Summary
Hematopoietic stem cells give rise to all blood cells in a differentiation process that involves widespread epigenome remodeling. Here we present genome-wide reference maps of the associated DNA methylation dynamics. We used a meta-epigenomic approach that combines DNA methylation profiles across many small pools of cells and performed single-cell methylome sequencing to assess cell-to-cell heterogeneity. The resulting dataset identified characteristic differences between HSCs derived from fetal liver, cord blood, bone marrow, and peripheral blood. We also observed lineage-specific DNA methylation between myeloid and lymphoid progenitors, characterized immature multi-lymphoid progenitors, and detected progressive DNA methylation differences in maturing megakaryocytes. We linked these patterns to gene expression, histone modifications, and chromatin accessibility, and we used machine learning to derive a model of human hematopoietic differentiation directly from DNA methylation data. Our results contribute to a better understanding of human hematopoietic stem cell differentiation and provide a framework for studying blood-linked diseases.
Keywords: reference epigenome mapping, DNA methylation profiling, whole genome bisulfite sequencing, single-cell sequencing, hematopoietic stem cell differentiation, lymphoid-myeloid lineage commitment, immature lymphoid progenitors, megakaryocyte maturation, cell type prediction, bioinformatic lineage reconstruction
Graphical Abstract
Highlights
-
•
Sequencing provides DNA methylation maps of hematopoietic stem and progenitor cells
-
•
Methylation differs in HSCs from fetal liver, bone marrow, cord, and peripheral blood
-
•
Myeloid and lymphoid progenitors are distinguished by enhancer-linked DNA methylation
-
•
Machine learning enables data-driven reconstruction of the hematopoietic lineage
As part of the IHEC consortium, Bock and colleagues present genome-wide reference maps of DNA methylation dynamics during human blood development. The characteristic DNA methylation patterns they see in the different cell types allow data-driven inference of an epigenome-based model of hematopoietic differentiation. Explore the IHEC web portal at http://www.cell.com/consortium/IHEC.
Introduction
All blood cells originate from hematopoietic stem cells (HSCs), which represent the apex of a differentiation cascade of progenitor cell types that gives rise to billions of new cells every day. HSC differentiation is believed to progress through stepwise restriction of lineage potential, a concept that is summarized by the classical tree model of murine hematopoiesis (Spangrude, 1991, Till and McCulloch, 1980).
HSC differentiation in human is less well understood than in mouse. Despite recent progress (reviewed in Doulatov et al., 2012, Theocharides et al., 2016, Vedi et al., 2016), several aspects of human hematopoiesis have remained controversial (Chen et al., 2014, Doulatov et al., 2010, McCracken et al., 2013, Notta et al., 2016, Park et al., 2008, Tanner et al., 2014, Woolthuis and Park, 2016).
We sought to use DNA methylation for in vivo dissection of human hematopoiesis. DNA methylation is well suited for studying cellular differentiation because its patterns are cell-type-specific and retain an epigenetic memory of a cell’s developmental history. For example, cell-of-origin-specific DNA methylation patterns are detectable among induced pluripotent stem cells (Kim et al., 2011, Polo et al., 2010), and such patterns of epigenetic tissue memory predict primary tumor location in metastatic cancers (Fernandez et al., 2012, Moran et al., 2016).
Previous studies have established a close connection between stem cell differentiation and widespread epigenome remodeling. DNA methylation has been studied in early mammalian development (Smallwood et al., 2011, Smith et al., 2012), mouse HSC differentiation (Bock et al., 2012, Cabezas-Wallscheid et al., 2014, Ji et al., 2010), neural differentiation (Lister et al., 2013), pluripotent stem cells (Bock et al., 2011, Habibi et al., 2013), and a broad collection of human tissue samples (Kundaje et al., 2015, Ziller et al., 2013). Chromatin accessibility has been mapped using the assay for transposase-accessible chromatin with high throughput sequencing (ATAC-seq) in multiple cell types of the human blood lineage (Corces et al., 2016), and three recent studies used chromatin immunoprecipitation sequencing (ChIP-seq) to map histone modifications in the developing mouse embryo (Dahl et al., 2016, Liu et al., 2016, Zhang et al., 2016).
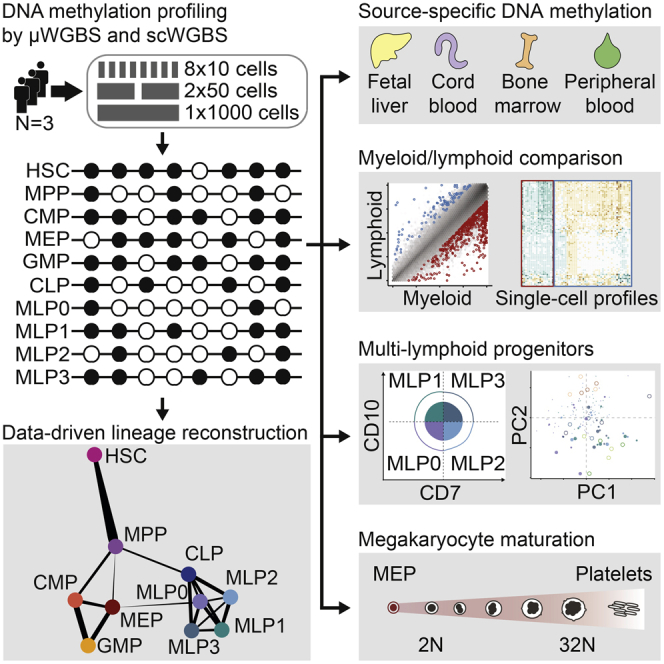
To establish a basis for epigenome-wide analysis and data-driven modeling of the human hematopoietic lineage, we applied our protocol for low-input and single-cell whole genome bisulfite sequencing (Farlik et al., 2015) to 17 hematopoietic cell types (Figure 1A). HSCs and multipotent progenitors (MPPs) were sorted from fetal liver, cord blood, bone marrow, and peripheral blood. Eight additional progenitor cell types and six differentiated cell types were sorted from peripheral blood, and megakaryocytes were sorted from bone marrow. For each stem and progenitor cell type, we sequenced an average of 32 low-input methylomes from three individuals, and we bioinformatically integrated them into meta-epigenomic profiles (Wijetunga et al., 2014). Additionally, we sequenced an average of 26 single-cell methylomes for seven cell types (HSC, MPP, common lymphoid progenitor [CLP], common myeloid progenitor [CMP], immature multi-lymphoid progenitor [MLP0], granulocyte macrophage progenitor [GMP], and megakaryocytes) to assess cell-to-cell heterogeneity.
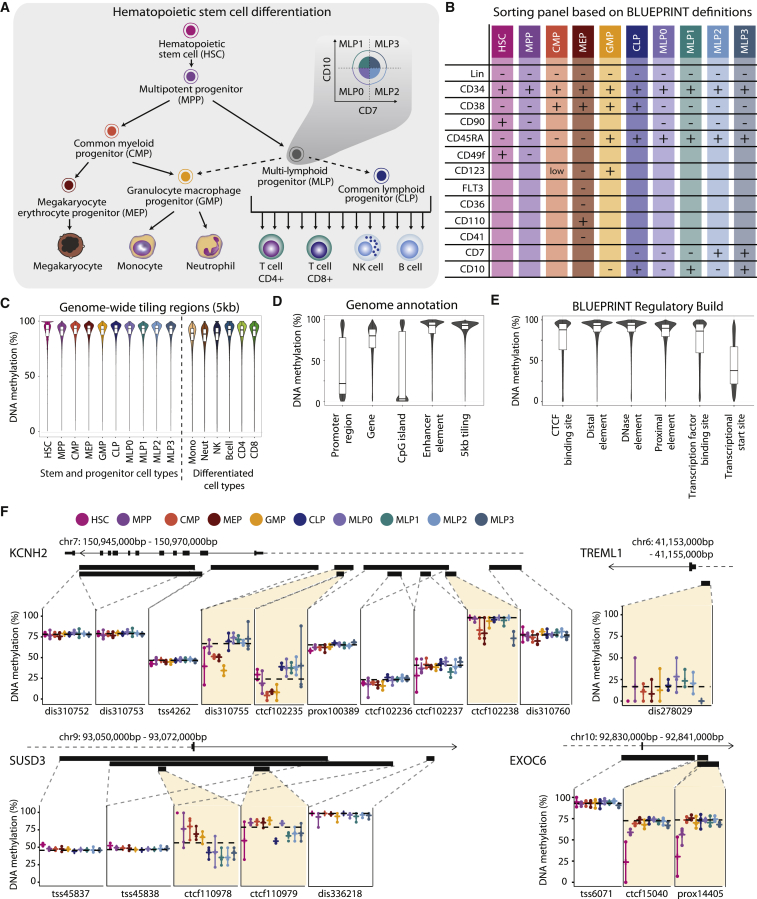
Figure 1.
Charting the DNA Methylation Landscape of Human Hematopoietic Differentiation
(A) Conceptual outline of human hematopoietic differentiation, highlighting the 17 hematopoietic cell types whose genome-wide DNA methylation patterns were profiled in this study. Arrows denote established differentiation trajectories, dashed arrows indicate uncertainty about the in vivo differentiation potential of lymphoid progenitors, and the inset illustrates the sorting of four subsets of immature multi-lymphoid progenitors.
(B) Fluorescence-activated cell sorting panel used to purify 10 stem and progenitor cell types from peripheral blood.
(C) Violin plots and boxplots showing the distribution of DNA methylation levels in 5-kb tiling regions for hematopoietic cell types sorted from peripheral blood.
(D) Distribution of DNA methylation levels across cell types for different sets of genomic regions. Gene and promoter annotations are based on GENCODE, CpG islands are from the UCSC Table Browser, enhancer elements are from Ensembl, and tiling regions were calculated with a custom script.
(E) Distribution of average DNA methylation levels across cell types for putative regulatory regions annotated by the Ensembl BLUEPRINT Regulatory Build.
