Abstract
The antifungal activity of citronellal, a typical terpenoid of plant essential oils, against Penicllium digitatum and the possible action mode involved were investigated. Results showed that the mycelial growth and spores’ germination of P. digitatum were inhibited by citronellal in a dose-dependent manner. The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) were determined to be 1.60 µL/mL and 3.20 µL/mL, respectively. It was found that the plasma membrane of citronellal-treated P. digitatum spores was damaged, as confirmed by the propidium iodide stain results, as well as a higher extracellular conductivity and release of cell constituents in citronellal-treated samples than those of control samples. Moreover, in vivo test results demonstrated that wax + citronellal (WC; 10 × MFC) treatment effectively reduced the incidence of green mold after 5 days of storage at 25 ± 2 °C. These findings suggested that the plasma damage mechanism contributed to the antifungal activity of citronellal against P. digitatum. In addition, citronellal was suggested to be a potential alternative to fungicidal agents in controlling green mold of citrus fruit.
Keywords: Antifungal, Activities, Citronellal, Green mold, Penicillium digitatum, Plasma membrane damage
Introduction
Citrus fruit are sensitive to Penicillium digitatum, P. italicum and Geotrichum citri-aurantii during postharvest handling of the fruit (Wuryatmo et al. 2014). Among them, P. digitatum is the most serious postharvest pathogen, accounting for up to 90% of product losses (Liu et al. 2010). Correspondingly, the control of postharvest diseases caused by these pathogens is vital for maintaining citrus quality and reducing economic losses. Synthetic fungicides, such as imazalil, thiabendazole, pyrimethanil, fludioxonil, are used to minimize postharvest decay effectively. However, their extensive application has led to the proliferation of resistant strains, which compromised their effectiveness, and also led to the increase of human health risks (Jhalegar et al. 2015). Therefore, there is a need to find alternatives to synthetic fungicides.
Plant essential oils might be a good choice. They have been widely reported to be an effective and safe strategy to control postharvest diseases (Pérez-Alfonso et al. 2012; Wang et al. 2012; Regnier et al. 2014; Tao et al. 2014a). Citronellal is a volatile constituent in citrus and other plant essential oils (Carrillo-Hormaza et al. 2015; Tolba et al. 2015). Its antifungal activity has been demonstrated by some researchers (Lee et al. 2008; Rammanee and Hongpattarakere 2011; Trindade et al. 2015). Previously, citronellal was found to exhibit inhibitory effect on the growth of P. digitatum, P. italicum, and P. ulaiense, with the inhibition zone of 43, 43 and 31 mm2, respectively (Scora and Scora 1998). Zore et al. (2011) demonstrated that citronellal could inhibit the growth of Candida albicans by affecting membrane integrity and arresting cell cycle. The growth of nine tested fungal stains, such as P. adametzii and P. citrinum, could be completely inhibited by citronellal at a dose of 112 mg/L (Nakahara et al. 2013). In recent studies, citronellal has been observed to be one of the principal components in some plant essential oils and showed good antifungal activity against the pathogen that cause severe destruction in apple, tomato and banana (Aguiar et al. 2014; Arancibia et al. 2014). Thus, citronellal might be a potential antifungal substance in controlling postharvest disease of citrus fruit.
The objective of present work is to evaluate the effect of citronellal on P. digitatum through in vitro and in vivo experiments.
Materials and methods
Plant materials
Fruit of Newhall navel orange (Citrus sinensis Osbeck) were purchased on November 2015 from a super market near the campus of Xiangtan University, Xiangtan, China. Defect-free fruit with uniform size were chosen for the experiments.
Chemicals and microbial strains
Citronellal (95%) was purchased from TCI Shanghai (Shanghai, China). P. digitatum was provided by the Department of Biotechnology and Food Engineering, Xiangtan University, Xiangtan, China. The test strain was purified and preserved at 25 ± 2 °C on potato dextrose agar (PDA).
Effect of citronellal on mycelial growth
The effects of citronellal on mycelial growth of P. digitatum were evaluated following the method of Tao et al. (2014b). The final concentrations of citronellal in the culture media were 0.00, 0.20, 0.40, 0.80, 1.60, and 3.20 μL/mL, respectively. The lowest concentration that completely prevented the growth of the fungus after 48 h of incubation at 25 ± 2 °C was regarded as the MIC (minimum inhibitory concentration). The MFC (minimum fungicidal concentration) was defined as the lowest concentration that inhibited pathogen growth after 96 h of incubation at 25 ± 2 °C.
