Abstract
Objectives
We tested the hypothesis that oxidative stress contributes to reductions in left ventricular diastolic function in estrogen-deficient postmenopausal women, related in part to reduced nitric oxide (NO) bioavailability.
Study design
LV diastolic function – recorded using transthoracic echocardiography and determined as the peak early (E) to late (A) mitral inflow velocity ratio and the E to peak early (e’) mitral annular velocity ratio – and brachial artery flow mediated dilation (FMD), a biomarker of NO bioavailability, were measured during acute systemic infusions of saline (control) and ascorbic acid (experimental model to decrease oxidative stress) in healthy premenopausal women (N=14, 18-40 years) and postmenopausal women (N=23, 45-75 years).
Results
The E/A ratio was lower (1.16[1.06−1.33] vs 1.65[1.5−2.3]; median[interquartile range]) and the E/e’ ratio was elevated (8.8[7.6−9.9] vs. 6.6[5.5−7.3]) in postmenopausal compared with premenopausal women, indicating reduced LV diastolic function. E/A and E/e’ were correlated with FMD (r=0.54 and r=−0.59, respectively, both P<0.01). Ascorbic acid infusion improved both FMD (5.4±2.0% to 7.8±2.6%) and E/e’ (to 8.1[7.2−9.7], P=0.01) in postmenopausal women but not in premenopausal women. Ascorbic acid did not change E/A in either group.
Conclusion
The current study provides evidence that oxidative stress contributes to reduced LV diastolic function in estrogen-deficient postmenopausal women, possibly by reducing the availability of NO.
Keywords: diastolic function, oxidative stress, postmenopausal women
1. Introduction
Aging is associated with an increased risk for the development of Heart failure (HF), a debilitating condition that affects nearly 6 million Americans and has been estimated to account for one-third of all disease-related mortality in American women [1],[2]. Of the two phenotypes of HF, older women (>65 years) are more likely to develop HF with a preserved ejection fraction (HFpEF), characterized by impaired left ventricular (LV) diastolic function [3-7]. Although LV diastolic function declines with age, postmenopausal women experience a more rapid decline compared to age-matched men [8]. Understanding the mechanisms that contribute to the decline in LV diastolic function in postmenopausal women is important for the development of strategies to preserve cardiac function and prevent heart failure in women. The biological processes underlying the reduction in LV diastolic function in estrogen-deficient postmenopausal women are not completely understood. Estrogen-deficient postmenopausal women have a greater oxidative burden than premenopausal women [9-11]. Elevated markers of reactive oxygen species (ROS) have been reported in the failing human myocardium [12], and LV diastolic dysfunction in ovariectomized (OVX) rats is associated with elevated cardiac ROS levels [13, 14]. These data suggest that oxidative stress may play a role in the reduction in LV diastolic function [14, 15]. Oxidative stress could impair LV diastolic function by decreasing the bioavailability of nitric oxide (NO), a key regulator of cardiac function. Elevated levels of ROS can scavenge NO decrease NO synthesis by suppressing the enzymatic function of nitric oxide synthase (NOS), the enzyme that catalyzes NO from L-arginine [16, 17] Whether oxidative stress is mechanistically linked to reduced LV diastolic function in postmenopausal women is unknown. Accordingly, we tested the hypothesis that oxidative stress contributes to the reduced LV diastolic function in estrogen-deficient postmenopausal women compared to premenopausal controls, related in part to reduced NO bioavailability.
2. Materials and Methods
2.1 Study Population
We studied 37 healthy women: 14 premenopausal (18-40 years) and 23 postmenopausal (45-75 years). Premenopausal women had regular menstrual cycles with no change in observed cycle length (21-35 days). Postmenopausal women had >12 months of amenorrhea. Women had not used oral contraceptives or hormone therapy for at least 6 months. Women were normotensive (resting blood pressure <140/90 mmHg), non-diabetic (fasted glucose <126 mg/dL), sedentary or recreationally active (<3 days/wk vigorous exercise), nonsmokers, and healthy as determined by medical history, physical examination, standard blood chemistries (chemistry panel, complete blood count and thyroid stimulating hormone) and electrocardiogram at rest and during incremental treadmill exercise. Additionally, women were not taking medications that could influence cardiovascular function (e.g., antihypertensive, lipid lowering medications) and had not used vitamin supplements or anti-inflammatory medications for at least 4 weeks prior to the study visit. The study was approved by the Colorado Multiple Institutional Review Board, and all participants provided a written informed consent form.
2.2 Measurements
Women were studied in the supine position following an overnight fast with proper hydration (water only). Participants were provided individualized meals based on a 3-day food intake record to ensure normal dietary patterns, including sodium intake, as described previously [18]. Meals were consumed 2-days immediately prior to any measurements. Premenopausal women were tested in the mid-follicular phase (e.g., 7-10 days after onset of menstruation) in an effort to perform measurements when estradiol was representative of average levels across the menstrual cycle. The study took place at the University of Colorado Anschutz Medical Campus Colorado Clinical Translational Sciences Institute Clinical and Translational Research Center.
