Abstract
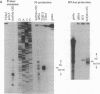
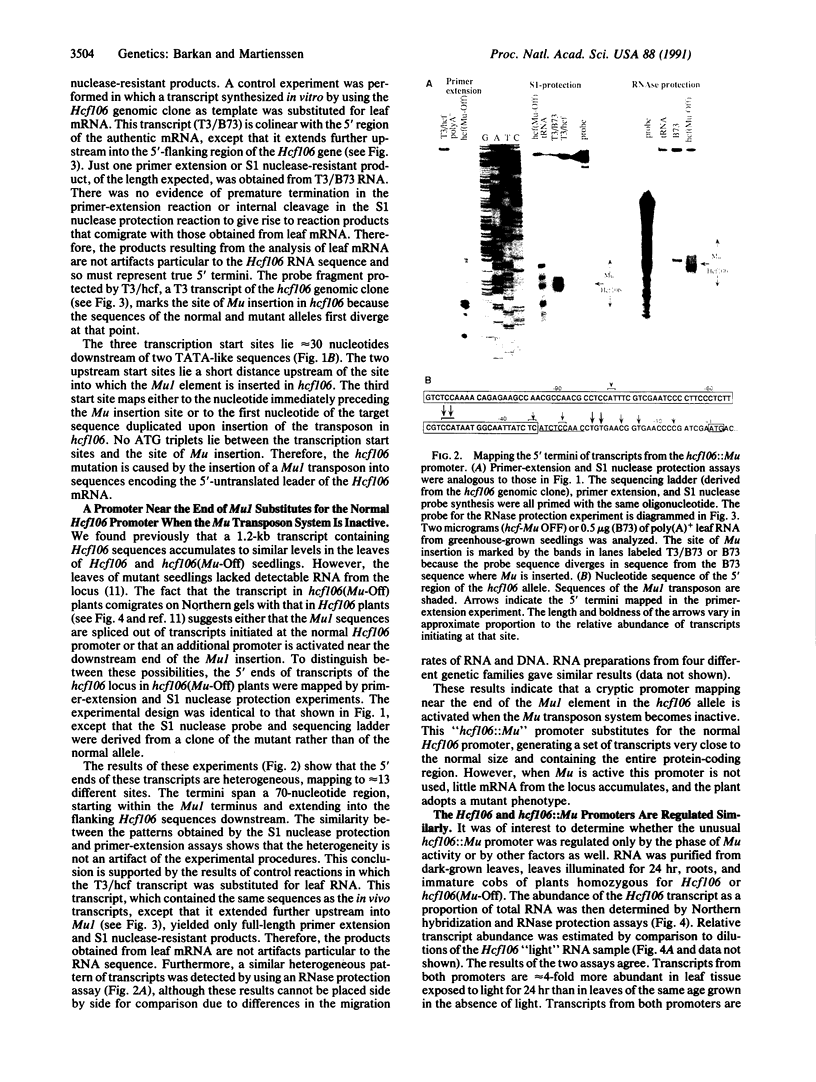
We described previously a mutation in maize, hcf106, caused by the insertion of a Mu1 transposon. When the Mu transposon system is in an active phase, hcf106 conditions a nonphotosynthetic, pale green phenotype. However, when the Mu system is inactive (a state correlated with hypermethylation of Mu elements), the plant adopts a normal phenotype despite the continued presence of the transposon within the gene. The molecular mechanisms that mediate this suppression of the mutant phenotype have now been investigated. We show here that the Mu element responsible for the hcf106 lesion lies within sequences encoding the 5'-untranslated leader of the Hcf106 mRNA. When the Mu transposon system is active, this insertion interferes with the accumulation of mRNA from the hcf106 allele. However, when Mu is inactive, mRNA similar in size and abundance to that transcribed from the normal allele accumulates. These transcripts initiate at many sites throughout a 70-base-pair region, within and immediately downstream of the Mu1 insertion. Thus, an unusual promoter spanning the downstream junction between Mu1 and Hcf106 substitutes for the normal Hcf106 promoter but only when Mu is inactive. The pattern of mRNA accumulation in different organs and in response to light suggests that the activity of this promoter is conditional not only upon the phase of Mu activity, but also upon signals that regulate the normal Hcf106 promoter.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayer D. E., Dynan W. S. Simian virus 40 major late promoter: a novel tripartite structure that includes intragenic sequences. Mol Cell Biol. 1988 May;8(5):2021–2033. doi: 10.1128/mcb.8.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A., Miles D., Taylor W. C. Chloroplast gene expression in nuclear, photosynthetic mutants of maize. EMBO J. 1986 Jul;5(7):1421–1427. doi: 10.1002/j.1460-2075.1986.tb04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A. Proteins encoded by a complex chloroplast transcription unit are each translated from both monocistronic and polycistronic mRNAs. EMBO J. 1988 Sep;7(9):2637–2644. doi: 10.1002/j.1460-2075.1988.tb03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R. F., Thompson D. V., Talbot D. R., Swanson J., Bennetzen J. L. Nucleotide sequence of the maize transposable element Mul. Nucleic Acids Res. 1984 Aug 10;12(15):5955–5967. doi: 10.1093/nar/12.15.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Swanson J., Taylor W. C., Freeling M. DNA insertion in the first intron of maize Adh1 affects message levels: cloning of progenitor and mutant Adh1 alleles. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4125–4128. doi: 10.1073/pnas.81.13.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W. A catalogue of splice junction and putative branch point sequences from plant introns. Nucleic Acids Res. 1986 Dec 22;14(24):9549–9559. doi: 10.1093/nar/14.24.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler V. L., Walbot V. DNA modification of a maize transposable element correlates with loss of activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1767–1771. doi: 10.1073/pnas.83.