Abstract
An important focus in vaccine research is the design of vaccine vectors with low seroprevalence and high immunogenicity. Replication-incompetent lymphocytic choriomeningitis virus (rLCMV) vectors do not elicit vector-neutralizing antibody responses, and homologous prime-boost regimens with rLCMV vectors induce boostable and protective T cell responses to model antigens in mice. However, cellular and humoral immune responses following homologous rLCMV vaccine regimens have not been rigorously evaluated in non-human primates (NHPs). To test whether rLCMV vectors constitute an effective vaccine platform in NHPs, we developed rLCMV vectors expressing SIVmac239 Env and Gag antigens and assessed their immunogenicity in mice and cynomolgus macaques. Immunization with rLCMV vaccine vectors expressing SIV Env and Gag was effective at generating SIV-specific T cell and antibody responses in both mice and NHPs. Epitope mapping using SIV Env in C57BL/6 mice demonstrated that rLCMV vectors induced sustained poly-functional responses to both dominant and subdominant epitopes. Our results suggest the potential of rLCMV vectors as vaccine candidates. Future SIV challenge experiments in rhesus macaques will be needed to assess immune protection by these vaccine vectors.
Keywords: LCMV, SIV, NHP
1. Introduction
The failure of the adenovirus serotype 5 (Ad5)-based vaccine in the STEP clinical trial has led to the search for alternative vaccine vectors with distinct immunological properties. Currently, the candidate vaccine portfolio is limited in terms of vector diversity, and a great focus has been to develop adenovirus-, cytomegalovirus-, and poxvirus-based vectors [1–5]. The characterization of novel alternative vaccine platforms may provide additional versatility for vaccine development, especially for use in populations with pre-existing immunity to other vaccine vectors [6].
In an attempt to expand the current vaccine portfolio, we have harnessed the immunogenicity of lymphocytic choriomeningitis virus (LCMV), which is a widely characterized murine virus that exhibits tropism for antigen presenting cells, especially macrophages and dendritic cells, and induces potent adaptive immune responses. The incidence of LCMV infections in human populations is low and clinical manifestations are rare, and thus, the virus does not represent a health concern [7,8]. However, severe LCMV infections have been reported in immunocompromised patients who receive solid organ transplants [9], and such infections have been shown to accelerate transplant rejection [10,11].
We have developed novel replication-defective LCMV vectors (rLCMV) that are not impeded by pre-existing humoral immunity following homologous boosting [12]. This is, at least in part, due to glycosylation of the LCMV surface protein (GP), which impairs the capacity of GP specific antibodies to neutralize the virus [13]. Moreover, rLCMV vectors do not encode the GP gene, and the vaccinee’s immune system is therefore only exposed to the minute amounts of viral GP present in the vector particle preparations, while de novo synthesis of GP as immunogen in the vaccinee is excluded. As a result, multiple homologous immunizations with rLCMV vectors result in undetectable rLCMV neutralization, thus allowing for robust transgene antigen delivery to boost vaccine-induced responses [12]. Moreover, we have previously shown several benefits of rLCMV vectors, including their potent CD8 T cell responses [12]. Although we have demonstrated partial immune protection against SIVsmE660 challenges following mismatch Env vaccination by Ad5 prime and rLCMV boost in rhesus macaques [14], the effect of homologous rLCMV boosting on adaptive immune responses in NHP models has not previously been evaluated.
In this study, we designed novel rLCMV vectors expressing SIVmac239 Env and Gag antigens, and we demonstrate their immunogenicity in mice and cynomolgus macaques. We also show that non-replicating rLCMV vectors can provide substantial immune protection against chronic LCMV challenges in mice. These results suggest that rLCMV vectors may be exploited for the development of prophylactic vaccines against infectious diseases or for immunotherapy of cancer, thus expanding the current vaccine portfolio.