2.2.1 Echocardiogram
Transthoracic echocardiographic measurements of LV diastolic function were obtained using a GE Vivid I ultrasound (GE Healthcare, Horten, Norway) using standard methods [19]. Briefly, 2 dimensional guided M-mode echocardiography was used to quantify LV structural characteristics and the Teichholz formula [20] to calculate LV volumes, ejection fraction, and fractional shortening. Pulsed-wave Doppler in the apical 4-chamber view was used to obtain mitral inflow velocities. The sample volume was placed between the mitral leaflet tips to quantify peak early filling (E) and late diastolic filling (A) velocities, E/A ratio, and deceleration time (interval from peak E to a point of intersection of the deceleration of flow with the baseline). Because mitral inflow patterns are sensitive to preload and can change dramatically with the progression of diastolic dysfunction, myocardial tissue Doppler imaging (TDI) was also performed in the apical 4 chamber view with a 2 mm sample volume at the septal and lateral mitral annulus. Septal and lateral values of peak early (e’) and late (a’) mitral annular velocities were calculated. The ratio between E and e’ was used as the primary parameter of diastolic performance. All measurements were performed by a single trained technician and all echocardiographic images were reviewed by a board eligible cardiologist.
2.2.2 Brachial artery flow mediated dilation
Ultrasound measurements of brachial artery FMD were performed as previously described in detail by our laboratory [21, 22], and according to published guidelines for assessing FMD in human participants [23]. Briefly, a pediatric cuff was placed on the upper forearm and brachial artery images were acquired ~3-6 cm above the antecubital fossa at baseline and following reactive hyperemia produced by inflating the cuff to 250 mmHg of pressure for 5 min. After the release of the arterial occlusion, the initial 10 Doppler blood flow velocity waveform envelopes were acquired and B-mode ultrasound brachial artery diameter images were measured continuously for two minutes. The dilation of the brachial artery in response to the stimulus of forearm ischemia has been shown to be dependent on the release of vasodilators, predominantly NO, from the vascular endothelium, and thus, is considered a biomarker of NO bioavailability [24]. Brachial images were analyzed for systolic and diastolic diameters using a computerized semi-automated edge-detection software that allows accurate identification and measurements of brachial artery lumen diameter (Vascular Analysis Tools v. 5.5; MIA LLC, Coralville, IA). Peripheral artery blood pressures were measured over the brachial artery using a semi-automated device (Dinamap; Johnson & Johnson, New Brunswick, NJ). All images were coded by number, blinded to menopause group and testing condition, and analyzed by the same individual. The coefficient of variation and intra-class correlation for trial-to-trial reliability measured in 10 individuals for FMD (%) were 2.2% and 0.99, respectively.
2.2.3 Body composition, physical activity, and blood sampling
Total and trunk fat percent were determined using dual energy X-ray absorptiometry (Hologic Discovery, version 12.6). Minimal waist and hip circumferences were measured and waist-to-hip ratio was calculated as previously described [21]. Leisure time physical activity was determined by the Modifiable Activity Questionnaire [25]. Fasting plasma concentrations of glucose, insulin, total cholesterol (Roche Diagnostic Systems, Indianapolis, IN), and high-density lipoprotein cholesterol (Diagnostic Chemicals Ltd, Oxford, CT) were determined using enzymatic/colorimetric methods. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation [26]. Serum concentrations of follicle-stimulating hormone (FSH), estradiol and progesterone were measured using chemiluminescence. Serum total antioxidant status (TAS), a measure of overall antioxidant defenses, was measured using an enzymatic kit (Randox Laboratories, Oceanside, CA). All blood samplings occurred on the day of vascular testing. All assays were performed at the University of Colorado Clinical Translational Research Center core laboratory.
2.3 Experimental design
To determine whether oxidative stress is mechanistically linked to the reduced LV diastolic function in estrogen-deficient postmenopausal women, we employed a common experimental model used to acutely suppress ROS as described previously by our laboratory and others [27-31]. Briefly, echocardiographic and brachial artery ultrasound measurements were obtained after 20 minutes of normal isovolumic saline infused systemically (control), and then repeated after 20 minutes of intravenous systemic infusion of a pharmacological dose of ascorbic acid. The concentration of the ascorbic acid solution prepared by the University of Colorado pharmacy was 0.06g ascorbic acid/kg fat-free mass/100ml of normal saline. All women received a bolus of 100ml ascorbic acid solution given at 5ml/min over 20 minutes followed by a “drip-infusion” given at 1.7ml/min to maintain ascorbic acid levels until cardiovascular testing was completed. This dose (~2-3g) of ascorbic acid has been previously shown to improve carotid artery compliance, femoral artery blood flow and endothelial function in estrogen-deficient postmenopausal women [29, 30, 32, 33]. The difference in E/A, E/e’, and brachial artery FMD following acute infusion of ascorbic acid versus saline represents the tonic suppression of LV diastolic function and FMD by ROS.
2.4 Statistics
Descriptive statistics were used to examine all data elements. Parameters with skewed distributions were log transformed and are presented as median (interquartile range). Independent t-tests were used to assess differences in participant characteristics. A mixed factor ANOVA, with group as a between-subject factor and saline vs. ascorbic acid as a within-subject factor, was used to determine the effects of ascorbic acid on LV diastolic function and brachial artery FMD. For a significant overall F-statistic (P<0.05), paired and independent t-tests were used to determine the within-group and between-group effect, respectively. Pearson and Spearman correlation analyses were used to test for the presence of significant linear bivariate relations between variables of interest (e.g., potential modulators of LV diastolic function) and basal LV diastolic function and the change in LV diastolic function with ascorbic acid. Data analysis was performed with IBM SPSS Statistics version 23.0.