Effect of citronellal on spores’ germination
Different concentrations of citronellal (0.00, 0.050, 0.10, 0.20, 0.40, 0.80, 1.60 μL/mL) were used to determine their effects on spores’ germination. Fungal spores were placed on glass slides in triplicate, and slides containing the spores were incubated in a moist chamber at 25 ± 2 °C for 12 h (Wang et al. 2012).
Effect of citronellal on cell morphology
Samples were taken from the periphery of the colony growing on potato dextrose broth (PDB), and then supplied with 1.60 and 3.20 μL/mL citronellal after incubation for 30 min (for mycelia) or 6 h (for spores), respectively. Samples from control plates without citronellal were also stained and observed.
Assay of plasma membrane integrity
Membrane integrity was conducted following the method of Liu et al. (2010). Aliquots of 100 μL of P. digitatum spores’ suspension collected at different culture time (0, 2, 4, and 6 h) were transferred to 20 mL PDB with 0.050 and 1.60 μL/mL citronellal, respectively. The PDB without citronellal served as the control group. P. digitatum spores’ suspension was adjusted to a final concentration of 106 spores/mL. The number of spores in bright-field was defined as the total number, and membrane integrity (MI) was calculated according to the formula:
Measurement of extracellular conductivity
Measurement of extracellular conductivity of P. digitatum mycelia and spores was assayed following the method of Tao et al. (2014a). Citronellal at various concentrations (0, MIC and MFC) was added and incubated for 0, 30, 60, 120 min (for mycelia) and 0, 2, 4, 6 h (for spores) at 25 ± 2 °C in an environmental incubator shaker (150 rpm), respectively. The extracellular conductivity at each time point was recorded and expressed as μS/cm. Each parameter was tested in triplicate.
Determination of the release of cell constituents
The release of cell constituents into the supernatants was measured according to the method described previously (Tao et al. 2014c). The suspensions were treated with citronellal at various concentrations (0, MIC and MFC) for 0, 30, 60, 120 min (for mycelia) and 0, 2, 4, 6 h (for spores). Each parameter was tested in triplicate.
In vivo assays