(F) DNA methylation at putative regulatory regions for illustrative gene loci. Black bars denote the position of regions annotated by the BLUEPRINT Regulatory Build, and dashed horizontal black lines indicate sample medians for the respective regions. Colored vertical bars connect the highest and lowest DNA methylation levels that have been measured in any sample of the indicated cell type.
dis, distal element; prox, proximal element; TSS, transcriptional start site. See also Figure S1 and http://blueprint-methylomes.computational-epigenetics.org.
Based on this dataset, which constitutes a community resource of the BLUEPRINT project (Adams et al., 2012) and the International Human Epigenome Consortium (IHEC; http://ihec-epigenomes.org), we compared DNA methylation between HSCs derived from different sources, and we studied changes in DNA methylation associated with commitment to the myeloid and lymphoid lineages. We also characterized novel subpopulations of immature multi-lymphoid progenitors and investigated the DNA methylation dynamics of megakaryocytes undergoing endomitotic replication. We linked the observed differences in DNA methylation to changes in gene expression, histone modifications, and chromatin accessibility, and we used machine learning to infer a model of human hematopoiesis directly from the DNA methylation data. These results highlight the power of DNA methylation analysis for in vivo dissection of cellular differentiation.
Results
Comprehensive DNA Methylation Maps of Human Hematopoietic Stem and Progenitor Cell Types
We established fluorescence-activated cell sorting panels for 10 hematopoietic stem and progenitor cell types that are present in the peripheral blood of healthy individuals (Figures 1A and 1B; Supplemental Experimental Procedures). Each cell type was sorted from three donors to account for inter-individual heterogeneity. To enhance data quality for these rare cell types, we processed many small pools of cells in parallel and combined the results. Specifically, for each donor and cell type, we sorted and sequenced eight pools of 10 cells, two pools of 50 cells, and one pool of 1,000 cells (or a lower cell number where the target of 1,000 cells could not be reached).
DNA methylation libraries were generated by whole genome bisulfite sequencing (WGBS) using the μWGBS protocol (Farlik et al., 2015) and sequenced at low coverage to minimize the number of PCR duplicates. In total, 639 DNA methylation libraries passed quality control, and 3.1 terabases of sequencing data were produced (Table S1). DNA methylation profiles clustered predominantly by cell type (Figure S1A), indicating that neither technical biases arising from the different cell numbers nor inter-individual variation between donors had a strong influence on our investigation of cell-type-specific DNA methylation patterns. For further analysis, the DNA methylation profiles of all replicates of a given cell type were computationally combined into meta-epigenomic maps that provide consensus DNA methylation levels as well as an initial assessment of variability within cell types and among individuals.
The distribution of DNA methylation levels was similar across all stem and progenitor cell types, while we observed a shift toward lower levels in differentiated cells of the myeloid lineage (Figure 1C). Genome-wide DNA methylation patterns followed the well-established characteristics observed in mammalian genomes (Suzuki and Bird, 2008), including high levels of DNA methylation in most parts of the genome (as illustrated by 5-kb tiling regions) and locally reduced levels at gene promoters and CpG islands (Figure 1D).
To provide a robust and biologically meaningful basis for analyzing DNA methylation differences between cell types, we aggregated all DNA methylation data at the genomic region level based on the BLUEPRINT version of the Ensembl Regulatory Build (Zerbino et al., 2015). The BLUEPRINT Regulatory Build integrates epigenome data across many cell types into region sets that reflect the organizing principles of the human genome, thus facilitating the detection of meaningful DNA methylation differences (Bock, 2012). This catalog comprises six types of putative regulatory regions, which exhibit broadly varying DNA methylation levels in our dataset (Figure 1E).
The BLUEPRINT Regulatory Build also provides a framework for visualization (Figure 1F) and interactive analysis (http://blueprint-methylomes.computational-epigenetics.org) of DNA methylation at individual genomic loci. For example, two CTCF sites and a distal element inside the KCNH2 gene (encoding a key factor for erythroid development) show decreased DNA methylation in the myeloid lineage, consistent with increased expression levels in CMP and GMP cells (Figure S1B). A putative enhancer of the myeloid-linked TREML1 gene displays decreased DNA methylation in HSCs, MPPs, and myeloid progenitors, which correlates with increased RNA expression levels. CTCF sites in the lymphoid-linked SUSD3 gene show lower DNA methylation in lymphoid progenitors, reflecting high expression in MLP0. Finally, a promoter-associated regulatory region in the EXOC6 gene illustrates the frequently observed case of large DNA methylation differences that occur in the absence of detectable changes in gene expression.
DNA Methylation Distinguishes HSCs from Fetal Liver, Cord Blood, Bone Marrow, and Peripheral Blood
HSCs are rare in peripheral blood, whereas they exist in higher frequencies in fetal liver, cord blood, and bone marrow. HSCs obtained from these different sources have been shown to vary in their differentiation capacity (Notta et al., 2016), which prompted us to search for concomitant differences in their DNA methylation profiles. We obtained CD34+ cells from fetal liver, cord blood, and bone marrow, and we sorted HSCs and MPPs in the same way as for peripheral blood (Figure 2A). DNA methylation analysis identified many more differences between peripheral blood and any of the other three sources (fetal liver, cord blood, and bone marrow) than between any two of the latter (Figure 2B; Table S2). Most of the genomic regions with source-dependent differences showed lower DNA methylation levels in HSCs and MPPs from peripheral blood, as compared with those obtained from the other sources.
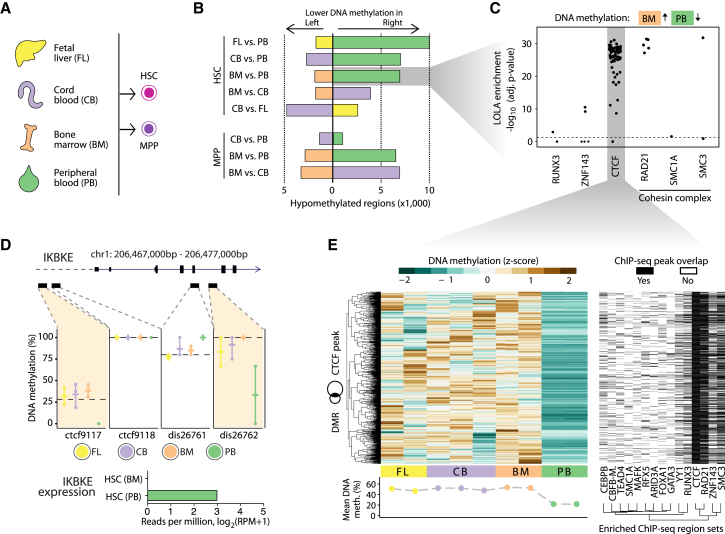
Figure 2.
Comparison of DNA Methylation Maps for HSCs and MPPs Isolated from Four Different Sources
(A) HSCs and MPPs were sorted from the peripheral blood of three healthy donors (Figures 1A and 1B), and in addition from fetal liver (HSCs only), cord blood (HSCs and MPPs), and bone marrow (HSCs and MPPs).
(B) Bar plots showing the numbers of differentially methylated regions in pairwise comparisons between HSCs and MPPs from different sources, based on the BLUEPRINT Regulatory Build regions (FDR-adjusted p ≤ 0.05, absolute difference ≥ 0.167 percentage points), calculated with RnBeads (Assenov et al., 2014).
(C) Region set enrichment analysis for genomic regions with lower DNA methylation in peripheral blood-derived HSCs compared with bone-marrow-derived HSCs. Enrichment was determined using LOLA (Sheffield and Bock, 2016). Each dot represents one ChIP-seq dataset, and the horizontal dashed line corresponds to a significance threshold of 0.05 on the adjusted p-value calculated by LOLA using Fisher’s exact test.
(D) Source-specific DNA methylation at the IKBKE gene locus. Reduced DNA methylation levels in peripheral blood-derived HSC at two putative regulatory regions (BLUEPRINT Regulatory Build) is associated with detectable expression of the IKBKE gene specifically in this cell population (bar plot).
(E) Heatmap showing DNA methylation levels for regions that have lower DNA methylation in peripheral blood-derived HSCs than in bone-marrow-derived HSCs and also overlap with CTCF binding sites in the LOLA Core database (left). The second heatmap (right) shows the overlap of these regions with transcription factor binding sites that a LOLA analysis of this region set identified as enriched. Rows were arranged by hierarchical clustering with complete linkage based on the Euclidean distances between the DNA methylation profiles.
See also Figure S2 and http://blueprint-methylomes.computational-epigenetics.org.
We tested the regions that were specifically hypomethylated in peripheral blood HSCs for associations with transcription factor binding and regulatory elements using LOLA enrichment analysis (Sheffield and Bock, 2016). Significant overlap was observed with binding sites of CTCF, members of the cohesin complex (RAD21, SMC1A, SMC3), and the transcription factors RUNX3 and ZNF143 (Figure 2C; Table S3). We detected similar patterns for both HSCs and MPPs, whereas no such enrichment could be found, for example, for regions differentially methylated between MPPs in bone marrow and cord blood (Figure S2B). An illustrative example of peripheral blood hypomethylation of CTCF binding sites is given by the IKBKE gene (Figure 2D), which encodes a key kinase for NF-κB activation.
To identify additional transcription factors that may be associated with this CTCF-linked difference in DNA methylation, we performed LOLA analysis on all regions with lower DNA methylation in HSCs from peripheral blood than from bone marrow that also overlapped CTCF-bound regions (Figure 2E). This analysis confirmed the strong enrichment of cohesin complex proteins, while also detecting significant overlap for transcriptions factors relevant for hematopoietic development (FOXA1, GATA3, MAFK) and immune cell function (ARID3A, CEBPB, RFX5).