3. Results
3.1 Participant characteristics
Among postmenopausal women, the reported mean±SD age at menopause and time since menopause were 51.3±5.0 and 6.1±5.2 years, respectively. Forty-four percent of postmenopausal women were prior hormone therapy users with a duration of 3.9±3.2 years. Seventy-nine percent of premenopausal women had used hormonal contraceptives for an average of 5.0±6.1 years. Postmenopausal women had a significantly greater BMI, total body fat percentage, trunk fat percentage, resting systolic blood pressure, and total cholesterol (all P<0.05; Table 1). Postmenopausal women had lower concentrations of estradiol and progesterone (both P<0.01), and higher concentrations of FSH compared to premenopausal women (P<0.001). There were no differences in reported micro- or macronutrient intake between groups (Table 2).
Table 1.
Participant characteristics
| Variable | Premenopausal (n = 14) | Postmenopausal (n = 23) | P |
|---|---|---|---|
| Age, y | 31.5 ± 6.0 | 57.4 ± 5.3 | <0.001 |
| Body mass, kg | 62.6 ± 9.1 | 70.6 ± 14.1 | 0.06 |
| BMI, kg/m2 | 23.1 ± 3.9 | 27.3 ± 5.3 | 0.01 |
| Total body fat, % | 29.2 ± 7.1 | 39.3 ± 5.4 | <0.001 |
| Trunk fat, % | 27.0 ± 8.5 | 38.0 ± 6.6 | <0.001 |
| Waist circumference, cm | 76.8 ± 9.8 | 84.9 ± 12.8 | 0.05 |
| WHR | 0.78 ± 0.07 | 0.81 ± 0.06 | 0.09 |
| LTPA, MET h/wk a | 17.8 (6.4 – 25.8) | 6.5 (3.0 – 16.0) | 0.19 |
| Seated systolic BP, mm Hg | 108 ± 7 | 119 ± 15 | 0.01 |
| Seated diastolic BP, mm Hg | 68 ± 5 | 73 ± 9 | 0.18 |
| Resting HR, bpm | 68 ± 9 | 64 ± 6 | 0.12 |
| Total cholesterol, mg/dl | 154± 32 | 176± 30 | 0.04 |
| LDL cholesterol, mg/dl | 91 ± 25 | 105± 30 | 0.15 |
| HDL cholesterol, mg/dl | 46± 9 | 49 ± 14 | 0.51 |
| Triglycerides, mg/dl a | 65 (54 – 94) | 86 (68 – 125) | 0.14 |
| Fasting insulin, μIU/ml a | 5.0 (3.0 – 8.3) | 5.0 (4.0 – 13.0) | 0.49 |
| Fasting glucose, mg/dl | 82± 10 | 87± 12 | 0.27 |
| FSH, μIU/ml | 5.5 ± 3.3 | 82.0 ± 35.7 | <0.001 |
| Estradiol, pg/ml a | 88 (61– 108) | 10 (10– 11.) | <0.001 |
| Progesterone, ng/ml a | 0.6 (0.3 – 0.7) | 0.3 (0.2 – 0.4) | 0.004 |
Data are mean ± SD, unless otherwise indicated. BMI, body mass index; WHR, waist to hip ratio; LTPA, leisure time physical activity; MET, metabolic equivalents; BP, blood pressure; HR, heart rate; bpm, beats per minute; LDL, low density lipoprotein; HDL, high density lipoprotein; FSH, follicle stimulating hormone;
Data are presented as median (interquartile range).
Table 2.
Reported micro and macro nutrient
| Variable | Premenopausal | Postmenopausal | P |
|---|---|---|---|
| Energy intake (kcal) | 1716 ± 397 | 1752 ± 397 | 0.79 |
| Total fat intake (g) | 69 ± 23 | 68 ± 21 | 0.85 |
| Total carbohydrate intake (g) | 204 ± 58.5 | 216 ± 77 | 0.64 |
| Total protein intake (g) | 73 ± 18.0 | 70 ± 17 | 0.63 |
| Vitamin D intake (mcg) | 5 ± 3.4 | 4 ± 3 | 0.26 |
| Total α-Tocopherol (mg) | 13 ± 10 | 10 ± 5 | 0.20 |
| Vitamin E intake (mg) | 10 ± 7 | 8 ± 4 | 0.25 |
| Vitamin C intake (mg) | 83 ± 50 | 93 ± 81 | 0.70 |
kcal, kilocalories; g, grams; mcg, micrograms; mg, milligrams
Postmenopausal women had lower TAS concentrations (1.31[1.16-1.43] nmol/L, P<0.05) compared to premenopausal women (1.40[1.24-1.59] nmol/L). TAS increased after the ascorbic acid infusion in both premenopausal (2.67[2.59-3.03] nmol/L) and postmenopausal women (2.40[2.22-3.10] nmol/L) (both P<0.05).
3.2 Effects of postmenopausal status on echocardiographic measurements and FMD
At baseline, there were no structural differences in diastole between pre- and postmenopausal women (Table 3). Examination of basal mitral inflow velocity patterns revealed a significantly lower E, higher A, and lower E/A in postmenopausal women compared to premenopausal women (P<0.01; Table 3). Basal TDI followed a similar pattern, with significantly lower e’ and higher a’ in postmenopausal compared to premenopausal women (P<0.01). E/e’ was higher (P<0.001) in postmenopausal compared to premenopausal women, indicating reduced LV diastolic function. Brachial artery FMD was reduced (P<0.001) in postmenopausal women compared to premenopausal women, indicating impaired endothelial function.