In vivo assays were conducted following the method of Fan et al. (2014). After fungi were inoculated, the fruit were sprayed with wax amended with citronellal at MFC or 10 × MFC. The incidence rate of disease (measured by counting the number of green mold-inflicted lesions) was calculated as follows:
Statistical analysis
Each parameter was tested in triplicate. Conventional statistical methods were used to calculate means and standard deviations. Comparisons between groups were tested by one-way ANOVA analysis and LSD test (P < 0.05) using SPSS statistical software package release 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Effect of citronellal on mycelial growth
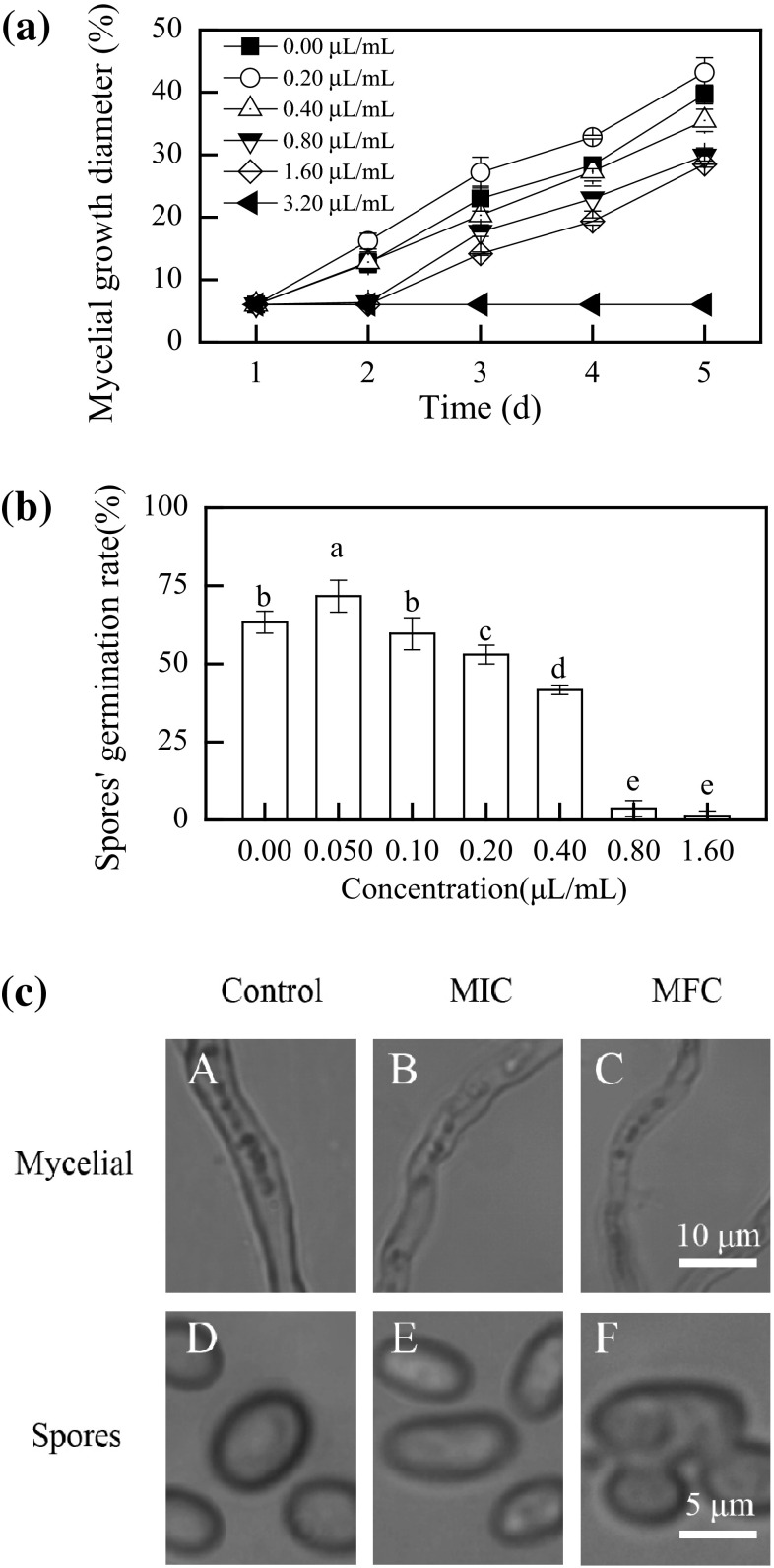
As shown in Fig. 1a, the mycelial growth of P. digitatum was affected by citronellal in a dose-dependent manner (P < 0.05). High citronellal concentration (≥1.60 μL/mL) completely inhibited the mycelial growth of P. digitatum, whereas citronellal at a concentration of 0.20 μL/mL showed stimulatory effect on the mycelial growth of P. digitatum after 2 days of incubation. After 4 days of incubation, the mycelial growth of P. digitatum was still totally inhibited by 3.20 μL/mL citronellal. MIC and MFC values of citronellal for P. digitatum were 1.60 and 3.20 μL/mL, respectively.
Fig. 1.
a Effect of citronellal at different concentrations on mycelial growth of P. digitatum incubated at 25 ± 2 °C for 5 days; b effect of different citronellal concentrations on the germination rate of P. digitatum spores incubated at 25 ± 2 °C for 12 h; c light microscopy image of P. digitatum mycelia after 30 min of incubation (A, B, and C) or spores after 6 h of incubation (D, E, and F) with or without citronellal. Data presented are the means of pooled data. Error bars indicate the standard deviations of the means (n = 3). Symbols with different letters at each time point indicate significant differences according to Duncan’s multiple range test (P < 0.05)
Effect of citronellal on spores’ germination
The percentage of germinated spores of P. digitatum is recorded in Fig. 1b. Citronellal at a concentration of 0.050 μL/mL stimulated the germination of spores. In contrast, the spores’ germination was visibly inhibited by citronellal at concentrations higher than 0.20 μL/mL. The germination rates for 0.40, 0.80, 1.60 μL/mL citronellal-treated samples were 53.0, 41.7 and 3.7%, respectively, which was significant lower than those of control (63.3%, P < 0.05).