Myeloid-Lymphoid Lineage Choice Is Marked by DNA Methylation Depletion at Key Transcription Factor Binding Sites
After the initial transition from HSC to MPP, one major step of hematopoietic differentiation is the commitment to either the myeloid or the lymphoid lineage. DNA methylation levels at regulatory regions were on average lower in myeloid progenitors (CMP, megakaryocyte erythrocyte progenitor [MEP], GMP) than in lymphoid progenitors (MLP0, MLP1, MLP2, MLP3, CLP), and the same was true for differentiated cells of the two lineages (Figure S3A). Focusing again on the BLUEPRINT Regulatory Build (Figure 3A; Table S2), we also identified many more genomic regions with lower DNA methylation in myeloid cells (n = 607) than in lymphoid cells (n = 101). On average, these regions retained their differential DNA methylation in differentiated cells of the two lineages (Figure 3B).
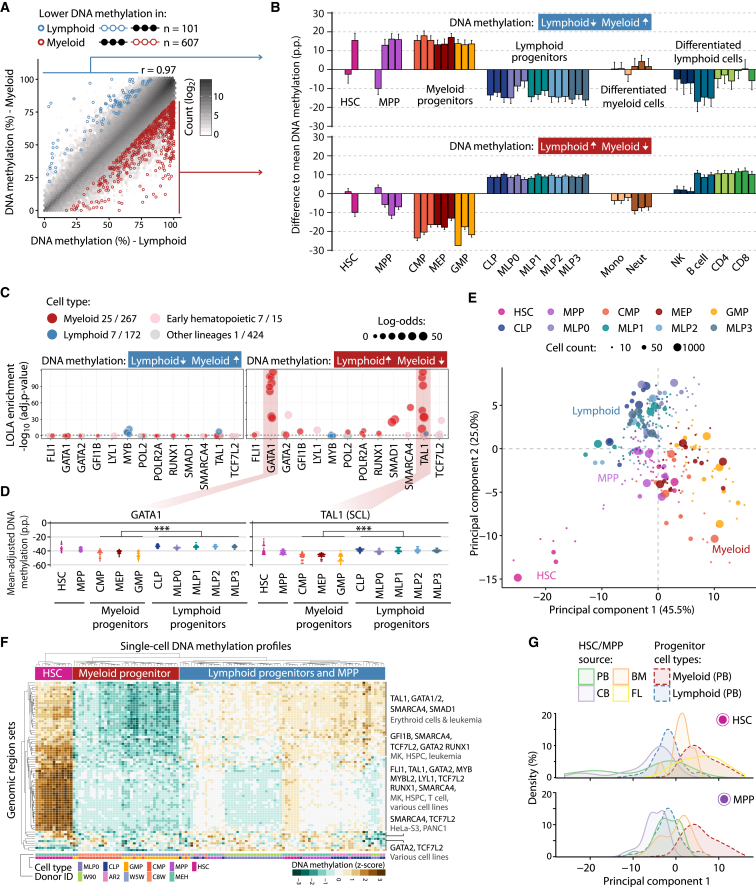
Figure 3.
DNA Methylation Differences Associated with Myeloid-Lymphoid Lineage Commitment
(A) Scatterplot showing average DNA methylation levels for myeloid progenitors (CMP, MEP, GMP) and lymphoid progenitors (CLP, MLP0, MLP1, MLP2, MLP3) across BLUEPRINT Regulatory Build regions (Pearson’s r = 0.97). Differentially methylated regions were identified with RnBeads (FDR-adjusted p ≤ 0.05, absolute difference ≥ 0.167 percentage points).
(B) Average DNA methylation in each cell type relative to the mean over all samples aggregated over all regions with lower DNA methylation in lymphoid progenitors (top) and myeloid progenitors (bottom). Error bars correspond to the standard error.
(C) Region set enrichment analysis for regions with lower DNA methylation in lymphoid progenitors (left) or myeloid progenitors (right). Enrichment was determined using LOLA. Colored dots represent ChIP-seq experiments for transcription factors in the indicated lineage. Dot size denotes the log-odds ratio, and the numbers in the legend (“X/Y”) refer to significantly enriched region sets (X) versus all analyzed region sets (Y). The horizontal dashed line represents the significance threshold (adjusted p ≤ 0.05).
(D) Mean-adjusted DNA methylation relative to the average CpG methylation levels for each individual 10-, 50-, and 1,000-cell sample averaged across ChIP-seq peaks for GATA1 (left) and TAL1 (right). ∗∗∗p ≤ 0.001 (two-tailed Wilcoxon test).
(E) Two-dimensional projection of all 10-, 50-, and 1,000-cell samples from peripheral blood using principal component analysis based on the mean-adjusted DNA methylation across all transcription factor binding datasets identified by LOLA. The first two principal components are shown, and the numbers in parentheses indicate the percentage of variance explained.
(F) Heatmap displaying mean-adjusted DNA methylation for all single-cell DNA methylation profiles across the same region sets as in (E). Rows and columns are arranged by hierarchical clustering with Euclidean distance and complete linkage. The labels on the right summarize the transcription factors and cell types for the major branches of the row dendrogram.
(G) Distribution of HSC (top) and MPP (bottom) samples derived from fetal liver (FL), cord blood (CB), bone marrow (BM), and peripheral blood (PB) when projected onto the first principal component from (E).
p.p., percentage points. See also Figure S3 and http://blueprint-methylomes.computational-epigenetics.org.
Differentially methylated regions between myeloid and lymphoid progenitors were enriched for binding sites of 11 transcription factors and for RNA polymerase II binding in hematopoietic cells (Figure 3C; Table S3). The most striking overlap was observed between regions with lower DNA methylation in myeloid cells and binding sites of myeloerythroid transcription factors such as GATA1 and TAL1. In contrast, regions with lower DNA methylation levels in lymphoid progenitors did not show such strong enrichment patterns for any transcription factor binding sites annotated in the LOLA Core database. The average DNA methylation levels across all binding sites of the myeloid-specific transcription factors were reduced in myeloid progenitors when compared with lymphoid progenitors (Figures 3D and S3B). For about half of the transcription factors, the lower DNA methylation in myeloid (as opposed to lymphoid) progenitors was mirrored in higher expression levels in myeloid progenitors (Figure S3B).
The average DNA methylation depletion at the enriched transcription factor binding sites enabled consistent grouping of the individual replicates (10-, 50-, and 1,000-cell pools) according to their cellular lineage (Figure 3E), whereas the segregation by lineage was less clear when we performed the same analysis on all transcription factors in the LOLA Core database (Figure S3C). The first five principal components calculated from the mean-adjusted DNA methylation at the enriched transcription factor binding sites accounted for 82.3% of the observed variation (Figure S3D), whereas this value was much lower when focusing on all transcription factor binding sites (63.4%) or on all regions in the LOLA Core database (29.6%).
Averaging across pre-defined regulatory region sets is also a powerful method for analyzing single-cell data (Bock et al., 2016a, Farlik et al., 2015), and we applied this method to our set of 122 single-cell DNA methylation profiles comprising HSCs and MPPs, two myeloid progenitors (CMP and GMP), and two lymphoid progenitors (MLP0 and CLP). Plotting all single-cell profiles based on their mean-adjusted DNA methylation at enriched transcription factor binding sites (Figure 3F), we observed that region sets with low levels of DNA methylation in myeloid progenitors had much higher levels in HSCs. About half of these region sets were highly methylated in lymphoid progenitors, whereas the other half showed low levels of DNA methylation in some lymphoid progenitors (MLP0).
Finally, we investigated how the source-specific differences in DNA methylation among HSCs and MPPs (Figure 2) relate to differences between the myeloid and lymphoid lineage. To this end, we projected the DNA methylation data for HSCs and MPPs onto the first principal component identified in our analysis of mean-adjusted DNA methylation at transcription factor binding sites (Figure 3E), and we plotted the distribution along the first principal component, which was most informative for the myeloid-lymphoid separation (Figure 3G). DNA methylation patterns in HSCs and MPPs from peripheral blood and cord blood were more similar to those of lymphoid progenitors, whereas cells from bone marrow and fetal liver showed higher similarity to myeloid progenitors.
Immature Multi-lymphoid Progenitors Show Characteristic DNA Methylation and Distinct Differentiation Propensities
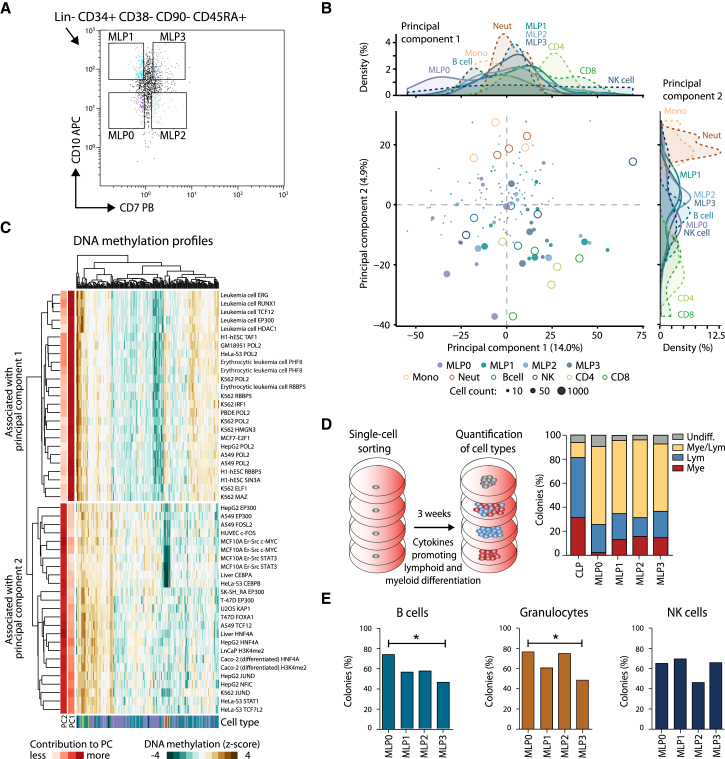
Recent research identified a population of immature multi-lymphoid progenitors (MLPs) that may be ancestral to CLPs in the differentiation hierarchy (Doulatov et al., 2010, Doulatov et al., 2012, Goardon et al., 2011, Kohn et al., 2012). We sorted MLPs using the published set of surface markers (Doulatov et al., 2010) and further subdivided this cell population into four subtypes based on their CD10 and CD7 levels (Figures 4A and S4A).
