Abstract
Maternal antibodies transported across the placenta can provide vital immunity against infectious pathogens for infants. We here examine maternal antibody (MA) levels and their association with neonatal antibody levels. Pregnant women of gestational age ≥35 weeks were enrolled at a Guangzhou China hospital and mother-infant paired sera were collected. Measles IgG antibody was detected using ELISA assay, neutralizing antibodies titers against coxsackievirus A16 (CA16), enterovirus 71 (EV71), PV I-III and HIV-1 were performed. 711 mother-infant pairs were enrolled and positive relationships for paired serums were found (r: 0.683–0.918). 81.6%, 87.0%, and 82.3% of mothers, and 87.3%, 72.7%, and 72.2% of newborns were positive for measles, CA16 and EV71 antibodies respectively. The highest Neonatal: maternal ratio (NMR) was found in measles (1.042) and the ratios for the other pathogens ranged from 0.84 to 1.00. Linear regressions showed that log(NMR) decreased by a factor of 0.04–15.43 as log(MA) levels increased. A second analysis restricted to maternal positive measles sera revealed that MA measles of was still inversely associated with NMR. Low NMR was found in high MA HIV + serums among 22 paired sera. MA levels appear to play a role determining transplacental antibody transfer; further study is needed to reveal the mechanism.
Maternal immunoglobulin G (IgG) is transported across the placenta by an active, neonatal Fc receptor (FcRn) mediated process during pregnancy. This transport can confer short-term passive immunity1,2,3 and protect infants against infections during their first months of life. Specific maternal antibodies provide immunity against infectious pathogens for infants until their own immune system has time to mature4.
Infectious diseases have been a threat to infants5. Over the past decade, measles, hand-foot-mouth disease (HFMD) and human immunodeficiency virus type 1 (HIV-1) infection have remained public health challenges among infants in some countries, including China6,7,8. Another disease, poliomyelitis, a crippling and potentially fatal infectious disease, may be nearing eradication by providing continued proper vaccination strategy among infants9.
Transplacental transport of antibodies has been shown to occur to various degrees for a variety of infectious diseases. For example, IgG transplacental transfer has been studied among preterm and term infants for certain antibodies, including tetanus, varicella, measles, and human papillomavirus (HPV). Preterm infants were found to benefit less from maternal antibodies, posing them at higher risk for infectious diseases in the first months after birth than term infants10,11,12,13. This difference may be related to the temporary decrease in total IgG during the second trimester of pregnancy due to hemodilution14.
Infections also influence maternal humoral immunity. Infants born to HIV-infected mothers have been found more likely to be measles antibody seronegative and had lower levels of antibodies than those born to HIV-negative mothers15. However, limited data are available on how maternal antibody (MA) levels influence transplacental transportation15. Decreasing transplacental transport of measles antibody has been reported associated with increasing levels of measles antibodies in maternal serums in some western countries and African countries14,16. However, studies of certain diseases, as well as studies in China, are lacking. Here we examine the MA levels for various antibodies of measles, HFMD, poliomyelitis virus (PV), and HIV infection, and their associations with neonatal antibody levels.
Results
Measles, CA16, EV71, and PV I, II, III antibodies
Demographic characteristics and seroprevalence of antibodies
Excluding 22 HIV + mothers, 711 mother-infant pairs were enrolled in this study with a median gestation period of 38.9 weeks (range 35–43), median delivery age of 27.7 years (range 16–45) and median birth weight of 3.2 kilograms (range 1.6–4.8). 62.7% (446) of infants were born by vaginal delivery. Antibody Levels for measles, coxsackievirus A16 (CoxA16), enterovirus 71 (EV71) and Poliomyelitis virus (PV) I, II, III in maternal and newborn serum samples are provided in Table 1. Less education (below college), diabetes and measles vaccination were related to higher maternal measles titers (p < 0.05). Less education was also linked to higher CA16 titer (p = 0.008), and women of older gestation age (≥39 weeks) were associated with higher PV II titers (p = 0.047).
