmacology. 2005;30:1861–1869. doi: 10.1038/sj.npp.1300732. [DOI] [PubMed] [Google Scholar]
- Tuplin EW, Stocco MR, Holahan MR. Attenuation of MK-801-induced behavioral perseveration by typical and atypical antipsychotic pretreatment in rats. Behav Neurosci. 2015;129:399–411. doi: 10.1037/bne0000066. [DOI] [PubMed] [Google Scholar]
- Yates JR, Batten SR, Bardo MT, Beckmann JS. Role of ionotropic glutamate receptors in delay and probability discounting in the rat. Psychopharmacology. 2015;232:1187–1196. doi: 10.1007/s00213-014-3747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, de Wit H. The prescription opioid, oxycodone, does not alter behavioral measures of impulsivity in healthy volunteers. Pharmacol Biochem Behav. 2009;94:108–113. doi: 10.1016/j.pbb.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]