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errede B., Company M., Hutchison C. A., 3rd Ty1 sequence with enhancer and mating-type-dependent regulatory activities. Mol Cell Biol. 1987 Jan;7(1):258–265. doi: 10.1128/mcb.7.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierl A. How maize transposable elements escape negative selection. Trends Genet. 1990 May;6(5):155–158. doi: 10.1016/0168-9525(90)90150-5. [DOI] [PubMed] [Google Scholar]
- Green M. R. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- Kunze R., Stochaj U., Laufs J., Starlinger P. Transcription of transposable element Activator (Ac) of Zea mays L. EMBO J. 1987 Jun;6(6):1555–1563. doi: 10.1002/j.1460-2075.1987.tb02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lers A., Bitoun R., Zamir A. Outreading promoters are located at both ends of the gamma-delta transposon. Mol Gen Genet. 1989 Mar;216(1):138–143. doi: 10.1007/BF00332242. [DOI] [PubMed] [Google Scholar]
- Luehrsen K. R., Walbot V. Insertion of Mu1 elements in the first intron of the Adh1-S gene of maize results in novel RNA processing events. Plant Cell. 1990 Dec;2(12):1225–1238. doi: 10.1105/tpc.2.12.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martienssen R. A., Barkan A., Freeling M., Taylor W. C. Molecular cloning of a maize gene involved in photosynthetic membrane organization that is regulated by Robertson's Mutator. EMBO J. 1989 Jun;8(6):1633–1639. doi: 10.1002/j.1460-2075.1989.tb03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martienssen R., Barkan A., Taylor W. C., Freeling M. Somatically heritable switches in the DNA modification of Mu transposable elements monitored with a suppressible mutant in maize. Genes Dev. 1990 Mar;4(3):331–343. doi: 10.1101/gad.4.3.331. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. Functional relationships between transcriptional control signals of the thymidine kinase gene of herpes simplex virus. Cell. 1982 Dec;31(2 Pt 1):355–365. doi: 10.1016/0092-8674(82)90129-5. [DOI] [PubMed] [Google Scholar]
- Ortiz D. F., Strommer J. N. The Mu1 maize transposable element induces tissue-specific aberrant splicing and polyadenylation in two Adh1 mutants. Mol Cell Biol. 1990 May;10(5):2090–2095. doi: 10.1128/mcb.10.5.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Retroviral elements and suppressor genes in Drosophila. Bioessays. 1986 Aug;5(2):52–57. doi: 10.1002/bies.950050203. [DOI] [PubMed] [Google Scholar]
- Rowland L. J., Strommer J. N. Insertion of an unstable element in an intervening sequence of maize Adh1 affects transcription but not processing. Proc Natl Acad Sci U S A. 1985 May;82(9):2875–2879. doi: 10.1073/pnas.82.9.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Walbot V. Inheritance of mutator activity in Zea mays as assayed by somatic instability of the bz2-mu1 allele. Genetics. 1986 Dec;114(4):1293–1312. doi: 10.1093/genetics/114.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler S. R. The splicing of maize transposable elements from pre-mRNA--a minireview. Gene. 1989 Oct 15;82(1):127–133. doi: 10.1016/0378-1119(89)90037-1. [DOI] [PubMed] [Google Scholar]