Table 1. Seroprevalence of measles, coxsackievirus A16, enterovirus 71, and poliovirus I, II, III antibodies in maternal and newborn serum samples.
| Level | NO | Maternal serum | Neonatal serum | Neonatal Maternal Ratio†§ | |
|---|---|---|---|---|---|
| GMT (95%CI) | GMT (95%CI) | ||||
| Measles | Maternal<200 | 131 | 78.8 (53.6, 98.6) | 162.3 (113.6, 196.6) | 1.128 (1.103, 1.162) |
| ≥200 | 580 | 963.9 (832.1, 1104.0) | 1246.8 (1123.5, 1367.3) | 1.036 (1.031, 1.042) | |
| Overall | 711 | 674.0 (596.8, 762.0) | 960.3 (819.9, 1044.1) | 1.042 (1.039, 1.050) | |
| CA16 | Maternal<1:8 | 29 | 4.0∫ | 4.0∫ | 1.000 |
| ≥1:8 | 195 | 32.0 (24.0, 32.0) | 16.0 (16.0, 24.0) | 0.857 (0.800, 0.896) | |
| Overall | 224 | 24.0 (24.0, 32.0) | 16.0 (12.0, 16.0) | 0.904 (0.848, 1.000) | |
| EV71 | Maternal<1:8 | 39 | 4.0∫ | 4.0∫ | 1.000 |
| ≥1:8 | 185 | 32.0 (24.0, 32.0) | 24.0 (16.0, 32.0) | 0.948 (0.911, 1.000) | |
| Overall | 224 | 24.0 (16.0, 32.0) | 16.0 (12.0, 24.0) | 1.000 (1.000, 1.000) | |
| PVI | Maternal<1:8 | 29 | 2.0 (2.0, 6.0) | 2.0 (2.0, 6.0) | 1.000 (1.000, 1.000) |
| ≥1:8 | 190 | 32.0 (32.0, 48.0) | 32.0 (24.0, 48.0) | 1.000 (0.941, 1.000) | |
| Overall | 219 | 32.0 (24.0, 32.0) | 24.0 (24.0, 32.0) | 1.000 (1.000, 1.000) | |
| PVII | Maternal<1:8 | 32 | 2.0 (2.0, 4.0) | 2.0 (2.0, 2.0) | 1.000 (1.000, 1.000) |
| ≥1:8 | 186 | 32.0 (32.0, 48.0) | 24.0 (16.0, 32.0) | 0.911 (0.884, 1.000) | |
| Overall | 218 | 32.0 (24.0, 32.0) | 16.0 (12.0, 24.0) | 0.941 (0.896, 1.000) | |
| PVIII | Maternal<1:8 | 61 | 2.0 (2.0, 4.0) | 2.0 (2.0, 2.0) | 1.000 (1.000, 1.000) |
| ≥1:8 | 153 | 32.0 (24.0, 32.0) | 12.0 (8.0, 16.0) | 0.764 (0.716, 0.837) | |
| Overall | 214 | 16.0 (12.0, 24.0) | 6.0 (4.0, 8.0) | 0.837 (0.764, 0.911) |
NOTE: The medians with the 95%CIs for CA16, EV71, PVI, PVII, and PVIII antibodies were the geometric mean concentrations.
†NMR = log (neonatal level): log (maternal level).
∫For CA16 and EV71 antibodies, all titers of maternal sera were 1:4.
§Comparisons for NMRs between two groups: Measles (t = 3.204, p = 0.002), CA16 (t = 5.422, p < 0.001), EV71 (t = 4.142, p < 0.001), PVI (t = 2.314, p = 0.028), PVII (t = 1.611, p = 0.117), PVIII (t = 3.467, p = 0.001).
Positive relationships between neonatal and maternal titers (geometric mean concentration) were found for all 7 antibodies (r: 0.918 for measles, 0.733 for CA16, 0.828 for EV71, 0.778 for PV I, 0.786 for PV II, and 0.683 for PV III; p < 0.001). 81.6% of pregnant women (95% confidence interval (CI): 78.6–84.3) and 87.3% of newborns (95%CI: 84.8–89.7) were measles antibody positive. 87.0% of pregnant women (95%CI: 82.3–90.9) and 72.7% of newborns (95%CI: 66.7–78.2) were positive for CA16 neutralizing antibodies. For EV71, the positive rates were 82.3% (95%CI: 77.0–86.8) and 72.2% (95%CI: 66.2–77.8) respectively.