Figure 4.
Characterization of MLP Populations by DNA Methylation and In Vitro Differentiation Assays
(A) Sorting panel for purifying four MLP populations from peripheral blood.
(B) Two-dimensional projection of all 10-, 50-, and 1,000-cell MLP samples using principal component analysis based on the mean-adjusted DNA methylation relative to the average CpG methylation levels across all region sets in the LOLA Core database. The first two principal components are shown, the numbers in parentheses indicate the percentage of variance explained, and the density plots (top and right) summarize the distribution of cell types along the two principal components.
(C) Heatmap displaying the mean-adjusted DNA methylation for all MLP samples across the 2 × 25 genomic region sets that contributed most strongly to the first principal component (PC1, top) and the second principal component (PC2, bottom). Rows and columns are arranged by hierarchical clustering with Euclidean distance and complete linkage. The row labels indicate the cell type and ChIP-seq target of the corresponding LOLA region sets.
(D) Differentiation potential of CLPs and four MLP populations measured by in vitro culture of single cells on MS-5 stroma with cytokines promoting lymphoid and myeloid differentiation. The percentage of colonies that show lymphoid (CD19+ or CD56+) as well as myeloid (CD14+ or CD15+) markers was determined by flow cytometry.
(E) Differentiation potential of the MLP populations measured as the percentage of colonies containing B cells (CD19+), granulocytes (CD15+), or NK cells (CD56+) in flow cytometry. ∗p ≤ 0.05 (Fisher’s exact test).
See also Figure S4 and http://blueprint-methylomes.computational-epigenetics.org.
To put the MLP subtypes into context with their differentiated progeny, we performed an unsupervised principal component analysis based on DNA methylation for all region sets contained in the LOLA Core database (Figure 4B). The first principal component segregated the MLP0 population (CD10−, CD7−) from the other progenitors and differentiated cell types. The second principal component discriminated between differentiated cell types of the myeloid and lymphoid lineage, placing the four MLP populations in an intermediate position.
We identified the region sets in the LOLA Core database that were most strongly associated with the first two principal components (Figure 4C). The first principal component comprised binding sites of broadly active transcription regulators and chromatin proteins (EP300, HDAC1, POL2, RBBP5, TAF1), whereas the second principal component included binding sites of transcription factors that are important for lymphoid and myeloid cell function (FOXA1, KAP1/TRIM28, MYC, STAT1, STAT3, TCF12).
We also assessed the differentiation capability of the lymphoid progenitors using in vitro colony formation assays (Figures 4D and S4B). CLPs from peripheral blood gave rise not only to lymphoid-restricted colonies, but also to a small number of myeloid and mixed myeloid and lymphoid colonies. This is in contrast with a previous analysis of cord-blood-derived cells (Doulatov et al., 2010) and highlights that the differentiation potential of progenitor populations in human is dependent on the cell source and stage of ontogeny (Notta et al., 2016). All MLP populations displayed higher proportions of mixed myeloid and lymphoid colonies than observed for the CLPs. The differentiation potential was similar among the four MLP subtypes, although MLP0 gave rise to the smallest number of myeloid-only colonies (Figure 4D) and had the highest potential for B cells and granulocytes (Figure 4E).
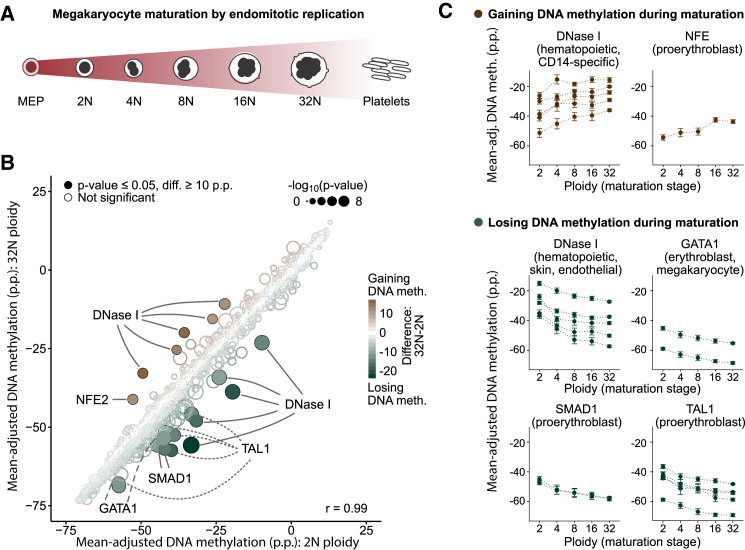
Endomitotic Replication of Megakaryocytes Is Accompanied by Progressive Changes in DNA Methylation Patterns
In the myeloid lineage, megakaryocyte maturation involves endomitotic replication and an exponential increase in cell ploidy (Figure 5A). Megakaryocytes are thought to be derived from MEPs, although evidence for mouse and human suggests an alternative origin directly from HSCs (Haas et al., 2015, Notta et al., 2016, Sanjuan-Pla et al., 2013). We collected megakaryocytes from the bone marrow of three donors, sorted them according to their ploidy (2N, 4N, 8N, 16N, 32N), and performed DNA methylome sequencing on 61 single cells and ten 5-cell pools (Table S1). The results were highly consistent between the single-cell and 5-cell samples (Figures S5A and S5B), arguing against technical biases caused by different DNA amounts influencing our analysis.
Figure 5.
DNA Methylation Analysis of Megakaryocyte Maturation
(A) Conceptual outline of megakaryocyte development from MEPs via a maturation phase involving endomitotic genome replication and a concomitant increase in ploidy.
(B) Scatterplot comparing mean-adjusted DNA methylation (relative to the average CpG methylation level in each sample) for all region sets in the LOLA Core database between megakaryocyte at the 2N and at the 32N stage of ploidy. Region sets that were significantly less methylated in 32N (n = 14, bottom right) or in 2N megakaryocyte (n = 6, top left) are highlighted with filled circles (p ≤ 0.05, Wilcoxon test, absolute difference ≥ 10 p.p.). Point colors indicate the magnitude of difference, and the size is proportional to statistical significance [−log10(p)].
(C) Mean-adjusted DNA methylation in region sets that gain (top) or lose DNA methylation (bottom) as identified in (B), plotted across ploidy stages of megakaryocyte maturation. Error bars correspond to the standard error.
p.p., percentage points. See also Figure S5 and http://blueprint-methylomes.computational-epigenetics.org.
Comparing DNA methylation for all LOLA Core region sets between diploid (2N) and polyploid (32N) megakaryocytes, we observed strong correlation (Pearson’s r = 0.99) and highly similar distributions of DNA methylation values (Figures 5B, S5A, and S5B), indicating that megakaryocyte maturation does not involve any large genome-wide changes in DNA methylation as previously observed for mouse erythroblast maturation (Shearstone et al., 2011). Nevertheless, a small number of region sets were differentially methylated, and these regions underwent consistent and progressive changes across the different ploidy stages of megakaryocyte maturation (Figure 5C).
Progressively increasing DNA methylation levels were observed for DNase I hypersensitive sites specific to hematopoietic cells and for binding sites of NFE2, which is a regulator of megakaryocyte maturation and platelet production (Lecine et al., 1998). Conversely, decreasing DNA methylation occurred in DNase I hypersensitive sites from a broader set of cell types and at the binding sites of hematopoietic transcription factors with an established role in megakaryocyte-erythroblast differentiation, including GATA1, SMAD1, and TAL1 (Tijssen et al., 2011).
The region sets that showed progressively decreasing DNA methylation levels in maturing megakaryocytes were on average more highly methylated in other progenitor cell types than in megakaryocytes (Figure S5C). In contrast, region sets with progressively increasing DNA methylation levels during megakaryocyte maturation moved toward the average levels in other progenitors rather than away from it.
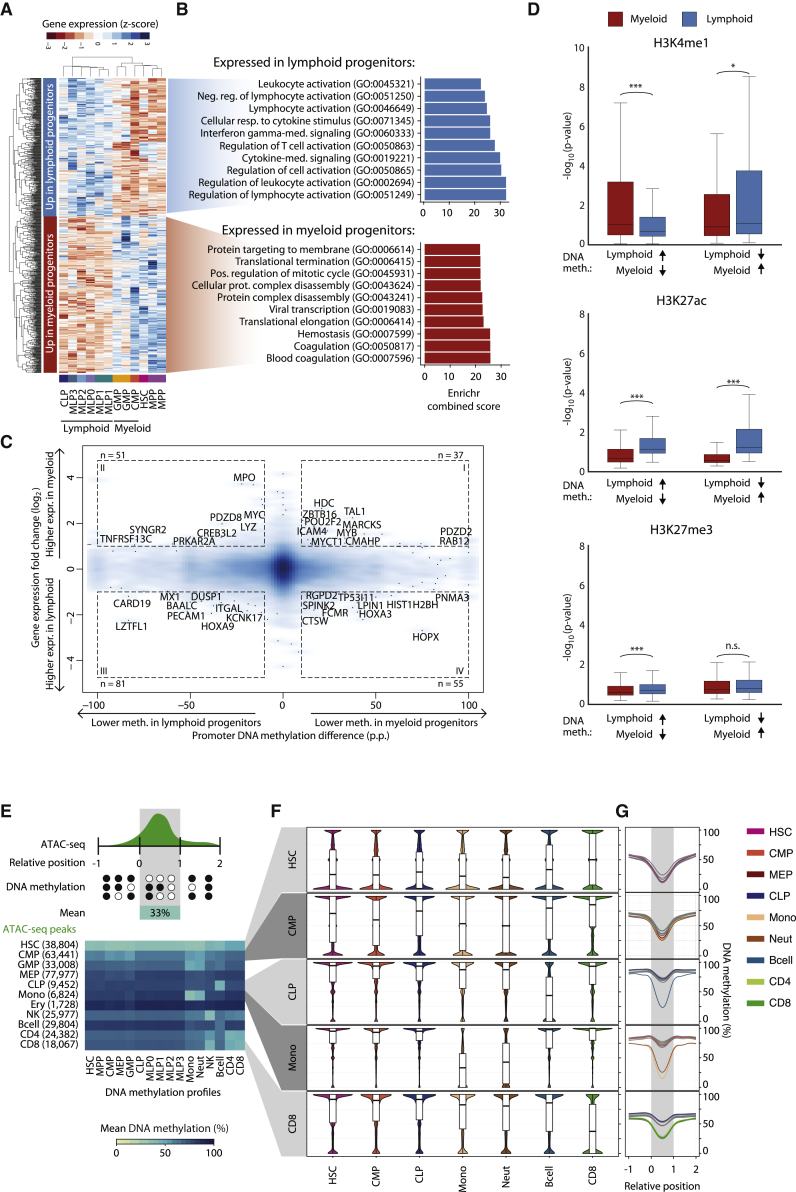
DNA Methylation Differences Are Linked to Cell-type-Specific Transcription Levels and Chromatin Signatures
DNA methylation at gene promoters can be associated with transcriptional repression, although the genome-wide correlation between DNA methylation and gene expression is low (Jones, 2012, Suzuki and Bird, 2008). To investigate this association in our dataset, we generated RNA-seq data for 100-cell pools of stem and progenitor cell types sorted from peripheral blood (Table S1) (http://blueprint-methylomes.computational-epigenetics.org), and we identified 656 genes that were differentially expressed between myeloid and lymphoid progenitors (false discovery rate [FDR]-adjusted p ≤ 0.05, |log2FC| ≥ 1). Gene Ontology analysis revealed an enrichment for genes associated with lymphocyte function in lymphoid progenitors and for genes associated with hemostasis in myeloid progenitors (Figures 6A and 6B).