Light microscopy
Light microscopy of the control P. digitatum hyphae showed normal morphology (Fig. 1c-A). Treatment with citronellal at MIC (Fig. 1c-B) or MFC (Fig. 1c-C) apparently altered the morphology of P. digitatum hyphae, including irregular hyphae, loss of linearity, and formation of warty surfaces. The control P. digitatum spores were normal and homogenous shape (Fig. 1c-D). By contrast, the MIC-treated spores showed a sunken surface and malformation (Fig. 1c-E), whereas the spores treated with MFC of citronellal were anomalous shape, with unordered structure and rough surface (Fig. 1c-F).
Effect of citronellal on plasma membrane integrity of P. digitatum spores
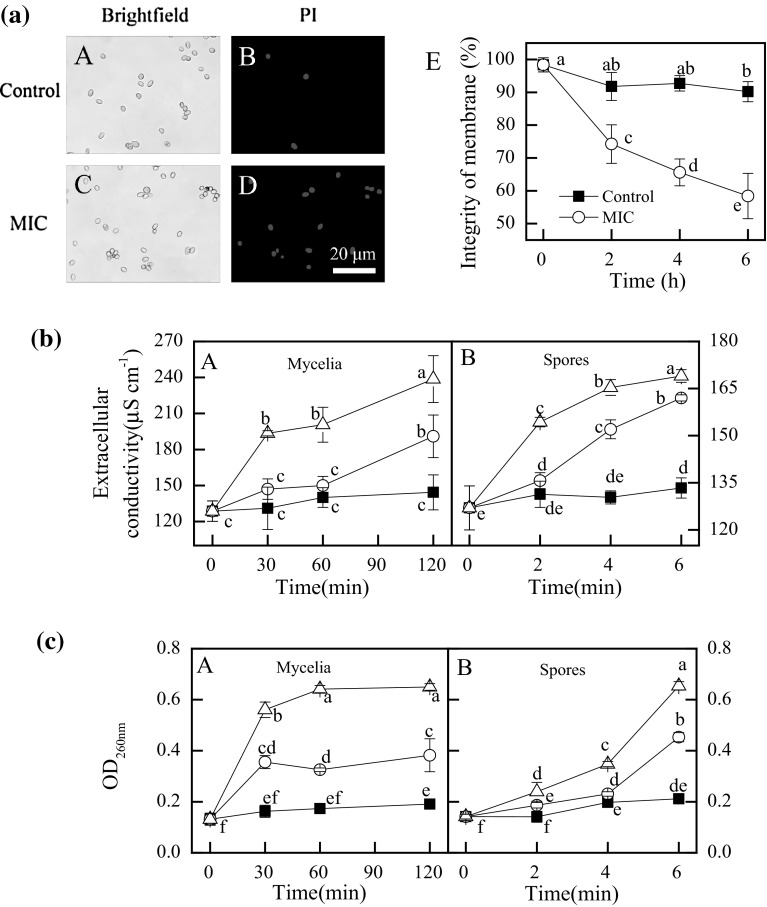
The plasma membranes of P. digitatum spores were markedly damaged by citronellal (P < 0.05) (Fig. 2a). The MI values of P. digitatum spores declined obviously with the increase of incubation time, in contrast to a relatively high level in those of control spores. After 6 h of incubation, the MI values of citronellal-treated and control P. digitatum spores were 58.4 and 90.2%, respectively.
Fig. 2.