2. Materials and methods
2.1. Animals and immunizations
Six to eight week old C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME), and were immunized intramuscularly (i.m) with 105 FFU of replication incompetent LCMV vectors (rLCMV) expressing SIVmac239 Env and Gag. For viral challenges, chronic LCMV Cl-13 was injected intravenously (i.v.) via the lateral tail vein at 2 × 106 PFU per mouse. The mouse experiments in Figs. 2–4 included a total of 7 rLCMV-Env vaccinated mice, 7 rLCMV-13-Gag vaccinated mice, and 7 unvaccinated mice (one experiment with n = 3 mice/per group and another experiment with n = 4 mice/per group). Cynomolgus macaques were used for testing the immunogenicity of rLCMV at different doses (Group I received 2 × 107 FFU, Group II received 2 × 106 FFU, and Group III received 2 × 105 FFU of rLCMV), each group consisting of 4 macaques. Cynomolgus macaques were housed at Bioqual (Rockville, MD). Immune responses were assessed at various time points using Env-specific ELISA, luciferase-based TZM-bl neutralization assays, microneutralization assay, ELISPOTs, and multiparameter intracellular cytokine (ICS) assays. Sera or peripheral blood mononuclear cells (PBMCs) were harvested and used for all immunological assays. All experiments were performed with approval of the appropriate Institutional Animal Care and Use Committees (IACUC).
Fig. 2.
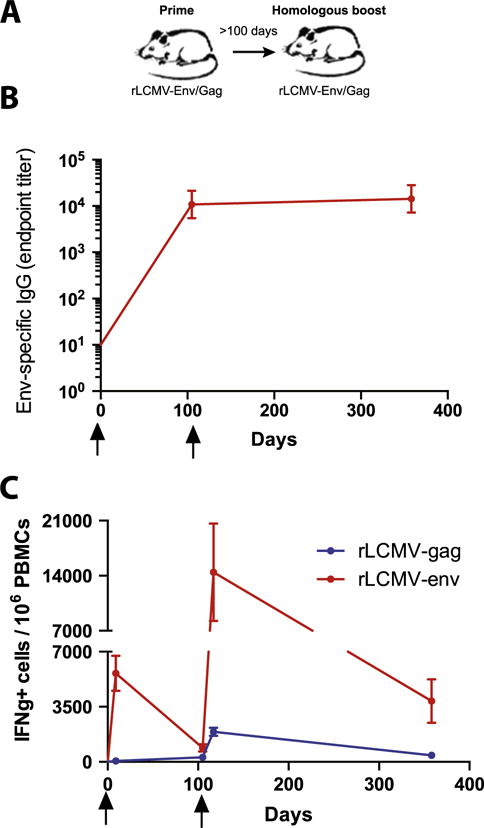
rLCMV vectors expressing SIVmac239 Gag and Env are immunogenic in mice. (A) Experimental layout. (B) Summary of SIV Env-specific antibody responses by ELISA. (C) Summary of Gag- and Env-specific CD8 T cell responses by ICS. Mice were first primed at day 0 and boosted homologously after day 100 to assess cellular immune responses in PBMC (ICS) and sera (ELISA). Error bars indicate SEM. Data are from 2 experiments, n = 3–4 per group/experiment.
Fig. 4.
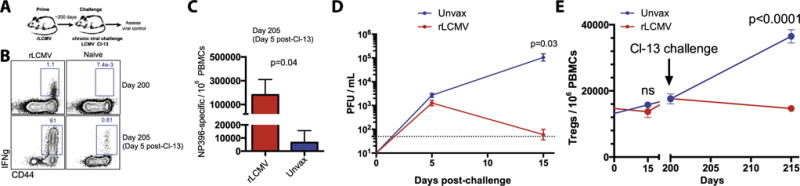
rLCMV vectors protect against a chronic LCMV Cl-13 challenge. (A) Experimental layout. (B) Representative FACS plots showing NP-specific CD8 and CD4 T cell responses before and after challenge by ICS. (C) Summary of NP-specific CD8 and CD4 T cell responses before and after challenge by ICS. (D) Viral control in sera. (E) Treg levels in PBMCs. Mice were challenged i.v. with 2 × 106 PFU LCMV Cl-13, and bled at day 5 to assess LCMV NP-specific CD8 T cell responses by ICS or viral loads by plaque assays. Error bars indicate SEM. Data are from 2 experiments, n = 3–4 per group/experiment.