Table 3.
Hemodynamic and cardiac parameters, and FMD during saline and ascorbic acid infusions.
| Premenopausal | Postmenopausal | |||
|---|---|---|---|---|
| Saline | Ascorbic Acid | Saline | Ascorbic Acid | |
| Hemodynamic Measures | ||||
| SBP (mm Hg) | 105 ± 9 | 104 ± 8 | 119 ± 13 | 118 ± 14 |
| DBP (mm Hg) | 66 ± 9 | 67 ± 7 | 70 ± 7 | 69 ± 8 |
| MAP (mm Hg) | 81 ± 10 | 80 ± 8 | 87 ± 8 | 86 ± 11 |
| Heart rate (beats·min−1) | 58 ± 7 | 59 ± 8† | 59 ± 6 | 59 ± 6 |
| Cardiac Parameters | ||||
| IVSd, cm | 0.91 ± 0.12 | – | 0.94 ± 0.18 | – |
| LVd, cm | 4.6 ± 0.4 | – | 4.9 ± 0.4 | – |
| LVEDV, ml | 98.9 ± 16.9 | – | 110.9 ± 21.8 | – |
| Stroke volume, ml | 64.0 ± 13.9 | – | 68.1 ± 17.1 | – |
| Ejection fraction, % | 64.8 ± 6.1 | – | 61.7 ± 9.9 | – |
| Fractional shortening, % | 35.4 ± 4.8 | – | 33.7 ± 9.9 | – |
| E (cm/s) | 84.6 ± 9.1 | 81.4 ± 8.3 | 71.2 ± 11.4* | 75.7 ± 14.2† |
| A (cm/s) | 47.3 ± 11.7 | 47.9 ± 10.1 | 60.1 ± 12.8* | 63.4 ± 15.7* |
| E/A a | 1.65 (1.53-2.30) | 1.65 (1.46-1.91) | 1.16 (1.06-1.33)* | 1.22 (1.11-1.35)* |
| Deceleration Time (ms) | 268.4 ± 48.5 | 229.1 ± 35.1† | 265.1 ± 44.8 | 233.1 ± 35.2† |
| e’ (cm/s) a | 12.75 (11.88-14.0) | 12.5 (11.5-13.5) | 8.5 (7.0-9.5)* | 9.0 (8.0-10.0)*† |
| a’ (cm/s) a | 7.25 (6.50-8.0) | 7.5 (6.88-8.0) | 8.5 (8.0-9.5)* | 9.0 (8.0-10.5)*† |
| E/e’ a | 6.6 (6.2-7.6) | 6.6 (5.5-7.3) | 8.8 (7.6-9.9)* | 8.1 (7.2-9.7)*† |
| FMD (%) | 10.0±2.1 | 10.2±1.9 | 5.4±2.0* | 7.8±2.6*† |
Different from premenopausal women
different from saline condition within group.
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; IVSd, interventricular septal thickness at diastole; LVd, left ventricular diastolic diameter; LVEDV, left ventricular end diastolic volume; E, peak early mitral inflow velocity; A, peak late mitral inflow velocity; e’, peak early mitral annular velocity; a’, peak late mitral annular velocity; FMD, flow mediated dilation;
Data are presented as median (interquartile range).
3.3 Effects of ascorbic acid on hemodynamics, LV diastolic function and FMD
There were no significant differences in systolic blood pressures, diastolic blood pressures, or mean arterial pressures between saline and ascorbic acid conditions in premenopausal or postmenopausal women (Table 3). There was a very small (1 beat), but statistically higher resting supine heart rate during the ascorbic acid conditions in premenopausal women. All echocardiographic measures, with the exception of late mitral inflow velocity (A) and the ratio between early and late mitral inflow velocities (E/A), were significantly improved in the postmenopausal women following the ascorbic acid infusion (Table 3). Measures were unchanged in premenopausal women, except for a decrease in deceleration time. Similarly, FMD improved following the ascorbic acid infusion in postmenopausal women (P=0.01), and did not change in premenopausal women (P=0.78).
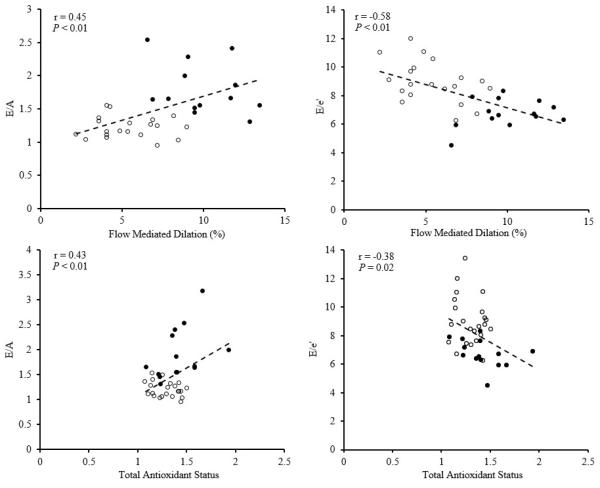
In the pooled population, both E/A and E/e’ were highly correlated with baseline FMD and with TAS (Figure 1). Changes in E/e’ and FMD with the ascorbic acid infusion were not significantly correlated (r=0.07, P=0.83).