a Effect of citronellal (1.60 μL/mL) on the plasma membrane integrity of P. digitatum spores(A, B, C, and D stand for the PI staining results at 6 h of exposure, whereas E stands for the percentage of plasma membrane integrity; b Effect of citronellal on the extracellular conductivity; c The 260 nm-absorbing material release of mycelia and spores. (Filed square) control; (open circle) citronellal at MIC; (filed triangle) citronellal at MFC. Data presented are the means of pooled data. Error bars indicate the SDs of the means (n = 3). Symbols with different letters at each time point indicate significant differences according to Duncan’s multiple range test (P < 0.05)
Extracellular conductivity
The extracellular conductivity in mycelia or spores suspensions was increased by the citronellal treatments (Fig. 2b-A). The extracellular conductivity in spores with MIC of citronellal for 6 h was 162.0 ± 1.0 μS/cm, which was significantly higher (P < 0.05) than those of control (133.3 ± 3.2 μS/cm) but significantly lower (P < 0.05) than those with MFC of citronellal (169.2 ± 2.0 μS/cm) (Fig. 2b-B)
Release of cell constituents
During the early exposure of MIC or MFC before 30 min (mycelia) and 6 h (spores), the release of cell constituents significantly increased (P < 0.05). The OD260nm value in P. digitatum mycelial suspensions with MIC of citronellal for 30 min was 0.36 ± 0.03, which was significantly higher (P < 0.05) than those of the control (0.16 ± 0.02) but significantly lower (P < 0.05) than those with MFC of citronellal (0.56 ± 0.03) (Fig. 2c-A). The OD260nm value of P. digitatum spores suspensions treated with MIC and MFC of citronellal maintained a smooth ascending trend after 2 h of exposure, whereas those with MFC continuously increased after 4 h of exposure, and reached to the highest absorbance of 0.45 ± 0.02 and 0.65 ± 0.17 at 6 h of exposure, respectively (Fig. 2c-D).
In vivo experiments
The ability of citronellal treatment to inhibit the disease development of citrus fruit inoculated with P. digitatum is presented in Table 1. The control fruit began to decay after 2 days of storage, whereas the incidence of fruit decay significantly (P < 0.05) delayed in citronellal-treated fruit in a dose-dependent manner. As incubation time was prolonged up to 4 days after inoculation, despite that the incidence of decay in control fruit was 100%, those in citronellal-treated (1 × and 10 × MFC) fruit were 100 and 5%, respectively.
Table 1.
Decay incidences in inoculated fruit treated with wax and wax + citronellal (WC; 1 × and 10 × MFC) treatment during storage at 25 ± 2 °C for 5 days and 85–90% relative humidity
| Treatment | Disease incidence (%) | ||||
|---|---|---|---|---|---|
| Inoculation period (days) | |||||
| 1 | 2 | 3 | 4 | 5 | |
| Wax | 0a | 25a | 80a | 100a | 100a |
| WC(1 × MFC) | 0a | 10b | 65b | 100a | 100a |
| WC(10 × MFC) | 0a | 0c | 0c | 5b | 30b |
Data presented are the means of pooled data (n = 10). Columns with different letters at each time point indicate significant differences according to Duncan’s multiple range test (P < 0.05)
Discussion
In the present study, citronellal exhibited a pronounced antifungal activity against the mycelial growth of P. digitatum, with MIC and MFC values being 1.60 μL/mL and 3.20 μL/mL, respectively. The MIC value was higher than those for citronellol, geraniol, neral, geranial and myrtaceae essential oils, but lower than those for citral, octanal, α-terpineol (Lee et al. 2008; Zhou et al. 2014). In addition, the MFC value was lower than those for citral, and α-terpineol (Zhou et al. 2014). The germination of P. digitatum spores was also visibly inhibited by citronellal at concentrations higher than 0.20 μL/mL. This result was in agreement with previous reports describing the antifungal activity of citronellal (Nakahara et al. 2013; Aguiar et al. 2014). Interestingly, citronellal at a concentration of 0.050 μL/mL could stimulate the germination of P. digitatum spores. This phenomenon was also observed in some previous studies. For example, Droby et al. (2008) reported that limonene, α-pinene, β-pinene and myrcene were stimulatory to P. digitatum and P. italicum at relatively low concentrations. In our previous studies, the spores’ germination and mycelial growth of P. italicum and P. digitatum were found to be mildly stimulated by the ‘Shatangju’ (C. reticulata Blanco) essential oil, β-myrcene, terpinen-4-ol, and citronellal (Wang et al. 2012; Tao et al. 2014a).