Figure 6.
Integrative Analysis of Gene Expression, Histone Modifications, and Chromatin Accessibility
(A) Heatmap showing row-normalized expression levels for 656 differentially expressed genes (FDR-adjusted p ≤ 0.05, |log2FC| ≥ 1) between myeloid progenitors (CMP, GMP) and lymphoid progenitors (CLP, MLP0, MLP1, MLP2, MLP3) determined using DEseq2 (Love et al., 2014). Rows and columns were arranged by hierarchical clustering with Euclidean distance and complete linkage.
(B) Top 10 most highly enriched Gene Ontology terms associated with genes overexpressed in lymphoid (top) and myeloid (bottom) progenitors based on the Enrichr software (Kuleshov et al., 2016).
(C) Density scatterplot contrasting myeloid-lymphoid differences in DNA methylation at gene promoters with expression differences of the corresponding genes. Selected genes with strong differences in DNA methylation (absolute difference ≥ 10 p.p.) and gene expression (|log2FC| ≥ 1) are highlighted.
(D) Boxplots showing histone modification levels for open chromatin-associated H3K4me1, active enhancer-linked H3K27ac, and polycomb-associated H3K27me3 in regions that were differentially methylated between myeloid and lymphoid progenitors (Figure 3A). Histone modification levels were calculated from multiple ChIP-seq datasets for myeloid cells (neutrophils, monocytes, and macrophages, in red) and lymphoid cells (NK cells, B cells, and CD4+/CD8+ T cells, in blue). Brackets identify two-tailed Mann-Whitney U tests. ∗p ≤ 0.05, ∗∗∗p ≤ 0.001.
(E) Heatmap showing DNA methylation levels (columns) in regions with cell-type-specific chromatin accessibility based on published ATAC-seq data for hematopoietic cell types (rows). Numbers in parentheses denote the number of chromatin accessible regions specific to each cell type.
(F) Distribution of DNA methylation levels across regions with cell-type-specific chromatin accessibility.
(G) Composite plots showing DNA methylation averages across regions with cell-type-specific chromatin accessibility. CpGs in the neighborhood of these regions were annotated with coordinates relative to their start and end (x axis). CpGs with a relative coordinate of 0 and 1 are located at the start and end of a region, respectively, and the coordinates −1 and 2 correspond to one region length upstream and downstream of the region. The curves show cubic spline smoothing of DNA methylation levels per cell type across accessible regions.
p.p., percentage points; n.s., not significant. See also Figure S6 and http://blueprint-methylomes.computational-epigenetics.org.
When we linked the observed differences in gene expression to DNA methylation differences at associated promoters, we found only a small number of genes with strong and concordant changes (Figure 6C), which is consistent with previous observations for mouse hematopoiesis (Bock et al., 2012). Among the genes whose promoters were less methylated and more highly expressed in myeloid progenitors were myeloid regulators such as TAL1, MYB, MARCKS, and ICAM4. Conversely, several genes that play a role in lymphocyte function—including ITGAL, DUSP1, and MX1—were less methylated and more highly expressed in lymphoid progenitors (Figure 6C).
We also investigated the link between DNA methylation and histone modifications. Using ChIP-seq profiles for differentiated blood cells types, which have been generated as part of the BLUEPRINT project (Adams et al., 2012), we calculated consensus maps for three histone modifications (H3K4me1, H3K27ac, and H3K27me3) in myeloid and lymphoid cells. Regions with lower DNA methylation levels in myeloid progenitors showed higher H3K4me1 levels in differentiated myeloid cells, and the opposite was true for regions with lower DNA methylation in lymphoid progenitors (Figure 6D). For H3K27ac, we observed consistently higher levels in lymphoid cells than in myeloid cells, whereas the observed differences for H3K27me3 were less pronounced than for the other marks.
Finally, we compared our DNA methylation data with a recently published chromatin accessibility dataset (Corces et al., 2016). This dataset includes ATAC-seq profiles for several hematopoietic stem and progenitor cell types, from which we derived cell-type-specific regions of open chromatin. Genomic regions with HSC-specific open chromatin had low DNA methylation levels across all cell types (Figures 6E–6G, S6A, and S6B), regions with open chromatin in differentiated cells showed reduced DNA methylation levels only in the corresponding cell type while being highly methylated in progenitors, and regions with accessible chromatin in myeloid or lymphoid progenitors were hypomethylated only in differentiated cells of the respective lineage.
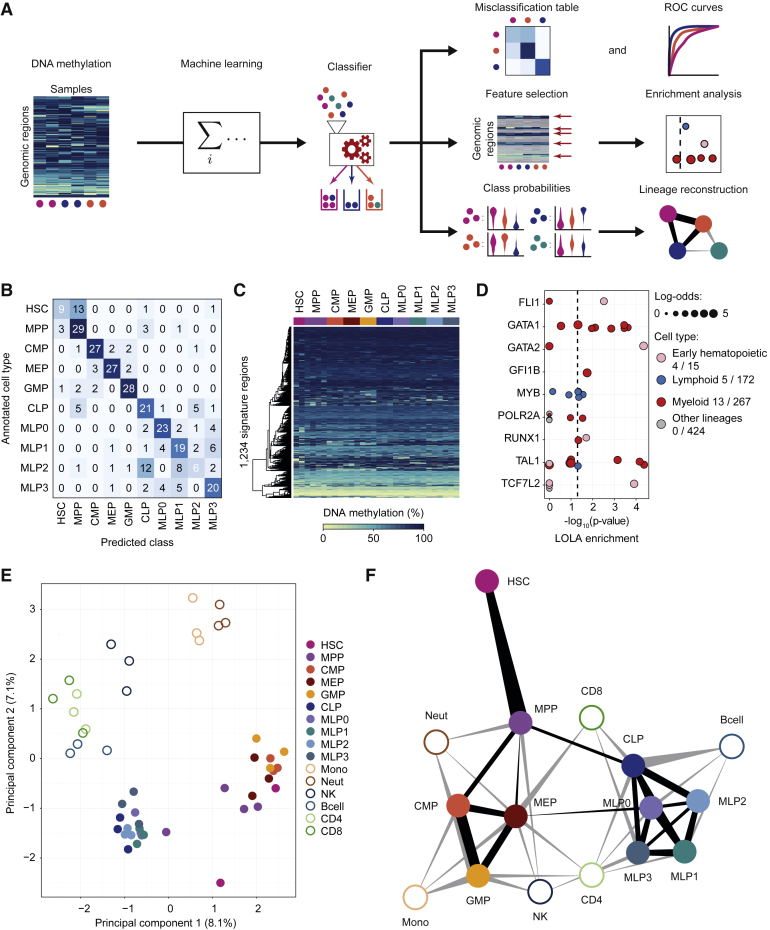
Computational Modeling Identifies Predictive Epigenetic Signatures that Support Data-Driven Lineage Reconstruction
Having identified characteristic DNA methylation dynamics in several branches of the human hematopoietic lineage, we employed machine learning methods in order to predict cell types from DNA methylation patterns, to quantify epigenetic similarity, and to infer cellular differentiation landscapes. We based this analysis on classifiers that were trained to predict cell type from genome-wide DNA methylation profiles in putative regulatory regions (Figure 7A).
Figure 7.
Data-Driven Reconstruction of the Human Hematopoietic Lineage using Machine Learning
(A) Conceptual outline of the machine learning approach used to predict cell type, to identify signature regions, and to infer cellular differentiation landscapes.
(B) Confusion matrix showing the frequency of misclassification based on 10-fold cross-validation of cell-type classifiers trained and evaluated on 319 stem and progenitor samples (all 10-, 50-, and 1,000-cell pools) from peripheral blood.
(C) Heatmap showing average DNA methylation levels of merged replicates (one column for each cell type in each donor) for the 1,234 signature regions extracted from a classifier trained on all peripheral blood-derived stem and progenitor samples. Regions (rows) were arranged using hierarchical clustering with Euclidean distance and complete linkage.