Figure 1.
Relation between baseline E/A and E/e’ with baseline FMD and total antioxidant status in premenopausal (•) and postmenopausal (○) women.
4. Discussion
The current study provides novel insight into a potential mechanism underlying reduced LV diastolic function in estrogen-deficient postmenopausal women. Specifically, acute infusion of the antioxidant ascorbic acid improved LV diastolic function (E/e’) in estrogen-deficient postmenopausal women, but not in premenopausal women. Moreover, the ascorbic acid infusion improved the surrogate marker of NO bioavailability, brachial FMD, in postmenopausal women but not in premenopausal women. These data suggest that oxidative stress contributes to the reduced LV diastolic function in estrogen-deficient postmenopausal women, possibly through reductions in NO bioavailability.
4.1 Oxidative stress and LV diastolic function
Consistent with previous observations [34, 35], in the present study postmenopausal women had a 30% lower E/A and a 33% higher E/e’ compared to premenopausal women, indicative of reduced LV diastolic function. The present study extends previous work by providing evidence for oxidative stress as one potential mechanism underlying these observed differences. First, we found that basal measures of LV diastolic function were moderately correlated with basal TAS, a marker of endogenous antioxidants. Second, improvements in parameters of LV diastolic function with an acute ascorbic acid infusion in postmenopausal women, but not premenopausal women, supports the notion of tonic suppression of LV diastolic function by ROS. These findings are consistent with previous investigations that showed an improvement in LV diastolic function following the chronic administration of the mitochondrial targeted antioxidant coenzyme Q10 in patients with hypercholesterolemia and hypertrophic cardiomyopathy [36, 37].
4.2 Potential mechanism for the tonic suppression of LV diastolic function by oxidative stress
We can only speculate on the mechanisms by which oxidative stress contributes to reduced LV diastolic function and how the acute ascorbic acid infusion improved LV diastolic function in estrogen-deficient postmenopausal women. Cardiac myocytes, as well as endothelial cells and neutrophils within the heart, generate multiple cellular sources of ROS in the plasma membrane (e.g., NADPH oxidase), cytoplasm (e.g., xanthine oxidase), peroxisomes (e.g., lipid oxidation) and mitochondria (superoxide production via oxidative phosphorylation) [38]. Excessive ROS has been shown to impair LV diastolic function by preventing the oxidation of SERCA (sarcoendoplasmic reticulum calcium transport ATPase) and increasing cytosolic calcium removal [10]. The overproduction of ROS can also scavenge nitric NO [39-42]. NO derived from NOS located in the sarcolemmal caveolae and in the sarcoplasmic reticulum (SR) of cardiac myocytes may modulate excitation-contraction coupling and diastolic function by enhancing SR re-uptake of calcium released during systole [39, 40]. Indeed, reductions in NO lead to changes in phospholamban, a key regulator of SR-dependent calcium handling, that contribute to increases in cytosolic calcium and impairments in LV diastolic function [41, 42]. Endothelium-derived NO also enhances cardiac myocyte relaxation and diastolic function through its effects on cGMP induced reduction in myofilament responsiveness to calcium [43]. Ascorbic acid is a potent water-soluble antioxidant, and when infused at supraphysiological levels, it has been shown to prevent the scavenging of NO by ROS. Moreover, infusing high doses of ascorbic acid has been shown to reduce markers of inflammation [44] which can inactivate NOS, as well as produce excess ROS [45]. Consistent with this, in the present study the reduced LV diastolic function in postmenopausal women was associated with reduced brachial artery FMD, a biomarker of NO bioavailability. .
The ascorbic acid infusion could have also increased NO and LV diastolic function by stabilizing NOS through recycling one of its essential cofactors, tetrahydrobiopterin (BH4). When BH4 is deficient, NOS becomes uncoupled, producing the ROS, superoxide, instead of NO. In this regard, reduced LV diastolic function observed in OVX rats was shown to be associated with reduced cardiac BH4 and elevated cardiac ROS, presumably due to NOS uncoupling and reduced NO [14]. Moreover, OVX rats that were supplemented with BH4 for 4 weeks had reduced levels of cardiac ROS and preserved LV diastolic function, presumably due to preservation of NOS coupling and NO production [13, 14]. The effect of BH4 supplementation on LV diastolic function and circulating ROS levels in women warrants future exploration.
Finally, oxidative stress could also contribute to reduced LV diastolic function in estrogen-deficient postmenopausal women via its effects on endothelium dependent vasodilation, large elastic artery stiffness and arterial-ventricular (AV) coupling, (a measure of cardiac efficiency and interaction between the LV and arterial system). Similar to the apparent accelerated decline in LV diastolic function in women after menopause, AV coupling also declines at a faster rate in postmenopausal women compared to age-matched men [8]. The impairment in AV coupling is partly due to endothelial dysfunction, alterations in arterial structure and function, diameter, wall thickness, and wall stiffness (e.g., reduced arterial compliance) [46]. These maladaptations increases the afterload on the heart, consequently increasing LV stiffening and further reducing LV function [46].