2.2. Viral vector purification
The rLCMV vectors were generated and titrated as described previously [23,24]. Briefly, the coding sequence (cDNA) of individual vaccine antigens was generated by chemical synthesis by Genscript (USA) and inserted into a plasmid encoding a GP-deleted S segment of LCMV clone 13 under the control of a murine pol I promoter. SIV gag and gp140 antigen sequences used were derived from SIV mac 239 (Genbank accession number M33262). BHK-21 cells genetically engineered to express the LCMV GP protein were transfected with plasmids of the vector rescue system and vectors were harvested from supernatant of transfected cell after a blind passage of cells and supernatant.
rLCMV-gag and rLCMV-env vector stocks were generated in 30 ml cultures of serum-free suspension HEK293 production cell lines (293-GP) genetically engineered to express the LCMV GP protein. Cells were seeded in shake flasks at densities of 3 × 105 cells/ml in 30 ml CDM4HEK293 medium (GE Healthcare) supplemented with 4 mM stable Glutamine and 100 μg/ml Geneticin, and infected with rescued vector material at an MOI of 0.001. At day 3 post infection, supernatants from shake flasks were cleared from cells and debris by low speed centrifugation (500g, 5 min at 2–8 °C), aliquoted and frozen at <−60 °C. To assess vector growth kinetics, 293-GP cells were seeded and infected as above. Aliquots from the individual cultures were drawn at 2, 24, 48, 72 and 96 h post-infection, cleared as above, and frozen at < −60 °C.
For nonhuman primate studies, viral vectors encoding the gag and env gp140 transgenes from SIV mac239 were each produced in 10 L disposable bioreactors (Eppendorf, Germany) using vector stocks as seed material. The cell substrate for production was a complementing HEK293 cell line expressing LCMV GP. The production cells were seeded in CDM4HEK293 medium, cultivated and infected using the aforementioned viruses. During the whole cultivation the parameters like pH, DOT, T and PIV were controlled. When the vector propagation peaked, the cell suspension was harvested using serial Sartobind PP3 depth filtration units. Following DNA degradation using Benzonase (EMPROVE, Merck Millipore), vector concentration and purification was performed by bind-elute mode chromatography using a convective interaction medium (CIM-) cation exchange column. Post chromatography buffer exchange into a physiological buffer was performed by 300 K Hollowfiber tangential flow filtration (GE Healthcare). The vector product was sterilized with a Millipak filter unit (Merck Millipore).
The gag and env sequences encoded by rLCMV vectors were analyzed by consensus sequencing and expression of the proteins was verified by Western blotting of vector infected cells using SIV mac239 gag- or env-specific antibodies.
2.3. Viral titration
Infectious vector titers were determined by a focus formation assay using an anti-LCMV NP antibody (VL-4; Bio X Cell, Lebanon, NH). Briefly, monolayers of adherent LCMV GP-expressing HEK293 cells in 24-well plates were infected with serial dilutions of virus stock, incubated for 48 h, then fixed and stained with the anti-NP antibody. The number of foci was determined, and the virus titer was calculated. Titration of LCMV in mouse challenge studies was performed on VERO E6 cell (ATCC) monolayers by standard plaque assay as previously shown [15]. In brief, three ten-fold serial dilutions from serum samples were distributed on top of the VERO E6 cell monolayers in six well plates (>80% confluent). Plates were incubated for a total of 60 min manually rocking every 10 min. After this, a 1:1 solution of 1% agarose in 2 × 199 media (Gibco, Life Technologies) was carefully overlaid on top of the monolayers, and four days after, a 1:1 solution of 1% agarose in 2 × 199 media with 1:50 neutral red was pipetted on top of each well. Plaques were counted at day 5 based on neutral red exclusion using a transluminator.
2.4. Flow cytometry
Mouse PBMCs were stained with anti-CD8α (53–6.7), -CD4 (RM4-5), -CD44 (IM7). All surface anti-mouse antibodies were purchased from BD Pharmingen, except for CD44 (Biolegend). Biotinylated MHC I monomers (to detect LCMV NP396- or SIV AL11-specific CD8 T cell responses) were obtained from the NIH Tetramer Facility. PBMCs from cynomolgus macaques were stained with anti-CD3 (SP34-2), -CD4 (L200), -CD8 (RPA-T8), -CD69 (FN50). Intracellular cytokine staining for IFN-γ, TNFα, and IL-2 was performed with the Cytofix/Cytoperm kit (BD Biosciences). Samples were acquired using an LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo (Treestar).
2.5. Elispots assays
IFNγ-ELISPOT assays were performed using PBMCs from cynomolgus macaques stimulated with SIVmac239 Gag, Env1, Env2 peptide pools. We mapped SIVmac239 Env CD8 T cell epitopes in C57BL/6 mice using overlapping peptide pools (JPT, Berlin, Germany). Peptide pools consisted of 15-mer peptides overlapping by 11, which spanned the entire SIVmac239 Env protein. Peptides were purified to >80% for routine usage, or >95% for “high purity” as specifically indicated in Fig. 3.