The permeability and integrity of cell plasma membrane serve important functions in maintaining fungal viability (Paul et al. 2011; Tao et al. 2014a). Previous studies have shown that the lipophilicity of essential oils enables them to preferentially partition from an aqueous phase into membrane structures of the fungi, resulting in membrane expansion, increased membrane fluidity and permeability, disturbance of membrane-embedded proteins, inhibition of respiration, alteration of ion transport processes in fungi and induced the leakage of other cellular contents (Tao et al. 2014b; Tyagi and Malik 2010; Zhou et al. 2014). In the current research, the results of PI staining and microscopy showed that the membrane integrity of P. digitatum spores obviously declined after citronellal treatment (Fig. 2a). In addition, exposure of P. digitatum to citronellal caused fast the loss of 260 nm absorbing materials and the increase of extracellular conductivity. These findings indicate that citronellal can induce damage to cell plasma membrane integrity of P. digitatum cells, which is in agreement with other studies (Zore et al. 2011; Tao et al. 2014c).
The in vivo results showed that citronellal elicited an evident inhibitory effect on green mold rot in navel orange fruit. These results confirmed those observed in the in vitro assay and also agreed with reports on other essential oils or their volatile compounds in controlling the diseases in citrus fruit (Fan et al. 2014; Pérez-Alfonso et al. 2012; Regnier et al. 2014; Tao et al. 2014b).
Conclusion
Our present research indicates that citronellal can inhibit the mycelial growth and spores’ germination of P. digitatum by a plasma damage mechanism. In addition, citronellal combined with wax treatment can reduce the incidence rate of postharvest green mold in citrus fruit. These results suggest that citronellal might be developed to be an alternative as natural fungicidal agents in controlling the postharvest disease of citrus fruit.
Acknowledgements
Funding was provided by National Natural Science Foundation of China (Grant Nos. 31271964, 31572172), Research Foundation of Education Bureau of Hunan Province (Grant No. 15A181).
References
- Aguiar RWDS, Ootani MA, Ascencio SD, Ferreira TP, Santos MMD, Santos GRD (2014) Fumigant antifungal activity of Corymbia citriodora and Cymbopogon nardus essential oils and citronellal against three fungal species. Sci World J 2014:1–8, Article ID 492138. doi:10.1155/2014/492138 [DOI] [PMC free article] [PubMed]
- Arancibia MY, López-Caballero ME, Gómez-Guillén MC, Montero P. Release of volatile compounds and biodegradability of active soy protein lignin blend films with added citronella essential oil. Food Control. 2014;44:7–15. doi: 10.1016/j.foodcont.2014.03.025. [DOI] [Google Scholar]
- Carrillo-Hormaza L, Mora C, Alvarez R, Alzate F, Osorio E. Chemical composition and antibacterial activity against Enterobacter cloacae of essential oils from Asteraceae species growing in the Páramos of Colombia. Ind Crop Prod. 2015;77:108–115. doi: 10.1016/j.indcrop.2015.08.047. [DOI] [Google Scholar]
- Droby S, Eick A, Macarisin D, Cohen L, Rafael G, Stange R, McColum G, Dudai N, Nasser A, Wisniewski M, Shapira R. Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol Technol. 2008;49:386–396. doi: 10.1016/j.postharvbio.2008.01.016. [DOI] [Google Scholar]
- Fan F, Tao NG, Jia L, He XL. Use of citral incorporated in postharvest wax of citrus fruit as a botanical fungicide against Penicillium digitatum. Postharvest Biol Technol. 2014;90:52–55. doi: 10.1016/j.postharvbio.2013.12.005. [DOI] [Google Scholar]
- Jhalegar MJ, Sharma RR, Singh D. In vitro and in vivo activity of essential oils against major postharvest pathogens of Kinnow (Citrus nobilis× C deliciosa) mandarin. J Food Sci Technol. 2015;52:2229–2237. doi: 10.1007/s13197-014-1281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Kim J, Shin SC, Lee SG, Park IK. Antifungal activity of Myrtaceae essential oils and their components against three phytopathogenic fungi. Flavour Fragr J. 2008;23:23–28. doi: 10.1002/ffj.1850. [DOI] [Google Scholar]