4.3 Clinical perspective
This study was designed to investigate the mechanistic contributions of ROS on LV diastolic function in postmenopausal women, through a common experimental model of acute ascorbic acid infusion. Although our findings support the hypothesis that LV diastolic function in postmenopausal women is partly reduced, due to ROS, they cannot provide insight into the effects of chronic antioxidant therapy on LV diastolic function. Numerous investigations have explored responses to long term antioxidant therapy on markers of cardiovascular disease in aging women with little to no benefit [47, 48]. Mixed findings in small [49-51] and large [52-54] scale studies suggest that oral antioxidant supplements, such as vitamin C and/or E, may not be efficacious treatments to combat cardiovascular disease. In this regard, attention has recently been focused on targeting sources of high ROS production, such as the mitochondria. Alternative mitochondria targeted antioxidant therapies (e.g., Coenzyme Q10 and MitoQ) have been increasingly investigated in populations with advanced cardiovascular disease [36, 55, 56] demonstrating improvements in LV function, endothelial function, and favorable cardiac remodeling. However, to our knowledge, there are no studies that have investigated the effects of mitochondrial targeted antioxidants on cardiovascular function in postmenopausal women, acutely or chronically, making it an attractive area of research for studying alternative methods of attenuating the decline in cardiovascular function with aging in women.
4.4 Experimental considerations and limitations
The current study is not without limitations. Echochardiographic indices of cardiac structure were not measured during the ascorbic acid infusion, as changes were not expected due to the acute nature of the experiment. Consequently, cardiac morphologic measures typically associated with LV diastolic function (e.g., left atrial volume index, LV mass index) were not measured. Typically, for cardiac structural modifications to occur in response to an intervention (e.g., aerobic exercise, drug intervention), multiple weeks to months of exposure are required [57, 58]. Acute administration (sublingual) of estrogen, which has antioxidant like properties, was shown to improve LV diastolic function in postmenopausal women despite lack of alterations in cardiac structural parameters [59]. Additionally, the ascorbic acid infusion did not significantly improve all echocardiographic indices of LV diastolic function, and those measures that were significantly improved in the postmenopausal group were not restored to premenopausal levels. It is possible that the acute ascorbic acid infusion did not completely suppress other sources of ROS (e.g., peroxynitrite). Our relatively small sample size (13 premenopausal and 24 postmenopausal women) may have also limited our ability to detect a significant difference in all echocardiographic indices. However, post hoc sample size calculations revealed 99% power to detect a within group (saline vs. ascorbic acid infusion) difference of 0.4±0.3 in E/e’ and 0.01± 0.01 in E/A for the postmenopausal women. . It is important to consider that the women enrolled in our study were healthy and free of any overt CVD, limiting the generalizability of our findings. However, this study sought to identify mechanisms that may partly explain the reduced LV diastolic function observed in estrogen-deficient postmenopausal women, specifically oxidative stress. Therefore, a healthy population was recruited to limit characteristics associated with increased ROS (e.g., hypertension, diabetes, CVD).
Our study design cannot isolate whether the reduced LV diastolic function and the oxidative stress-tonic suppression of LV diastolic function in postmenopausal women is related to menopause and estrogen deficiency, and/or chronological age. Moreover, the current study design did not test potentially important sex-specific differences in the role of oxidative stress and LV diastolic function. . Future studies are encouraged to isolate the effects of menopause and age on LV diastolic function, and the role of factors that change with menopause and age (i.e., adiposity, blood pressure). Additionally, future studies should explore whether the potential beneficial effects of hormone therapy on LV diastolic function are related to antioxidant effects, and whether sex differences in LV diastolic function with aging are related to oxidative stress. Additionally, the authors recognize that nutraceuticals and long term dietary intake can influence cardiovascular health as previously suggested [60, 61]. Accordingly, the current study only included participants that had not taken antioxidant or anti-inflammatory supplements for at least 4 weeks. Moreover, in the present study there were no reported differences in micro- or macronutrient intake between the pre- and postmenopausal women (Table 2). Finally, we used brachial artery FMD as a surrogate measure of NO and did not measure cardiac or circulating levels of NO. However, measuring NO in the heart would require invasive techniques. In addition, blood measures of NO may not always provide an accurate assessment of whole body or tissue NO due to the extremely short half-life of NO [62].
5. Conclusions
In conclusion, the current study provides initial evidence that oxidative stress and reduced NO, contributes to reduced LV diastolic function in estrogen-deficient postmenopausal women. These data contribute to the growing literature supporting oxidative stress as an important mediator of cardiovascular function in this population. Understanding the biological processes that promote oxidative stress will help to identify potential strategies to preserve LV diastolic function and decrease the risk of HF in postmenopausal women.
Highlights.
Ascorbic acid infusion improved diastolic function in postmenopausal women.
Ascorbic acid infusion also improved the surrogate marker of nitric oxide (NO) bioavailability.
Oxidative stress contributes to reduced diastolic function in postmenopausal women.
Oxidative stress is an important mediator of cardiovascular function.
Acknowledgments
Funding
This work was supported by the National Institutes of Health awards R01AG027678, T32ST63009794; Colorado Clinical and Translational Sciences Institute UL1 TR001082; Colorado Nutrition and Obesity Research Center P30 DK048520
This work was supported by the National Institutes of Health awards R01AG027678, T32ST63009794; Colorado Clinical and Translational Sciences Institute UL1 TR001082; Colorado Nutrition and Obesity Research Center P30 DK048520
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
CO participated in the data analysis and manuscript composition, and saw and approved the final version.
KLH participated in the data collection and manuscript composition, and saw and approved the final version.
DWG participated in the data analysis and saw and approved the final version.