(D) Region set enrichment analysis for the signature regions using LOLA. Colored dots represent ChIP-seq experiments for transcription factors in the indicated lineage. Dot size denotes the log-odds ratio, and the numbers in the legend (“X/Y”) refer to significantly enriched region sets (X) versus all analyzed region sets (Y). The vertical dashed line represents the significance threshold (adjusted p ≤ 0.05).
(E) Two-dimensional projection of merged replicates (one point for each cell type in each donor) using principal component analysis based on average DNA methylation levels in the signature regions. The first two principal components are shown, and the numbers in parentheses indicate the percentage of variance explained.
(F) Hematopoietic lineage reconstruction using the prediction propensities of DNA methylation-based classifiers as a measure of similarity between cell types. Nodes in the graph represent cell types, and edges are weighted by class probabilities of cross-prediction. An automated edge-weighted graph layout algorithm was used to define the positions of the nodes.
See also Figure S7 and http://blueprint-methylomes.computational-epigenetics.org.
Specifically, we used elastic net-regularized general linear models (Friedman et al., 2010) for predicting the cell type of each individual stem and progenitor sample in our dataset. These classifiers were trained on the DNA methylation levels of all BLUEPRINT Regulatory Build regions in each 10-, 50-, and 1,000-cell sample, and the model performance was evaluated using 10-fold cross-validation (Figures 7B and S7A).
We observed high prediction accuracies for all cell types, with receiver operating characteristic (ROC) area under curve (AUC) values for individual cell types ranging from 0.85 to 1.00 (Figure S7A). Highest accuracies were obtained for myeloid progenitors (CMP, GMP, and MEP) and for the MLP0 population. Lymphoid progenitors (CLP, MLP1, MLP2, and MLP3) were more difficult to distinguish, consistent with their similar DNA methylation profiles (Figure S1A) and similar functional properties (Figure 4E). Lowest AUC values were observed for the HSC and MLP2 cell populations, which were frequently confused with MPPs and CLPs, respectively (Figure 7B).
The regularized classifiers weigh all genomic regions by their discriminatory power, thus establishing a measure of their importance for cell-type prediction. Based on this measure, we identified a set of 1,234 signature regions whose DNA methylation levels collectively distinguished hematopoietic cell types with high accuracy and robustness (Figure 7C; Table S4). Individual DNA methylation differences were small for most of these regions, highlighting that many weak but complementary differences can support accurate cell-type prediction.
LOLA enrichment analysis for the signature regions identified significant overlap with the binding sites of key hematopoietic transcription factors such as FLI1, GATA1/2, MYB, RUNX1, and TAL1 (Figure 7D). Unsupervised analysis based on the signature regions identified strong separation between myeloid and lymphoid progenitors, but no clear clustering within each group (Figure 7E). Moreover, differentiated cell types of the myeloid and lymphoid lineage formed separate clusters in the vicinity of their corresponding progenitors.
To quantify the similarity between cell types, we trained 10 additional classifiers, each excluding one of the stem and progenitor cell types (“leave-one-out-classifiers”), and we calculated the class probabilities for the samples that were withheld from the analysis (see Experimental Procedures). These class probabilities (Figure S7B; Table S5) define a data-driven network model of the human hematopoietic lineage, which emerges from the characteristic DNA methylation patterns of each cell type and their relationship with each other (Figure 7F).
Discussion
We established genome-wide maps of the DNA methylation dynamics in human hematopoietic differentiation, which comprise 17 cell types, four different sources of HSCs, and a total of 639 DNA methylation profiles. This resource, accessible via public repositories and a dedicated website (http://blueprint-methylomes.computational-epigenetics.org), provides insights into the role of epigenetic regulation in HSCs and their differentiating progeny, and it constitutes a reference for biomedical research focusing on diseases of the blood.
A key outcome of our study is the high accuracy with which DNA methylation profiles predict cell type throughout the human hematopoietic lineage. This is not merely due to the correlation between DNA methylation and gene expression (which was low in our dataset), but rather suggests that DNA methylation itself reflects a cell’s differentiation trajectory at the epigenetic level. We showed that prediction based on DNA methylation in regulatory regions can place sorted cell populations into a developmental context. DNA methylation analysis thus complements studies of human hematopoietic differentiation that were based on gene expression profiling (Chen et al., 2014, Notta et al., 2016, Novershtern et al., 2011) and chromatin accessibility mapping (Corces et al., 2016).
To illustrate the value of our dataset for biological hypothesis generation and for guiding mechanistic studies on specific aspects of hematopoietic differentiation, we focused on four areas of the human hematopoietic lineage.
First, we compared HSCs from four different sources. Peripheral blood is readily accessible and therefore highly relevant for clinical diagnostics. To establish a broadly useful reference, we thus based most of our dataset on stem and progenitor cell populations purified from the peripheral blood of healthy donors. Nevertheless, the microenvironment of peripheral blood differs markedly from that of bone marrow, cord blood, and fetal liver, which are commonly used sources of HSCs in basic research. HSCs from peripheral blood showed lower DNA methylation levels at the binding sites of CTCF and cohesin complex proteins than HSCs from other sources, which may reflect changes in chromatin 3D architecture that influence gene expression. These differences stress the importance of taking cell source and microenvironment into account when studying human hematopoietic stem and progenitor cells.
Second, we investigated the DNA methylation dynamics of myeloid-lymphoid lineage choice, observing an asymmetric pattern: regulatory regions that showed reduced DNA methylation levels in myeloid progenitors were enriched for binding sites of transcription factors associated with hematopoietic differentiation, myeloid lineage fate, and leukemia as well as lymphoma, whereas there was no strong enrichment among regions that had reduced DNA methylation levels in lymphoid progenitors. This observation is consistent with DNA methylation data for mouse hematopoiesis (Bock et al., 2012), and together with the finding that lymphoid differentiation is compromised in transgenic mice with impaired maintenance DNA methylation (Bröske et al., 2009), it supports the view that DNA methylation may epigenetically shield lymphoid progenitors from the default program of myeloid differentiation.
Third, we combined DNA methylation mapping and in vitro differentiation assays to characterize four populations of immature multi-lymphoid progenitors that appear to constitute epigenetically and functionally distinguishable cell types. MLP0 (CD7− CD10−) showed the most distinctive DNA methylation signature and highest levels of multi-lineage differentiation potential from individual cells. The observed patterns of multi-lineage differentiation among MLPs and CLPs may reflect an underappreciated level of epigenetic plasticity in human hematopoietic differentiation (Notta et al., 2016, Paul et al., 2015).
Fourth, we analyzed the DNA methylation dynamics over the course of megakaryocyte maturation, which involves multiple rounds of endomitotic replication and consequent increases in ploidy. Whereas the cellular morphology of maturing megakaryocytes changes dramatically, DNA methylation levels at regulatory regions showed only mild, but consistent and progressive, changes. Certain genomic regions (counter-intuitively including NFE2 binding sites) started off with low levels in 2N megakaryocytes but gained DNA methylation up to a level comparable with HSCs, whereas the majority of region sets started with myeloid-like DNA methylation levels that were lost during maturation.
In summary, we have established a comprehensive catalog of DNA methylation in human hematopoietic differentiation, which provides a resource and framework for studying the different cell types of the blood, as well as their associated diseases. Given the medical relevance (Laird, 2003) and technical feasibility (Bock et al., 2016b) of using DNA methylation as a clinical biomarker, it is expected that detailed DNA methylation analysis of immunodeficiencies, cardiovascular diseases, and blood cell malignancies will help advance precision medicine.
Experimental Procedures
Sample Preparation Summary
Peripheral blood cells were isolated from apheresis filters of healthy platelet donors belonging to the National Institute for Health Research (NIHR) Cambridge BioResource after informed consent and with ethical approval (REC 12/EE/0040). Cells were stained with antibodies and sorted on either BD Influx or BD FACSAria III fluorescence-activated cell sorting instruments. Library preparation followed the μWGBS/scWGBS protocol as described previously (Farlik et al., 2015). A detailed description of the sample collection, purification, library preparation, and sequencing is provided in the Supplemental Experimental Procedures.
Data Analysis Summary
Bisulfite sequencing reads were aligned with Bismark v0.12.2 (Krueger and Andrews, 2011) and processed with RnBeads v1.5 (Assenov et al., 2014) to aggregate DNA methylation values on regulatory regions annotated by the August 2015 release of the BLUEPRINT Ensembl Regulatory Build (Zerbino et al., 2015). Elastic net-regularized general linear models implemented in the R package glmnet (Krishnapuram et al., 2005) were used for cell-type prediction, and the cell-type similarity graph (Figure 7F) was derived from average class probabilities assigned by leave-one-class-out classifiers trained separately for each cell type. A detailed description of the sequencing data processing, differential DNA methylation analysis, genomic region enrichment analysis, single-cell DNA methylation analysis, and cell-type prediction is provided in the Supplemental Experimental Procedures.
Data Availability and Accession Numbers
The presented dataset can be accessed through five alternative and complementary sources:
-
1.
A supplemental website with additional diagrams and tables, which also contains direct links to the other data sources, is available at http://blueprint-methylomes.computational-epigenetics.org.
-
2.
The genome browser track hub, which is linked at http://blueprint-methylomes.computational-epigenetics.org, provides the processed DNA methylation data for interactive visualization and processing with online tools such as Galaxy.
-
3.
Preprocessed data (DNA methylation calls and gene expression levels) can be downloaded without any restrictions from GEO: GSE87197.
-
4.
The raw sequencing data from which the DNA methylation calls and gene expression levels have been derived are available from the European Genome-phenome Archive (EGA): EGAS00001002070 (controlled access to protect patient privacy).
-
5.
The dataset is included in the epigenome registry of IHEC (http://www.ebi.ac.uk/vg/epirr, accession numbers IHECRE00002734 to IHECRE00002810), the DeepBlue Epigenomic Data Server (http://deepblue.mpi-inf.mpg.de), and the IHEC Data Portal (http://epigenomesportal.ca/ihec).