Neonatal: maternal ratio (NMR)
The highest NMR was found for measles (1.042). The NMRs for CA16, PV I-III and HIV-1 antibodies ranged from 0.84 to 1.00. Subjects with positive maternal measles, CA16, EV71, PV I, and III antibody titers, were found to have significant lower NMRs compared with subjects with negative maternal serum levels (Table 1). Negative maternal sera also had a higher NMR for PV II antibody than the positive sera; however, the difference did not reach statistical significance. Multiple linear regressions results showed that MA titer was the common, statistically significant factor related to NMR. Indeed, NMR decreased by a factor of 0.04–15.43 as log(MA) levels increased (Table 2).
Table 2. Linear models for NMRs as a function of maternal levels for different antibodies.
| Model 1# |
Model 2* |
|||||||
|---|---|---|---|---|---|---|---|---|
| NO. | Beta (95%CI) | t | p | NO. | Beta (95%CI) | t | p | |
| Measles | 516 | −15.43 (−18.17, −12.69) | −11.05 | <0.001 | 421 | −0.06 (−0.07, −0.04) | −7.69 | <0.001 |
| CA16 | 208 | −0.10 (−0.16, −0.04) | −3.22 | 0.001 | 180 | −0.07 (−0.14, 0.02) | −1.61 | 0.109 |
| EV71 | 208 | −0.04 (−0.12, 0.03) | −1.21 | 0.226 | 171 | 0.06 (−0.02, 0.14) | 1.49 | 0.138 |
| PVI | 203 | −0.08 (−0.16, −0.01) | −2.11 | 0.037 | 175 | 0.03 (−0.06, 0.12) | 0.73 | 0.467 |
| PVII | 202 | −0.09 (−0.20, 0.01) | 0.12 | 0.087 | 172 | 0.06 (−0.03, 0.14) | 1.24 | 0.217 |
| PVIII | 198 | −0.24 (−0.39, −0.10) | −3.30 | 0.001 | 145 | 0.18 (0.05, 0.31) | 2.79 | 0.006 |
NOTE: The ENTER (forced Entry) method for all variables was used for the multiple linear regression models.
In both models, the dependent variable was NMR and the independent variables were maternal age, education, vaccination history (measles), hypertension, diabetes, anemia, gestation week, newborn’s gender, birth weight and Log (maternal level).
All maternal sera were analyzed in model 1 and only positive maternal sera were analyzed in model 2.
#In model 1, education (Beta = 0.071, p = 0.044) and birth weight (Beta = 0.123, p = 0.012) were also significant for NMR of CA16, gestation week (Beta = −2.971, p = 0.003) and maternal age (Beta = −0.014, p = 0.004) were significant for NMR of EV71, and hypertension was significant for PV II (Beta = 0.780, p = 0.005).
*In model 2, maternal age (Beta = −0.011, p = 0.022) for PV I and gestation week (Beta = −0.041, p = 0.031) for PVII were significant.
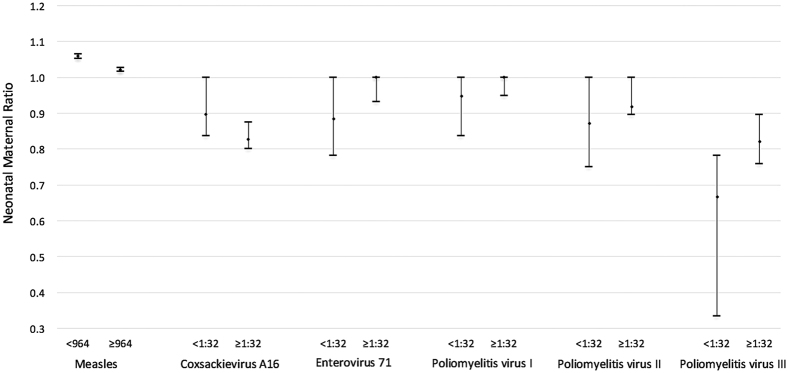
After we restricted the analysis to the positive maternal sera, and using the median as a cut-off, serum levels in the lower quantile showed a higher NMR than serum levels in the upper quantile for measles and CA16 (p < 0.05) (Fig. 1). The median for log (maternal antibody level) was taken as the cut-off value (2.984 for measles antibody and 1.505 for CA16, EV71, PVI, PVII and PVIII antibodies). Number of paired serums, measles: 290 (concentration <964) and 290(≥ 964); CA16: 87(<1:32) and 108(≥1:32); EV71: 84 (<1:32) and 101 (≥1:32); PV I: 87 (<1:32) and 102 (≥1:32); PVII: 75(<1:32) and 111(≥1:32); PV III:74(<1:32) and 79(≥1:32). Comparisons for NMRs between two groups: measles (t = 7.579, p < 0.001), CA16 (t = 2.436, p = 0.016), EV71 (t = −1.568, p = 0.119), PVI (t = 1.031, p = 0.304), PVII (t = −0.914, p = 0.363), and PVIII (t = −1.527, p = 0.129). For EV71 and PV I-III antibodies, maternal serum levels in the lower quantile appeared to have lower NMRs; however, the differences were not significant. In the linear regression models, NMR decreased by 0.06 as log(MA) level increased (p < 0.001) for measles, and an increase of 0.18 in NMR against log(MA) was observed for PV III antibody (p = 0.006).