Fig. 3.
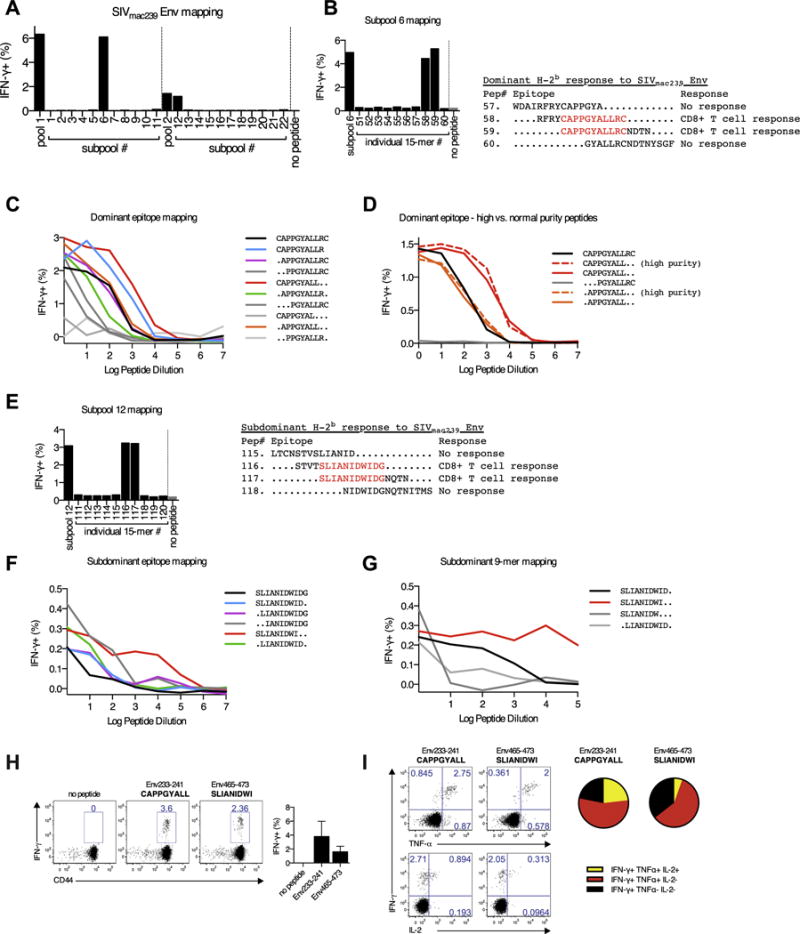
Mapping of SIVmac239 Env CD8 T cell epitopes in C57BL/6 mice. In panels A-G, C57BL/6 mice were immunized intramuscularly with Ad26-SIVmac239 Env. Purified splenocytes were re-stimulated with the indicated SIVenv239 peptide or peptide pool. (A) IFNγ expression by CD8 T cells in response to stimulation with the indicated SIVmac239 peptide pool or the constituent subpools of the indicated pool. (B) Mapping of the dominant peptide epitope within subpool 6. (C) Identification of the minimal CD8 T cell epitope within the peptide identified in subpool 6, as measured by IFNγ expression in response to log-fold dilution of peptide. (D) Response kinetics for subpool 6 mapping using high purity peptides against the two strongest responding peptides identified in panel C. This was performed to confirm that the stronger responses in the “CAPPGYALL” epitope did not reflect degradation impurities. (E) Mapping of the subdominant peptide epitope within subpool 12. (F) Identification of the minimal CD8 T cell epitope within the peptide identified in subpool 12, as measured by IFNγ expression in response to log-fold dilution of peptide. Mapping was performed down to the 9-mer level. (G) Identification of the minimal CD8 T cell epitope within the 9-mer peptide identified in subpool 12 (“SLIANIDWI”), as measured by IFNγ expression in response to log-fold dilution of peptide. (H, I) C57BL/6 mice were immunized intramuscularly with rLCMV-SIVmac239 Env and >1 year post-immunization Env-specific CD8 T cell responses were assessed against the dominant (Env233–241; CAPPGYALL) or subdominant (Env465–473; SLIANIDWI) epitopes. (H) Representative staining and group summaries for IFNγ expression by CD8 T cells in response to ex vivo stimulation with the dominant and subdominant epitope peptides. (I) Polyfunctionality of CD8 T cells from rLCMV-SIVmac239 Env immunized animals specific for the dominant and subdominant epitopes. Data are from two experiments, n = 3–4 per group/experiment.