- Liu J, Zong Y, Qin G, Li B, Tian S. Plasma membrane damage contributes to antifungal activity of silicon against Penicillium digitatum. Curr Microbiol. 2010;61:274–279. doi: 10.1007/s00284-010-9607-4. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Alzoreky NS, Yoshihashi T, Nguyen HT, Trakoontivakorn G. Chemical composition and antifungal activity of essential oil from Cymbopogon nardus (citronella grass) JARQ-Jpn Agric Res Q. 2013;37:249–252. doi: 10.6090/jarq.37.249. [DOI] [Google Scholar]
- Paul S, Dubey RC, Maheswari DK, Kang SC. Trachyspermum ammi (L) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control. 2011;22:725–731. doi: 10.1016/j.foodcont.2010.11.003. [DOI] [Google Scholar]
- Pérez-Alfonso CO, Martínez-Romero D, Zapata PJ, Serrano M, Valero D, Castillo S. The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. italicum involved in lemon decay. Int J Food Microbiol. 2012;158:101–106. doi: 10.1016/j.ijfoodmicro.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Rammanee K, Hongpattarakere T. Effects of tropical citrus essential oils on growth, aflatoxin production, and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Bioprocess Technol. 2011;4:1050–1059. doi: 10.1007/s11947-010-0507-1. [DOI] [Google Scholar]
- Regnier T, Combrinck S, Veldman W, du Plooy W. Application of essential oils as multi-target fungicides for the control of Geotrichum citri-aurantii and other postharvest pathogens of citrus. Ind Crop Prod. 2014;61:151–159. doi: 10.1016/j.indcrop.2014.05.052. [DOI] [Google Scholar]
- Scora KM, Scora RW. Effect of volatiles on mycelium growth of Penicillium digitatum, P. italicum, and P. ulaiense. J Basic Microbiol. 1998;38:405–413. doi: 10.1002/(SICI)1521-4028(199811)38:5/6<405::AID-JOBM405>3.0.CO;2-2. [DOI] [Google Scholar]
- Tao NG, Jia L, Zhou HE. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014;153:265–271. doi: 10.1016/j.foodchem.2013.12.070. [DOI] [PubMed] [Google Scholar]
- Tao NG, Fan F, Jia L, Zhang ML. Octanal incorporated in postharvest wax of Satsuma mandarin fruit as a botanical fungicide against Penicillium digitatum. Food Control. 2014;45:56–61. doi: 10.1016/j.foodcont.2014.04.025. [DOI] [Google Scholar]
- Tao NG, OuYang QL, Jia L. Citral inhibits mycelial growth of Penicillium italicum by a membrane damage mechanism. Food Control. 2014;41:116–121. doi: 10.1016/j.foodcont.2014.01.010. [DOI] [Google Scholar]
- Tolba H, Moghrani H, Benelmouffok A, Kellou D, Maachi R. Essential oil of Algerian Eucalyptus citriodora: chemical composition, antifungal activity. J Med Mycol. 2015;25:e128–e133. doi: 10.1016/j.mycmed.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Trindade LA, de Araújo Oliveira J, de Castro RD, de Oliveira Lima E. Inhibition of adherence of C albicans to dental implants and cover screws by Cymbopogon nardus essential oil and citronellal. Clin Oral Invest. 2015;19:2223–2231. doi: 10.1007/s00784-015-1450-3. [DOI] [PubMed] [Google Scholar]
- Tyagi AK, Malik A. Liquid and vapour-phase antifungal activities of selected essential oils against Candida albicans: microscopic observations and chemical characterization of Cymbopogon citratus. BMC Complement Altern Med. 2010;10:1. doi: 10.1186/1472-6882-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tao NG, Huang SR, Liu YJ. Effect of Shatangju (Citrus reticulata Blanco) essential oil on spore germination and mycelium growth of Penicillium digitatum and P. italicum. J Essent Oil Bear Plants. 2012;15:715–723. doi: 10.1080/0972060X.2012.10644111. [DOI] [Google Scholar]
- Wuryatmo E, Able AJ, Ford CM, Scott ES. Effect of volatile citral on the development of blue mould green mould and sour rot on navel orange. Australas Plant Pathol. 2014;43:403–411. doi: 10.1007/s13313-014-0281-z. [DOI] [Google Scholar]
- Zhou HE, Tao NG, Jia L. Antifungal activity of citral octanal and α-terpineol against Geotrichum citri-aurantii. Food Control. 2014;37:277–283. doi: 10.1016/j.foodcont.2013.09.057. [DOI] [Google Scholar]
- Zore GB, Thakre AD, Jadhav S, Karuppayil SM. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine. 2011;18:1181–1190. doi: 10.1016/j.phymed.2011.03.008. [DOI] [PubMed] [Google Scholar]