Figure 1. Neonatal Maternal Ratio (NMR) with 95% CIs grouped for subjects with maternal antibody levels above and below the overall median.
HIV-1 neutralization antibodies
For the 22 paired mother-newborn sera for which HIV-1 neutralization antibodies were detected, the titers for 45.5% (10/22) of pairs in both mothers and newborns were 10.0. Positive relationships between neonatal and maternal titers (geometric mean concentration) were found (r: 0.819, p < 0.001). Antibody titers were found to be higher in mothers (median:19.8, 95%CI:10.0–71.9) than paired newborns (median:10.0, 95%CI: 10.0–23.2) (Z = −2.411, p = 0.016), with an average NMR of 0.957 (95%CI: 0.780–1.000). When the median of 1.2965 for log (MA titer) was used as a cutoff, the maternal sera with lower titer levels had an NMR of 1.000, and lower NMR (0.780, 95%CI: 0.627–0.913) was found in the higher titer level quantile (Mann-Whitney U = 14.000, p = 0.001).
Discussion
Over the last decade in countries such as China, a greater number of measles, HFMD and HIV infected cases among infants have been reported7,17. At the same time new challenges arise for shifting the immunization schedule to better protect against ‘old’ infectious diseases such as poliomyelitis. MA level is an important predictor of neonatal antibody level, and maternal vaccination strategies should be recommended to provide better immunity (IgG) to neonates in the months before they are vaccinated against vaccine preventable diseases under the current national immunization schedule. For example, nearly twenty percent of pregnant women in our study were measles antibody negative. The measles vaccine with HU-191 and CHANG-47 strains has been used since 1967, and the national Expanded Program on Immunization (EPI), which used a 2-dose monovalent measles vaccine schedule, was implemented in 1986. As a consequence, maternal immunity in our study is primarily vaccine rather than naturally acquired (the oldest study subject was 45 years); however, vaccine acquired immunity may leave some women at risk to measles virus. Women of childbearing age, especially those of higher education status—a group found to have lower maternal measles titers and who are not currently advised to receive vaccination under the national schedule—should be recommended to receive a measles-containing booster, such as Measles, Mumps, and Rubella virus (MMR) vaccine, especially in the countries targeted for measles elimination in the near future17.
The seropositive rates of neutralizing antibody against EV71 (82.3%) and CA16 (87.0%) in women are similar with results from other areas in China (85.3% and 89.1%)18 but higher than other Asian and Pacific regions19, suggesting a higher prevalence of EV71 among the study subjects. This finding indicates that adults in China may get persistent immunity to HFMD disease by exposure to the CA16 and EV71 viruses. Because the majority of HFMD cases are reported in infants7, the EV71 vaccine has been licensed for use at 6–35 months.
The overall measles NMR (1.56) found here is similar to that of developed countries (>1.0) while higher than for some developing countries (<1.0)15,20. As measured by the association between cord and maternal levels, the transplacental transfer efficiency for the CA16, PV, and HIV-1 antibodies (r < 0.85) was lower than found for measles (r > 0.85). Our findings were similar to previous reporting, which showed neutralization antibody could be efficiently transferred and was strongly correlated between mothers and infants with 1.3-fold higher antibody levels in maternal plasmas21. More efficient transplacental transfer of measles was also reported by Goncalves22 for the transfer difference between measles antibody and total IgG antibodies. IgG subclasses do not cross the placenta with equal efficiency; for example, IgG1 and IgG4 are transported more efficiently than IgG3 and IgG22,3,10. Such differences in IgG subclass production and accumulation may have led to the preferential transfer of measles antibody from mother to newborns. However, we did not have access to maternal IgG subclasses for this study and thus could not evaluate the associations between the IgG subclasses and NMRs for different antibodies.