2.6. Luciferase-based TZM-bl neutralization assays
These assays were performed using pseudotyped SIV, and CCR5 and CD4 expressing Hela cells as previously shown [16].
2.7. LCMV microneutralization assay
ARPE-19 cells (ATCC CRL-2302) were seeded in DMEM:F12 with 10% heat-inactivated FBS in half-area flat-bottom 96-well tissue culture plates three days prior to infection. Serial 2-fold dilutions of test sera, pre-incubated at 56 °C for 30 min to inactivate complement, were prepared in DMEM:F12 containing 25% heat-inactivated FBS in order to maintain a constant concentration of serum. GFP-expressing rLCMV was diluted in serum-free DMEM: F12 medium to obtain a suspension that generates 80–150 NP-positive cells per well and incubated with the serum dilutions at 37 °C for 1 h. The culture medium was removed and replaced with 50 μl of virus/serum mixture. 50 μl of DMEM:F12 medium containing 10% heat-inactivated FBS was added to each well and the plates were incubated overnight at 37 °C. The medium was removed, the cells were washed with PBS and fixed with cold 80% acetone for 25–30 min at 4 °C. Fifty μl of anti-NP monoclonal antibody (VL-4; Bio X cell, West Lebanon, NH) diluted to 1 μg/ml in PBS with 2% dry milk was added for 1 h at 37 °C before washing three times in PBS. Fifty μl of biotin-labeled goat anti-rat IgG (Sigma) diluted 1:1000 in PBS with 2% dry milk was added for 1 h at 37 °C. Following washing three times in PBS, 50 μl of HRP-streptavidin (Dako, Glostrup, Denmark) diluted 1:1000 in PBS with 2% dry milk was added for 30 min at 37 °C. Following washing four times in PBS, TrueBlue (KPL, Gaithersburg, MD) was added for 15 min at room temperature in the dark. Stained plates were rinsed with distilled water, air dried, and stored in the dark until manual reading under a microscope.
2.8. Statistical analysis
Statistical analyses were performed using two-tailed parametric Mann-Whitney tests in GraphPad Prism software.
3. Results
3.1. Construction of rLCMV vectors
Replication incompetent rLCMV Cl-13 vectors were designed as previously shown [12]. The LCMV GP gene was exchanged with the SIV mac239 Env and Gag transgenes, respectively, producing two individual vectors (Fig. 1A). Correctness of the gag and env sequences encoded by LCMV vectors was confirmed by consensus sequencing and expression of the proteins was verified by Western blotting of vector infected cells using SIV mac239 gag- or env-specific antibodies (data not shown). The two vectors replicated robustly in cultured trans-complementing LCMV GP-expressing HEK293 cells (Fig. 1B).
Fig. 1.
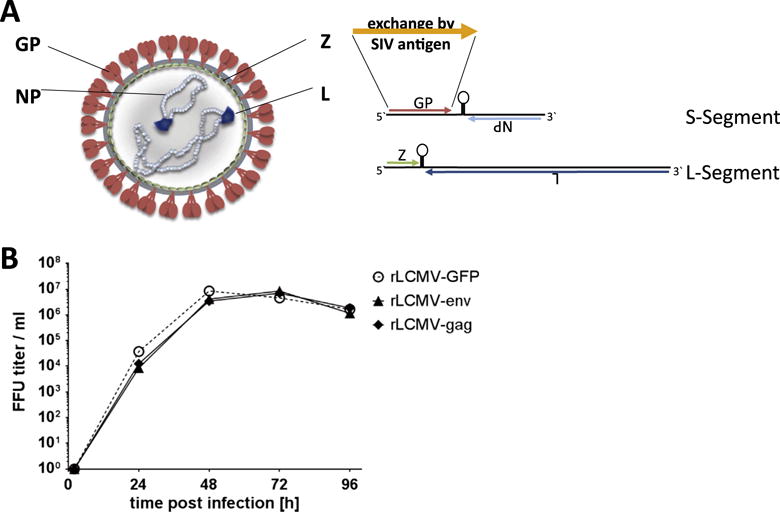
Generation of rLCMV vectors expressing SIV antigens. (A) Diagram adapted from Flatz and Pinschewer (Nature Medicine 2010) depicting how vectors were made. (B) Replication kinetics of the vectors in 293-GP cells. Cells were infected with vectors at MOI of 0.001 and aliquots were drawn at 2, 24, 48, 72 and 96 h post-infection for titration. A rLCMV vector expressing green fluorescent protein (rLCMV-GFP) was used as a control to show that the SIV transgenes did not affect vector propagation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. rLCMV vectors are immunogenic in mice