KLM participated in the study conceptualization, data collection, data analysis, and manuscript composition, and saw and approved the final version.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Colorado Multiple Institutional Review Board. Informed consent was obtained from all participants.
Provenance and peer review
This article has undergone peer review.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bybee KA, Stevens TL. Matters of the heart: cardiovascular disease in U.S. women. Mo Med. 2013;110(1):65–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26(6):562–8. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 4.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115(1):79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen-Solal A, Caviezel B, Laperche T, Gourgon R. Effects of aging on left ventriculararterial coupling in man: assessment by means of arterial effective and left ventricular elastances. J Hum Hypertens. 1996;10(2):111–6. [PubMed] [Google Scholar]
- 6.Cohen-Solal A. Left ventricular diastolic dysfunction: pathophysiology, diagnosis and treatment. Nephrol Dial Transplant. 1998;13(Suppl 4):3–5. doi: 10.1093/ndt/13.suppl_4.3. [DOI] [PubMed] [Google Scholar]
- 7.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32(5):1221–7. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 8.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112(15):2254–62. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 9.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45(6):1107–12. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 10.Bednarek-Tupikowska G, Bohdanowicz-Pawlak A, Bidzinska B, Milewicz A, Antonowicz-Juchniewicz J, Andrzejak R. Serum lipid peroxide levels and erythrocyte glutathione peroxidase and superoxide dismutase activity in premenopausal and postmenopausal women. Gynecol Endocrinol. 2001;15(4):298–303. [PubMed] [Google Scholar]
- 11.Signorelli SS, Neri S, Sciacchitano S, Pino LD, Costa MP, Marchese G, Celotta G, Cassibba N, Pennisi G, Caschetto S. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas. 2006;53(1):77–82. doi: 10.1016/j.maturitas.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM. Redox signaling in cardiac myocytes. Free Radic Biol Med. 2011;50(7):777–93. doi: 10.1016/j.freeradbiomed.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jessup JA, Zhang L, Chen AF, Presley TD, Kim-Shapiro DB, Chappell MC, Wang H, Groban L. Neuronal nitric oxide synthase inhibition improves diastolic function and reduces oxidative stress in ovariectomized mRen2.Lewis rats. Menopause. 2011;18(6):698–708. doi: 10.1097/gme.0b013e31820390a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jessup JA, Zhang L, Presley TD, Kim-Shapiro DB, Wang H, Chen AF, Groban L. Tetrahydrobiopterin restores diastolic function and attenuates superoxide production in ovariectomized mRen2.Lewis rats. Endocrinology. 2011;152(6):2428–36. doi: 10.1210/en.2011-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam KK, Ho ST, Yen MH. Tetrahydrobiopterin improves vascular endothelial function in ovariectomized rats. J Biomed Sci. 2002;9(2):119–25. doi: 10.1007/BF02256022. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell VB, Freeman BA. Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ Res. 2001;88(1):12–21. doi: 10.1161/01.res.88.1.12. [DOI] [PubMed] [Google Scholar]
- 17.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43(3):562–71. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson ET, Davy KP, Seals DR. Hemostatic, metabolic, and androgenic risk factors for coronary heart disease in physically active and less active postmenopausal women. Arterioscler Thromb Vasc Biol. 1995;15(5):669–77. doi: 10.1161/01.atv.15.5.669. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10(2):165–93. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 20.Meller J, Herman MV, Teichholz LE. Noninvasive assessment of left ventricular function. Adv Intern Med. 24(1979):331–57. [PubMed] [Google Scholar]
- 21.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol. 2012;302(5):H1211–8. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreau KL, Deane KD, Meditz AL, Kohrt WM. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis. 2013;230(2):390–396. doi: 10.1016/j.atherosclerosis.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 24.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 2001;101(6):629–35. [PubMed] [Google Scholar]
- 25.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc. 1997;29(6 Suppl):S1–205. [PubMed] [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 27.Hornig B, Arakawa N, Kohler C, Drexler H. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;97(4):363–8. doi: 10.1161/01.cir.97.4.363. [DOI] [PubMed] [Google Scholar]
- 28.Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol. 2004;286(4):H1528–34. doi: 10.1152/ajpheart.00879.2003. [DOI] [PubMed] [Google Scholar]
- 29.Moreau KL, Depaulis AR, Gavin KM, Seals DR. Oxidative stress contributes to chronic leg vasoconstriction in estrogen-deficient postmenopausal women. J Appl Physiol. 2007;102(3):890–5. doi: 10.1152/japplphysiol.00877.2006. [DOI] [PubMed] [Google Scholar]
- 30.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic Acid Selectively Improves Large Elastic Artery Compliance in Postmenopausal Women. Hypertension. 2005;45(6):1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 31.Moreau KL, Gavin KM, Plum AE, Seals DR. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause. 2006;13(6):951–8. doi: 10.1097/01.gme.0000243575.09065.48. [DOI] [PubMed] [Google Scholar]
- 32.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97(12):4692–700. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–24. doi: 10.1113/jphysiol.2003.057042. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okura H, Takada Y, Yamabe A, Kubo T, Asawa K, Ozaki T, Yamagishi H, Toda I, Yoshiyama M, Yoshikawa J, Yoshida K. Age- and gender-specific changes in the left ventricular relaxation: a Doppler echocardiographic study in healthy individuals. Circ Cardiovasc Imaging. 2009;2(1):41–6. doi: 10.1161/CIRCIMAGING.108.809087. [DOI] [PubMed] [Google Scholar]