We also confirm the evidence of a decrease in NMR with increasing levels of measles antibodies in maternal serum samples in China, which is consistent with findings from rural Kenya and European countries20,22. We additionally report associations between MA levels and placental transfer rates for CA16, EV71, PV I-III, and HIV-1 antibodies. The transplacental transfer efficiency for the CA16 (NMR: 0.86), EV71 (0.95), PV (0.76–1.0), and HIV-1 antibodies (0.96) was lower than found for measles (NMR:1.04). Stronger association was also found in measles antibody (r>0.9) than other antibodies (r < 0.85) in our study. It is not yet clear why MA levels for different viruses are associated with different NMRs. One hypothesis is that MA levels are associated with the total amount of IgG antibody for different viruses; IgG antibodies may compete for a finite number of FcRn receptors, which normally divert bound endocytosed IgG from catabolic degradation within the placenta by transporting them to the fetal circulation, consequently leading to different NMRs2,4,10,16. However, this mechanism was not assessed in this study. We also did not measure maternal hypergammaglobulinemia levels, which has been reported to significantly impair the transfer of IgG1 and IgG2 but not of IgG3 or IgG416.
Compared with other primates, human placenta has greater active transport that concentrates IgG in the fetus during the final weeks of pregnancy, which leads to higher IgG levels in neonates than adult23. However, the human ability to concentrate may be impaired by gestational age, as well as infections status such as HIV and malaria infection15,16. It is possible “regression to the mean” bias, in which the observed change might depend on the initial value24, may have impacted this study and should be viewed as a potential limitation. Further studies of the differential concentration of antibodies as a function of age, geography, and population for various infectious diseases are needed.
To our knowledge, this is among the first studies to report the NMRs for CA16 and EV71, and to assess the associations between placental transfer rates and maternal levels for PV and HIV-1. For the 7 infectious disease antibodies examined, our study indicates that transplacental transport of antibodies occurs to various degrees and maternal antibody levels play an important role determining transfer efficiency.
Methods
During July 2013 to April 2014, pregnant women of gestational age ≥35 weeks at Liwan District Maternal and Child Health Hospital, Guangzhou, China were randomly enrolled for this study. Maternal venous blood was sampled 2 days prior to delivery, and cord blood was obtained from the placenta directly after delivery. Sera were excluded from individuals known to be affected by an immunodepressive condition or an acute infection. Subject demographic and health information, including maternal age, education, vaccination history (measles), hypertension, diabetes, anemia, gestation week, and newborn’s gender and birth weight, was collected by study nurses.
We used an enzyme linked immunosorbent assay (ELISA; Anti-Measles IgG and IgM, Virion/Serion, Germany) for quantitative measurement of measles antibodies25. A modified cytopathogenic effect assay was performed to detect neutralizing antibodies titers against CoxA16/G10 (A genotpye) and EV71/H07 (C4 genotpye)18. In brief, sera were firstly diluted to 1:8, inactivated at 56 °C (30 minutes), then serially diluted from 1:8 to 1:2048 and mixed with equal volumes of 100 TCID50 EV71 and CoxA16. The mixture was added into a 96-well microplate and incubated at 37 °C (2 hours). Human Rhabdomyosarcoma (RD) cell suspension (105 cells/ml) was added to the mixture. The plates were then placed in a CO2 incubator at 35 °C for 7 days, and the cytopathogenic effect was observed under microscopy. Cell control, serum control, virus control, and virus backdrops were all established on each plate. If the backdrops showed 32–320 TCID50/well, the test was considered successful. Neutralizing antibody titers were defined as the dilution rate showing 50% inhibition on the cytopathogenic effect.
PV I, II, and III antibody titers were assessed by virus-neutralizing antibody assay with Sabin or wild-type strains26. We also collected samples from 22 pairs of HIV + mothers and their infants in Guangzhou during 2006 to 2013. HIV-1 titer (IC50) neutralization antibody levels were determined from the curve generated by dilution and luciferase activity21. HIV-1 subtype B env (JRFL) plasmid and backbone plasmid pNL4.3Δenv egfp were co-transfected into 293 ft cells with 1:2 molar ratio by lipofectamine 2000. After 3 days, virus supernatant was harvested and stored at −70 °C. The virus titer was determined by blue cell assay in TZMbl cell. Plasma was 3-fold serial diluted in triplicate and incubated with 20–30 TCID50 virus per well for 1 hour in 96-well plates, then 1 × 104 suspended TZMbl cells were added. After 48 h incubation, cells were lysed and luciferase activity was measured. The titer (IC50) of neutralization antibody was determined by the curve generated by dilution and luciferase activity. Equivocal assay results were retested once. Positive antibody cut-off values of 200 mIU/ml for measles, and 1:8 for CA16, EV71 and PV I-III antibodies were used in the analysis18,27,28,29,30.