We immunized C57BL/6 mice i.m. with 105 FFU of rLCMV vectors expressing SIVmac239 Gag or Env, followed by homologous boosting after more than 100 days (Fig. 2A). This resulted in robust induction of SIV-specific antibody responses (Fig. 2B), and CD8 T cell responses (Fig. 2C). Of note, CD8+ T cell responses peaked on day 9 after rLCMV prime, contracted by day 100, but were potently recalled following boost immunization. rLCMV-env vectors induced higher levels of IFNγ expressing CD8 T cells than rLCMV-gag vectors (Fig. 2C). Hence, we sought to determine if this reflected a potent response against a single epitope, or responses against multiple epitopes. We identified a dominant and subdominant CD8 T cell epitope within the SIVmac239 Env response (Fig. 3A). Subsequent mapping identified the minimal dominant epitope as Env233–241 (CAPPGYALL) (Fig. 3B–D) and the minimal subdominant epitope as Env465–473 (SLIANIDWI) (Fig. 3E–G). With these novel epitopes mapped, PBMCs were collected from rLCMV-env homologous prime-boosted mice on day 400 post-immunization and restimulated individually with the dominant Env233–241 and subdominant Env465–0473 peptides. Robust IFN-γ production by vaccine-elicited CD8 T cells was detected in response to both epitopes (Fig. 3H). Additionally, a majority of CD8 T cells specific for Env233–241 and Env465–473 produced TNF-α and/or IL-2 in addition to IFN-γ (Fig. 3I). Thus, rLCMV vector vaccination can induce poly-functional memory CD8 T cell responses against dominant and subdominant epitopes, which can be efficiently boosted upon re-administration of the vector.
3.3. rLCMV vectors protect against chronic LCMV challenge in mice
In the experiments above, we confirmed that non-replicating rLCMV vectors induce cellular and humoral responses to heterologous vaccine antigens in mice. To assess protection by vaccine-induced immune responses to the vector, we performed LCMV challenges in rLCMV-vaccinated mice (Fig. 4A). We first vaccinated mice with non-replicating rLCMV expressing SIVmac239 Gag and Env (105 FFU), and after 200 days, we challenged them intravenously with the persistence-prone LCMV strain Clone 13 (Cl-13), which normally induces a chronic infection in mice [17,18]. rLCMV vaccinated mice exhibited 27.8-fold greater numbers of LCMV-specific CD8 T cells (H-2Db-NP396) in blood at day 5 following chronic LCMV Cl-13 challenge relative to unvaccinated mice (Fig. 4B and C). Although LCMV titers were not statistically different at day 5 post-challenge (p = 0.07), mice that had received the non-replicating rLCMV vaccine exhibited significant viral control by day 15 post-challenge (1763-fold lower viral loads, p = 0.03) relative to unvaccinated mice (Fig. 4D). Of note, rLCMV vectors (which are based on the chronic LCMV Cl-13 backbone) did not increase T regulatory cells, which normally occurs after infection with replicating Cl-13 [19], suggesting that rLCMV vectors are safe and do not induce an immunosuppressive environment (Fig. 4E). These results demonstrate that non-replicating rLCMV vaccines based on the Cl-13 backbone can protect against a persistent viral challenge and do not induce increased Treg levels.
3.4. rLCMV vectors are immunogenic in cynomolgous macaques
LCMV is a prototypic arenavirus that is highly immunogenic in mice and also replicates efficiently in non-human primates [20]. To validate the immunogenicity of our rLCMV vectors in NHPs, we vaccinated cynomolgus macaques (Macaca fascicularis) with non-replicating rLCMV vectors expressing SIVmac239 Gag and Env. We primed three groups of 4 macaques each with high (group I), mid (group II), or low (group III) doses (2 × 107, 2 × 106, and 2 × 105 FFU, respectively) of rLCMV-gag/env vectors, followed by repeated homologous boosting, as indicated in Fig. 5A. As expected, repetitive homologous boosting with rLCMV-gag and rLCMV-env vectors resulted in increased T cell responses detected by intracellular cytokine staining (Fig. 5B) and IFN-γ ELISPOT assays (Fig. 5C). There was a pattern of increased responses in the high dose group (Group I), but the difference was not statistically significant when compared to the other 2 groups. Importantly, high doses of rLCMV vaccines induced enhanced effector memory differentiation (CD28-CD95+) on total, as well as SIV-specific CD8 T cells (Fig. 6A and B).