Author Contributions
Conceptualization, M. Farlik, F.H., F.M., H.G.S., M. Frontini, and C.B.; Methodology, M. Farlik, F.H., F.M., M. Frontini, and C.B.; Formal analysis, F.H., F.M., P.E., and J.K.; Investigation, M. Farlik, F.A.C., S.F., A.S., V.C., A.M., and R.U.; Writing – Original Draft, M. Farlik, F.H., F.M., P.E., M. Frontini, and C.B.; Writing – Review & Editing, F.A.C., J.K., S.F., A.S., V.C., A.M., R.U., H.G.S., W.H.O, E.L., and T.L.; Supervision, E.L., T.L., M. Frontini, and C.B.; Funding acquisition, H.G.S., W.H.O., E.L., T.L., M. Frontini, and C.B.
Acknowledgments
This work was performed in the context of, and on behalf of, the BLUEPRINT project. We gratefully acknowledge the participation of NIHR Cambridge BioResource volunteers, and we thank the Cambridge BioResource staff for help with volunteer recruitment. We also thank Emily F. Calderbank and the Cambridge Biomedical Research Centre’s Cell Phenotyping Hub for contributions to cell purification, Thomas Krausgruber for help with data visualization, Nathan C. Sheffield and André F. Rendeiro for providing bioinformatic methods and software, Sebastian Ullrich and Roderic Guigó for help with RNA data processing, Paul Flicek and his team for providing the BLUEPRINT Regulatory Build, the members of the Cambridge BioResource Scientific Advisory Board and Management Committee for their support, the Biomedical Sequencing Facility at CeMM for assistance with next generation sequencing, and many members of the BLUEPRINT and IHEC consortia for helpful advice and generous data sharing. This work was funded by the BLUEPRINT project (European Union’s Seventh Framework Programme grant 282510), the NIHR Cambridge Biomedical Research Centre, and the Austrian Academy of Sciences. F.A.C. is supported by a Medical Research Council Clinical Training Fellowship (grant MR/K024043/1). F.H. is supported by a postdoctoral fellowship of the German Research Council (DFG; grant HA 7723/1-1). J.K. is supported by a DOC Fellowship of the Austrian Academy of Sciences. W.H.O. is supported by the NIHR, BHF (grants PG-0310-1002 and RG/09/12/28096), and NHS Blood and Transplant. E.L. is supported by a Wellcome Trust Sir Henry Dale Fellowship (grant 107630/Z/15/Z) and core support grant from the Wellcome Trust and MRC to the Wellcome Trust-Medical Research Council Cambridge Stem Cell Institute. M. Frontini is supported by the BHF Cambridge Centre of Excellence (grant RE/13/6/30180). C.B. is supported by a New Frontiers Group award of the Austrian Academy of Sciences and by a European Research Council (ERC) Starting Grant (European Union’s Horizon 2020 research and innovation program; grant 679146).
Published: November 17, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and five tables and can be found with this article online at http://dx.doi.org/10.1016/j.stem.2016.10.019.
Contributor Information
Mattia Frontini, Email: mf471@cam.ac.uk.
Christoph Bock, Email: cbock@cemm.oeaw.ac.at.
Supplemental Information
Table listing the annotation data and sequencing details for 639 DNA methylation profiles based on the μWGBS/scWGBS protocol and for 13 gene expression profiles based on the Smart-seq2 protocol.
Table listing all regulatory regions from the BLUEPRINT Regulatory Build that were differentially methylated in at least one pairwise comparison between HSCs and MPPs derived from four different sources or between the myeloid and lymphoid lineages in peripheral blood. An extended version of this table with additional comparisons is available from http://blueprint-methylomes.computational-epigenetics.org.
Region set enrichment analysis for differentially methylated regions (Table S2) and cell type signature regions (Table S4) calculated using the LOLA software tool and the LOLA Core database.
Table listing all regulatory regions from the BLUEPRINT Regulatory Build that contributed to the cell type classifier trained on 319 stem/progenitor samples (all 10-cell, 50-cell, and 1,000-cell pools) from peripheral blood, together with the average DNA methylation level of each region in each sample.
Class probabilities for each stem/progenitor sample by ten classifiers trained on datasets that excluded all samples of one specific cell type (“leave-one-class-out classifiers”).
References
- Adams D., Altucci L., Antonarakis S.E., Ballesteros J., Beck S., Bird A., Bock C., Boehm B., Campo E., Caricasole A. BLUEPRINT to decode the epigenetic signature written in blood. Nat. Biotechnol. 2012;30:224–226. doi: 10.1038/nbt.2153. [DOI] [PubMed] [Google Scholar]
- Assenov Y., Müller F., Lutsik P., Walter J., Lengauer T., Bock C. Comprehensive analysis of DNA methylation data with RnBeads. Nat. Methods. 2014;11:1138–1140. doi: 10.1038/nmeth.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C. Analysing and interpreting DNA methylation data. Nat. Rev. Genet. 2012;13:705–719. doi: 10.1038/nrg3273. [DOI] [PubMed] [Google Scholar]
- Bock C., Kiskinis E., Verstappen G., Gu H., Boulting G., Smith Z.D., Ziller M., Croft G.F., Amoroso M.W., Oakley D.H. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144:439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C., Beerman I., Lien W.H., Smith Z.D., Gu H., Boyle P., Gnirke A., Fuchs E., Rossi D.J., Meissner A. DNA methylation dynamics during in vivo differentiation of blood and skin stem cells. Mol. Cell. 2012;47:633–647. doi: 10.1016/j.molcel.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C., Farlik M., Sheffield N.C. Multi-omics of single cells: strategies and applications. Trends Biotechnol. 2016;34:605–608. doi: 10.1016/j.tibtech.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C., Halbritter F., Carmona F.J., Tierling S., Datlinger P., Assenov Y., Berdasco M., Bergmann A.K., Booher K., Busato F., BLUEPRINT Consortium Quantitative comparison of DNA methylation assays for biomarker development and clinical applications. Nat. Biotechnol. 2016;34:726–737. doi: 10.1038/nbt.3605. [DOI] [PubMed] [Google Scholar]
- Bröske A.-M., Vockentanz L., Kharazi S., Huska M.R., Mancini E., Scheller M., Kuhl C., Enns A., Prinz M., Jaenisch R. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat. Genet. 2009;41:1207–1215. doi: 10.1038/ng.463. [DOI] [PubMed] [Google Scholar]
- Cabezas-Wallscheid N., Klimmeck D., Hansson J., Lipka D.B., Reyes A., Wang Q., Weichenhan D., Lier A., von Paleske L., Renders S. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell. 2014;15:507–522. doi: 10.1016/j.stem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Chen L., Kostadima M., Martens J.H., Canu G., Garcia S.P., Turro E., Downes K., Macaulay I.C., Bielczyk-Maczynska E., Coe S., BRIDGE Consortium Transcriptional diversity during lineage commitment of human blood progenitors. Science. 2014;345:1251033. doi: 10.1126/science.1251033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces M.R., Buenrostro J.D., Wu B., Greenside P.G., Chan S.M., Koenig J.L., Snyder M.P., Pritchard J.K., Kundaje A., Greenleaf W.J. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nat. Genet. 2016;48:1193–1203. doi: 10.1038/ng.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J.A., Jung I., Aanes H., Greggains G.D., Manaf A., Lerdrup M., Li G., Kuan S., Li B., Lee A.Y. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature. 2016;537:548–552. doi: 10.1038/nature19360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulatov S., Notta F., Eppert K., Nguyen L.T., Ohashi P.S., Dick J.E. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat. Immunol. 2010;11:585–593. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- Doulatov S., Notta F., Laurenti E., Dick J.E. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10:120–136. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Farlik M., Sheffield N.C., Nuzzo A., Datlinger P., Schönegger A., Klughammer J., Bock C. Single-cell DNA methylome sequencing and bioinformatic inference of epigenomic cell-state dynamics. Cell Rep. 2015;10:1386–1397. doi: 10.1016/j.celrep.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A.F., Assenov Y., Martin-Subero J.I., Balint B., Siebert R., Taniguchi H., Yamamoto H., Hidalgo M., Tan A.C., Galm O. A DNA methylation fingerprint of 1628 human samples. Genome Res. 2012;22:407–419. doi: 10.1101/gr.119867.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Goardon N., Marchi E., Atzberger A., Quek L., Schuh A., Soneji S., Woll P., Mead A., Alford K.A., Rout R. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell. 2011;19:138–152. doi: 10.1016/j.ccr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Haas S., Hansson J., Klimmeck D., Loeffler D., Velten L., Uckelmann H., Wurzer S., Prendergast Á.M., Schnell A., Hexel K. Inflammation-induced emergency megakaryopoiesis driven by hematopoietic stem cell-like megakaryocyte progenitors. Cell Stem Cell. 2015;17:422–434. doi: 10.1016/j.stem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Habibi E., Brinkman A.B., Arand J., Kroeze L.I., Kerstens H.H.D., Matarese F., Lepikhov K., Gut M., Brun-Heath I., Hubner N.C. Whole-genome bisulfite sequencing of two distinct interconvertible DNA methylomes of mouse embryonic stem cells. Cell Stem Cell. 2013;13:360–369. doi: 10.1016/j.stem.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Ji H., Ehrlich L.I.R., Seita J., Murakami P., Doi A., Lindau P., Lee H., Aryee M.J., Irizarry R.A., Kim K. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467:338–342. doi: 10.1038/nature09367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Kim K., Zhao R., Doi A., Ng K., Unternaehrer J., Cahan P., Huo H., Loh Y.H., Aryee M.J., Lensch M.W. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat. Biotechnol. 2011;29:1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn L.A., Hao Q.-L., Sasidharan R., Parekh C., Ge S., Zhu Y., Mikkola H.K.A., Crooks G.M. Lymphoid priming in human bone marrow begins before expression of CD10 with upregulation of L-selectin. Nat. Immunol. 2012;13:963–971. doi: 10.1038/ni.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnapuram B., Carin L., Figueiredo M.A.T., Hartemink A.J. Sparse multinomial logistic regression: fast algorithms and generalization bounds. IEEE Trans. Pattern Anal. Mach. Intell. 2005;27:957–968. doi: 10.1109/TPAMI.2005.127. [DOI] [PubMed] [Google Scholar]