Seroprevalence of measles, CA16, EV71, and PV I, II, III antibodies in maternal and newborn serum samples were described. NMR was defined as the geometric mean of the individual ratios, i.e. log (neonatal): log(maternal). NMRs between two groups with different maternal antibody levels were compared, in which a t test or nonparametric tests were used (related or dependent sample test) when appropriate. A multiple linear regression analysis was performed to determine the influence of maternal age, education, vaccination (measles), hypertension, diabetes, anemia, gestational age, newborn gender, birth weight and log(MA) on NMR. Two models were used: model 1 was analyzed based on all maternal sera, and model 2 was based on only positive maternal sera.
The sampling and experimental protocols were approved by Ethics Board of Guangzhou Center for Disease Control and Prevention (GZCDC) and all methods were performed in strict accordance with the protocol (Permit Number: 2014–007). All experimental methods were carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from the enrolled pregnant women (ClinicalTrials.gov Identifier number NCT02219061).
Additional Information
How to cite this article: Fu, C. et al. Placental antibody transfer efficiency and maternal levels: specific for measles, coxsackievirus A16, enterovirus 71, poliomyelitis I-III and HIV-1 antibodies. Sci. Rep. 6, 38874; doi: 10.1038/srep38874 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We appreciate the participation of the parents and newborns enrolled in this study. All authors had full access to all of the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by research grants from the Project for Key Medicine Discipline Construction of Guangzhou Municipality (2017-2019-07), the National Natural Science Foundation (81273139), and National Science and Technology Major Projects (2012ZX10004213-005).
Footnotes
Author Contributions C.F. and M.W. designed the study; L.L., H.W. and Y.C. did the experiments and collected the data; Q.Y., Q.H. and Z.Y. built the dataset; C.F. and F.F. analyzed the data, C.F. wrote the manuscript text and J.S. revised it.
References
- Brambell F. W. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet 2, 1087–1093 (1966). [DOI] [PubMed] [Google Scholar]
- Roopenian D. C. & Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 7, 715–725, doi: 10.1038/nri2155 (2007). [DOI] [PubMed] [Google Scholar]
- Morphis L. G. & Gitlin D. Maturation of the maternofoetal transport system for human gamma-globulin in the mouse. Nature 228, 573 (1970). [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med 345, 1331–1335, doi: 10.1056/NEJMra012493 (2001). [DOI] [PubMed] [Google Scholar]
- Collaborators G. B. D. C. M. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1725–1774, doi: 10.1016/S0140-6736(16)31575-6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuridan E. et al. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ 340, c1626, doi: 10.1136/bmj.c1626 (2010). [DOI] [PubMed] [Google Scholar]
- Xing W. J. et al. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis 14, 308–318, doi: 10.1016/S1473-3099(13)70342-6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. J. et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 1005–1070, doi: 10.1016/S0140-6736(14)60844-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booy R. & Tashani M. Edging ever closer to polio eradication. Lancet Glob Health 4, e592–593, doi: 10.1016/S2214-109X(16)30172-3 (2016). [DOI] [PubMed] [Google Scholar]
- van den Berg J. P., Westerbeek E. A., van der Klis F. R., Berbers G. A. & van Elburg R. M. Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Hum Dev 87, 67–72, doi: 10.1016/j.earlhumdev.2010.11.003 (2011). [DOI] [PubMed] [Google Scholar]
- Linder N. et al. Placental transfer of maternal rubella antibodies to full-term and preterm infants. Infection 27, 203–207 (1999). [DOI] [PubMed] [Google Scholar]
- Glick C., Feldman S., Norris M. R. & Butler J. Measles, mumps, and rubella serology in premature infants weighing less than 1,000 grams. Southern Med J 91, 159–160 (1998). [DOI] [PubMed] [Google Scholar]