Fig. 5.
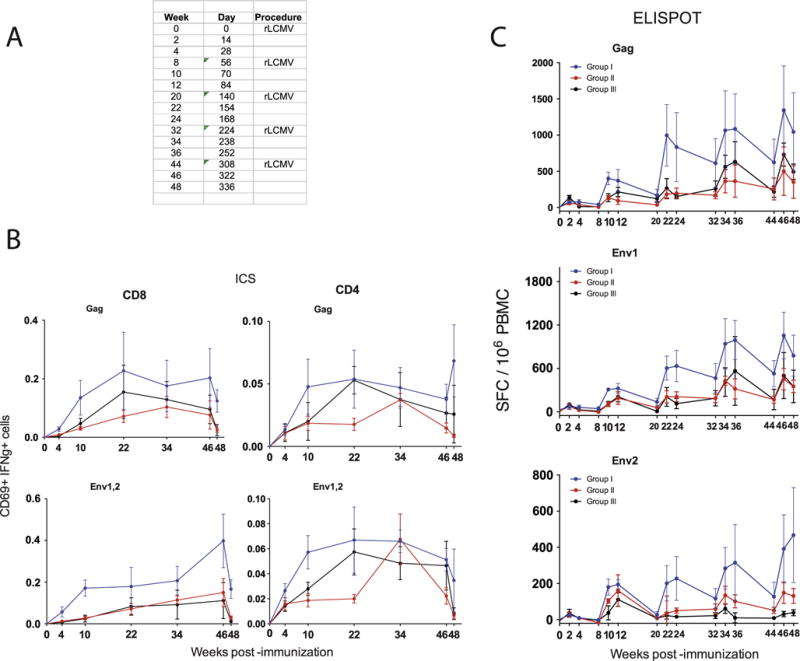
rLCMV vectors induce SIV-specific T cell responses in cynomolgus macaques. (A) Experiment outline. (B) Longitudinal analyses of Gag and Env-specific CD8 and CD4 T cell responses by ICS at week 49. (C) Representative FACS plots of CD8 and CD4 T cell responses by ICS. (D) Longitudinal analyses of Gag and Env-specific T cell responses by ELISPOTs. Error bars indicate SEM.
Fig. 6.
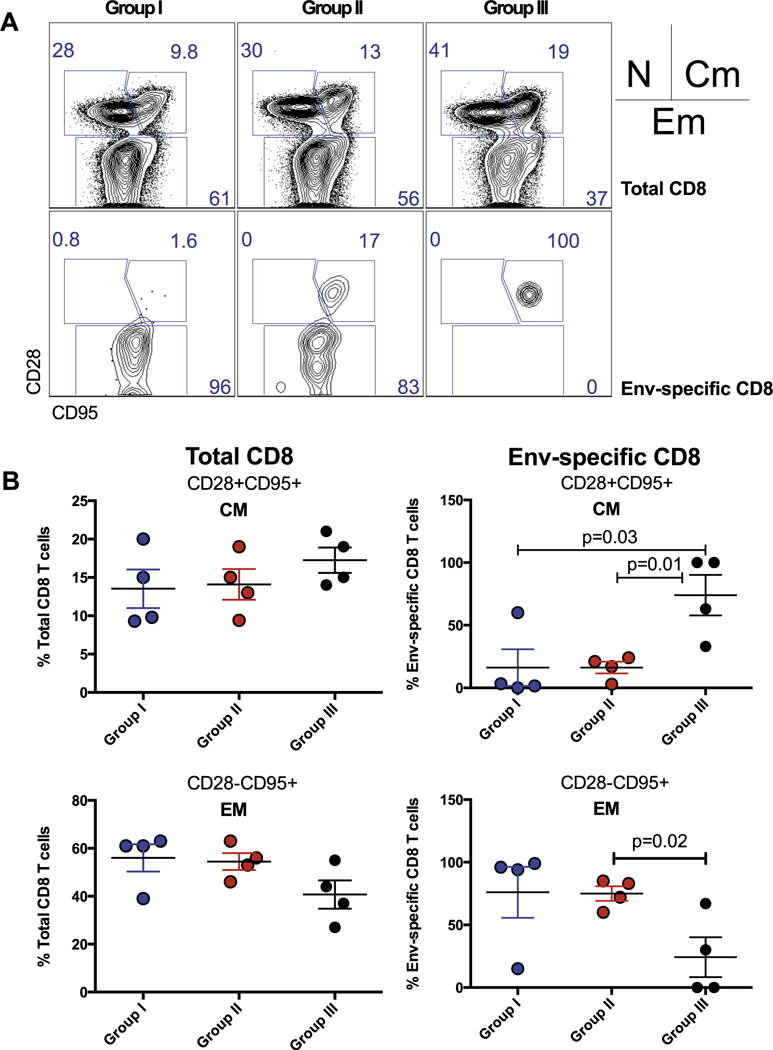
Effect of rLCMV vaccine dose on T cell subset differentiation. (A) Representative FACS plots showing effector memory and central memory subsets on total CD8 T cells and SIV Env-specific CD8 T cells. (B) Summary of effector memory and central memory subsets on total CD8 T cells and SIV Env-specific CD8 T cells. Data are from week 48 from the same macaque study as in Fig. 4, which included 4 macaques per group. Error bars indicate SEM.
In addition, we evaluated humoral responses following immunization with non-replicating rLCMV vectors. The 2 × 107 FFU dose (group I) resulted in high Env-specific IgG antibody responses by ELISA (Fig. 7A). Moreover, this high dose (2 × 107 FFU) induced substantial Tier 1A (SIVmac251.TCLA.15) neutralization in standard luciferase-based TZM-bl assays (Fig. 7B). Tier 1A-neutralizing responses were also consistently mounted upon administration of rLCMV vectors at low or medium