- Krueger F., Andrews S.R. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–1572. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuleshov M.V., Jones M.R., Rouillard A.D., Fernandez N.F., Duan Q., Wang Z., Koplev S., Jenkins S.L., Jagodnik K.M., Lachmann A. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundaje A., Meuleman W., Ernst J., Bilenky M., Yen A., Heravi-Moussavi A., Kheradpour P., Zhang Z., Wang J., Ziller M.J., Roadmap Epigenomics Consortium Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P.W. The power and the promise of DNA methylation markers. Nat. Rev. Cancer. 2003;3:253–266. doi: 10.1038/nrc1045. [DOI] [PubMed] [Google Scholar]
- Lecine P., Villeval J.L., Vyas P., Swencki B., Xu Y., Shivdasani R.A. Mice lacking transcription factor NF-E2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and show a platelet production defect that is intrinsic to megakaryocytes. Blood. 1998;92:1608–1616. [PubMed] [Google Scholar]
- Lister R., Mukamel E.A., Nery J.R., Urich M., Puddifoot C.A., Johnson N.D., Lucero J., Huang Y., Dwork A.J., Schultz M.D. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang C., Liu W., Li J., Li C., Kou X., Chen J., Zhao Y., Gao H., Wang H. Distinct features of H3K4me3 and H3K27me3 chromatin domains in pre-implantation embryos. Nature. 2016;537:558–562. doi: 10.1038/nature19362. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken M.N., Gschweng E.H., Nair-Gill E., McLaughlin J., Cooper A.R., Riedinger M., Cheng D., Nosala C., Kohn D.B., Witte O.N. Long-term in vivo monitoring of mouse and human hematopoietic stem cell engraftment with a human positron emission tomography reporter gene. Proc. Natl. Acad. Sci. USA. 2013;110:1857–1862. doi: 10.1073/pnas.1221840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran S., Martínez-Cardús A., Sayols S., Musulén E., Balañá C., Estival-Gonzalez A., Moutinho C., Heyn H., Diaz-Lagares A., de Moura M.C. Epigenetic profiling to classify cancer of unknown primary: a multicentre, retrospective analysis. Lancet Oncol. 2016;17:1386–1395. doi: 10.1016/S1470-2045(16)30297-2. [DOI] [PubMed] [Google Scholar]
- Notta F., Zandi S., Takayama N., Dobson S., Gan O.I., Wilson G., Kaufmann K.B., McLeod J., Laurenti E., Dunant C.F. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2016;351:aab2116. doi: 10.1126/science.aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novershtern N., Subramanian A., Lawton L.N., Mak R.H., Haining W.N., McConkey M.E., Habib N., Yosef N., Chang C.Y., Shay T. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell. 2011;144:296–309. doi: 10.1016/j.cell.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.Y., Majeti R., Weissman I.L. In vivo evaluation of human hematopoiesis through xenotransplantation of purified hematopoietic stem cells from umbilical cord blood. Nat. Protoc. 2008;3:1932–1940. doi: 10.1038/nprot.2008.194. [DOI] [PubMed] [Google Scholar]
- Paul F., Arkin Y., Giladi A., Jaitin D.A., Kenigsberg E., Keren-Shaul H., Winter D., Lara-Astiaso D., Gury M., Weiner A. Transcriptional heterogeneity and lineage commitment in myeloid progenitors. Cell. 2015;163:1663–1677. doi: 10.1016/j.cell.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Polo J.M., Liu S., Figueroa M.E., Kulalert W., Eminli S., Tan K.Y., Apostolou E., Stadtfeld M., Li Y., Shioda T. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat. Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan-Pla A., Macaulay I.C., Jensen C.T., Woll P.S., Luis T.C., Mead A., Moore S., Carella C., Matsuoka S., Bouriez Jones T. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502:232–236. doi: 10.1038/nature12495. [DOI] [PubMed] [Google Scholar]
- Shearstone J.R., Pop R., Bock C., Boyle P., Meissner A., Socolovsky M. Global DNA demethylation during mouse erythropoiesis in vivo. Science. 2011;334:799–802. doi: 10.1126/science.1207306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield N.C., Bock C. LOLA: enrichment analysis for genomic region sets and regulatory elements in R and Bioconductor. Bioinformatics. 2016;32:587–589. doi: 10.1093/bioinformatics/btv612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood S.A., Tomizawa S., Krueger F., Ruf N., Carli N., Segonds-Pichon A., Sato S., Hata K., Andrews S.R., Kelsey G. Dynamic CpG island methylation landscape in oocytes and preimplantation embryos. Nat. Genet. 2011;43:811–814. doi: 10.1038/ng.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Z.D., Chan M.M., Mikkelsen T.S., Gu H., Gnirke A., Regev A., Meissner A. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature. 2012;484:339–344. doi: 10.1038/nature10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G.J. Hematopoietic stem-cell differentiation. Curr. Opin. Immunol. 1991;3:171–178. doi: 10.1016/0952-7915(91)90046-4. [DOI] [PubMed] [Google Scholar]
- Suzuki M.M., Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat. Rev. Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- Tanner A., Taylor S.E., Decottignies W., Berges B.K. Humanized mice as a model to study human hematopoietic stem cell transplantation. Stem Cells Dev. 2014;23:76–82. doi: 10.1089/scd.2013.0265. [DOI] [PubMed] [Google Scholar]
- Theocharides A.P.A., Rongvaux A., Fritsch K., Flavell R.A., Manz M.G. Humanized hemato-lymphoid system mice. Haematologica. 2016;101:5–19. doi: 10.3324/haematol.2014.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijssen M.R., Cvejic A., Joshi A., Hannah R.L., Ferreira R., Forrai A., Bellissimo D.C., Oram S.H., Smethurst P.A., Wilson N.K. Genome-wide analysis of simultaneous GATA1/2, RUNX1, FLI1, and SCL binding in megakaryocytes identifies hematopoietic regulators. Dev. Cell. 2011;20:597–609. doi: 10.1016/j.devcel.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till J.E., McCulloch E.A. Hemopoietic stem cell differentiation. Biochim. Biophys. Acta. 1980;605:431–459. doi: 10.1016/0304-419x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- Vedi A., Santoro A., Dunant C.F., Dick J.E., Laurenti E. Molecular landscapes of human hematopoietic stem cells in health and leukemia. Ann. N Y Acad. Sci. 2016;1370:5–14. doi: 10.1111/nyas.12981. [DOI] [PubMed] [Google Scholar]
- Wijetunga N.A., Delahaye F., Zhao Y.M., Golden A., Mar J.C., Einstein F.H., Greally J.M. The meta-epigenomic structure of purified human stem cell populations is defined at cis-regulatory sequences. Nat. Commun. 2014;5:5195. doi: 10.1038/ncomms6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolthuis C.M., Park C.Y. Hematopoietic stem/progenitor cell commitment to the megakaryocyte lineage. Blood. 2016;127:1242–1248. doi: 10.1182/blood-2015-07-607945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino D.R., Wilder S.P., Johnson N., Juettemann T., Flicek P.R. The Ensembl Regulatory Build. Genome Biol. 2015;16:56. doi: 10.1186/s13059-015-0621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zheng H., Huang B., Li W., Xiang Y., Peng X., Ming J., Wu X., Zhang Y., Xu Q. Allelic reprogramming of the histone modification H3K4me3 in early mammalian development. Nature. 2016;537:553–557. doi: 10.1038/nature19361. [DOI] [PubMed] [Google Scholar]
- Ziller M.J., Gu H., Müller F., Donaghey J., Tsai L.T.-Y., Kohlbacher O., De Jager P.L., Rosen E.D., Bennett D.A., Bernstein B.E. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500:477–481. doi: 10.1038/nature12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table listing the annotation data and sequencing details for 639 DNA methylation profiles based on the μWGBS/scWGBS protocol and for 13 gene expression profiles based on the Smart-seq2 protocol.
Table listing all regulatory regions from the BLUEPRINT Regulatory Build that were differentially methylated in at least one pairwise comparison between HSCs and MPPs derived from four different sources or between the myeloid and lymphoid lineages in peripheral blood. An extended version of this table with additional comparisons is available from http://blueprint-methylomes.computational-epigenetics.org.
Region set enrichment analysis for differentially methylated regions (Table S2) and cell type signature regions (Table S4) calculated using the LOLA software tool and the LOLA Core database.
Table listing all regulatory regions from the BLUEPRINT Regulatory Build that contributed to the cell type classifier trained on 319 stem/progenitor samples (all 10-cell, 50-cell, and 1,000-cell pools) from peripheral blood, together with the average DNA methylation level of each region in each sample.
Class probabilities for each stem/progenitor sample by ten classifiers trained on datasets that excluded all samples of one specific cell type (“leave-one-class-out classifiers”).
Data Availability Statement
The presented dataset can be accessed through five alternative and complementary sources:
-
1.
A supplemental website with additional diagrams and tables, which also contains direct links to the other data sources, is available at http://blueprint-methylomes.computational-epigenetics.org.
-
2.
The genome browser track hub, which is linked at http://blueprint-methylomes.computational-epigenetics.org, provides the processed DNA methylation data for interactive visualization and processing with online tools such as Galaxy.
-
3.
Preprocessed data (DNA methylation calls and gene expression levels) can be downloaded without any restrictions from GEO: GSE87197.
-
4.
The raw sequencing data from which the DNA methylation calls and gene expression levels have been derived are available from the European Genome-phenome Archive (EGA): EGAS00001002070 (controlled access to protect patient privacy).
-
5.
The dataset is included in the epigenome registry of IHEC (http://www.ebi.ac.uk/vg/epirr, accession numbers IHECRE00002734 to IHECRE00002810), the DeepBlue Epigenomic Data Server (http://deepblue.mpi-inf.mpg.de), and the IHEC Data Portal (http://epigenomesportal.ca/ihec).