- Matys K. et al. Mother-infant transfer of anti-human papillomavirus (HPV) antibodies following vaccination with the quadrivalent HPV (type 6/11/16/18) virus-like particle vaccine. Clin Vaccine Immunol 19, 881–885, doi: 10.1128/CVI.00002-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder N. et al. Placental transfer of measles antibodies: effect of gestational age and maternal vaccination status. Vaccine 22, 1509–1514, doi: 10.1016/j.vaccine.2003.10.009 (2004). [DOI] [PubMed] [Google Scholar]
- Scott S. et al. Neonatal measles immunity in rural Kenya: the influence of HIV and placental malaria infections on placental transfer of antibodies and levels of antibody in maternal and cord serum samples. J Infect Dis 191, 1854–1860, doi: 10.1086/429963 (2005). [DOI] [PubMed] [Google Scholar]
- Okoko B. J. et al. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J Infect Dis 184, 627–632, doi: 10.1086/322808 (2001). [DOI] [PubMed] [Google Scholar]
- Fu C. X. et al. Low measles seropositivity rate among children and young adults: A sero-epidemiological study in southern China in 2008. Vaccine 28, 8219–8223, doi: 10.1016/j.vaccine.2010.07.071 (2010). [DOI] [PubMed] [Google Scholar]
- Zhu F. C. et al. Retrospective Study of the Incidence of HFMD and Seroepidemiology of Antibodies against EV71 and CoxA16 in Prenatal Women and Their Infants. Plos One 7, doi: ARTN e37206 10.1371/journal.pone.0037206 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S. T. et al. Enterovirus 71 Maternal Antibodies in Infants,Taiwan. Emerg Infect Dis 15, 581–584, doi: 10.3201/eid1504.081550 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black F. L. et al. Geographic-Variation in Infant Loss of Maternal Measles Antibody and in Prevalence of Rubella Antibody. Am J Epidemiol 124, 442–452 (1986). [DOI] [PubMed] [Google Scholar]
- Omenda, M M. et al. Evidence for Efficient Vertical Transfer of Maternal HIV-1 Envelope-Specific Neutralizing Antibodies but No Association of Such Antibodies With Reduced Infant Infection. Jaids-J Acq Imm Def 64, 163–166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves G. et al. Transplacental transfer of measles and total IgG. Epidemiol Infect 122, 273–279, doi: 10.1017/S0950268899002046 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe C. L., Lubach G. R. & Izard K. M. Progressive Improvement in the Transfer of Maternal Antibody across the Order Primates. Am J Primatol 32, 51–55, doi: 10.1002/ajp.1350320106 (1994). [DOI] [PubMed] [Google Scholar]
- Hayes R. J. Methods for assessing whether change depends on initial value. Stat Med 7, 915–927 (1988). [DOI] [PubMed] [Google Scholar]
- Xu G. Z. et al. [Levels of transition on maternal transferred measles antibody in infants in 3 cities in China]. Zhonghua Liu Xing Bing Xue Za Zhi 29, 1074–1077 (2008). [PubMed] [Google Scholar]
- Verdijk P. et al. Safety and immunogenicity of inactivated poliovirus vaccine based on Sabin strains with and without aluminum hydroxide: A phase I trial in healthy adults. Vaccine 31, 5531–5536, doi: 10.1016/j.vaccine.2013.09.021 (2013). [DOI] [PubMed] [Google Scholar]
- Ma C. et al. Measles vaccine coverage estimates in an outbreak three years after the nation-wide campaign in China: implications for measles elimination, 2013. BMC Infect Dis 15, 23, doi: 10.1186/s12879-015-0752-z (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardemil C. V. et al. Poliovirus Immunity Among Pregnant Females Aged 15–44 Years, Namibia, 2010. Journal of Infectious Diseases 210, S136–S142, doi: 10.1093/infdis/jiu086 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H. et al. Epidemiologic and clinical features of non-polio enteroviral infections in northern Taiwan in 2008. J Microbiol Immunol Infect 44, 265–273, doi: 10.1016/j.jmii.2011.01.029 (2011). [DOI] [PubMed] [Google Scholar]
- Chang L. Y. et al. Transmission and clinical features of enterovirus 71 infections in household contacts in Taiwan. JAMA 291, 222–227, doi: 10.1001/jama.291.2.222 (2004). [DOI] [PubMed] [Google Scholar]