- 35.Carvalho JC, Farand P, Do HD, Brochu MC, Bonenfant F, Lepage S. Effect of age and sex on echocardiographic left ventricular diastolic function parameters in patients with preserved ejection fraction and normal valvular function. Cardiol J. 2013;20(5):513–8. doi: 10.5603/CJ.2013.0137. [DOI] [PubMed] [Google Scholar]
- 36.Silver MA, Langsjoen PH, Szabo S, Patil H, Zelinger A. Effect of atorvastatin on left ventricular diastolic function and ability of coenzyme Q10 to reverse that dysfunction. Am J Cardiol. 2004;94(10):1306–10. doi: 10.1016/j.amjcard.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 37.Adarsh K, Kaur H, Mohan V. Coenzyme Q10 (CoQ10) in isolated diastolic heart failure in hypertrophic cardiomyopathy (HCM) Biofactors. 2008;32(1-4):145–9. doi: 10.1002/biof.5520320117. [DOI] [PubMed] [Google Scholar]
- 38.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301(6):H2181–90. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 39.Ziolo MT, Kohr MJ, Wang H. Nitric oxide signaling and the regulation of myocardial function. J Mol Cell Cardiol. 2008;45(5):625–32. doi: 10.1016/j.yjmcc.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silberman GA, Fan TH, Liu H, Jiao Z, Xiao HD, Lovelock JD, Boulden BM, Widder J, Fredd S, Bernstein KE, Wolska BM, Dikalov S, Harrison DG, Dudley SC., Jr. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121(4):519–28. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban, American journal of physiology. Cell physiology. 2008;294(6):C1566–75. doi: 10.1152/ajpcell.00367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias EG, Casadei B. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 2008;102(2):242–9. doi: 10.1161/CIRCRESAHA.107.164798. [DOI] [PubMed] [Google Scholar]
- 43.Layland J, Li JM, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol. 2002;540:457–67. doi: 10.1113/jphysiol.2001.014126. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikirova N, Casciari J, Rogers A, Taylor P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med. 2012;10:189. doi: 10.1186/1479-5876-10-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittermayer F, Pleiner J, Schaller G, Zorn S, Namiranian K, Kapiotis S, Bartel G, Wolfrum M, Brugel M, Thiery J, MacAllister RJ, Wolzt M. Tetrahydrobiopterin corrects Escherichia coli endotoxin-induced endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289(4):H1752–1757. doi: 10.1152/ajpheart.00057.2005. [DOI] [PubMed] [Google Scholar]
- 46.Chantler PD, Lakatta EG. Arterial-ventricular coupling with aging and disease. Front Physiol. 2012;3:90. doi: 10.3389/fphys.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goszcz K, Deakin SJ, Duthie GG, Stewart D, Leslie SJ, Megson IL. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front Cardiovasc Med. 2015;2:29. doi: 10.3389/fcvm.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gori T, Munzel T. Oxidative stress and endothelial dysfunction: therapeutic implications. Ann Med. 2011;43(4):259–72. doi: 10.3109/07853890.2010.543920. [DOI] [PubMed] [Google Scholar]
- 49.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583–92. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 50.Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, Liu CH, Hwang J, Selzer RH, Azen SP. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106(12):1453–9. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 51.Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, Atkins RC, Nicholls K, Fraenkel M, Hutchison BG, Walker R, McNeil JJ. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47(6):1108–16. doi: 10.1016/j.jacc.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 52.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–8. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Sleight P, Peto R, Collins R. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303(24):2486–94. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- 54.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300(18):2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belardinelli R, Mucaj A, Lacalaprice F, Solenghi M, Principi F, Tiano L, Littarru GP. Coenzyme Q10 improves contractility of dysfunctional myocardium in chronic heart failure. Biofactors. 2005;25(1-4):137–45. doi: 10.1002/biof.5520250115. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32(5):810–2. doi: 10.2337/dc08-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arbab-Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams-Huet B, Haykowsky MJ, Levine BD. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation. 2014;130(24):2152–61. doi: 10.1161/CIRCULATIONAHA.114.010775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoshikawa E, Matsumura Y, Kubo T, Okawa M, Yamasaki N, Kitaoka H, Furuno T, Takata J, Doi YL. Effect of left ventricular reverse remodeling on long-term prognosis after therapy with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers and beta blockers in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2011;107(7):1065–70. doi: 10.1016/j.amjcard.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 59.Fisman EZ, Tenenbaum A, Shapira I, Motro M, Pines A. The acute effects of sublingual estradiol on left ventricular diastolic function in normotensive and hypertensive postmenopausal women. Maturitas. 1999;33(2):145–52. doi: 10.1016/s0378-5122(99)00051-1. [DOI] [PubMed] [Google Scholar]
- 60.Ciccone MM, Cortese F, Gesualdo M, Carbonara S, Zito A, Ricci G, De Pascalis F, Scicchitano P, Riccioni G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm. 20132013:782137. doi: 10.1155/2013/782137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sciacchitano S, Cameli M, Maiello M, Modesti PA, Muiesan ML, Novo S, Palmiero P, Saba PS, Pedrinelli R, Ciccone MM. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. Journal of Functional Foods. 2014;6:11–32. [Google Scholar]
- 62.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43(5):645–57. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]