dose (Group II, III), respectively, although at somewhat lower titers than in the high-dose group (Group I). No neutralizing antibody responses were detected against Tier 2 pseudoviruses using luciferase-based TZM-bl assays (SIVmac251.30 ID50 titer <1:20; data not shown). We also assessed the ability of sera from animals immunized with one or two doses of the vaccines to neutralize LCMV infection of human epithelial cells in vitro. Sera from week 0 (baseline), week 8 (following one dose), and week 12 (following two doses) showed no neutralizing ability at a dilution of 1:8, the lowest dilution tested (data not shown).
Fig. 7.
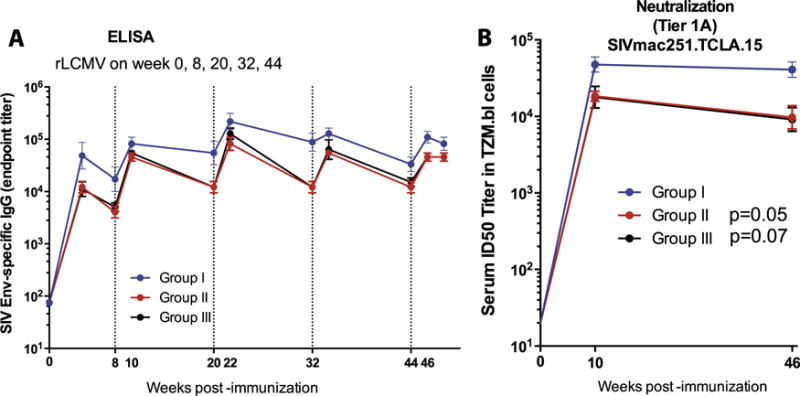
rLCMV vectors induce SIV-specific antibody responses in cynomolgus macaques. (A) Longitudinal analyses of Env-specific antibody responses by ELISA. SIVmac239 Env protein was used as coating antigen and secondary antibody was conjugated to HRP. (B) Longitudinal analyses of SIV neutralizing antibody responses by TZM-bl assays. P-values at week 10 post-immunization are indicated relative to Group I. Error bars indicate SEM.
4. Discussion
We have evaluated the immunogenicity and boosting capacity of replication-incompetent rLCMV vectors in mice and NHPs. Immunization of mice with rLCMV vectors expressing SIVmac239 Env and Gag immunogens resulted in persistent CD8+ T cell responses (Figs. 2–4). We were also able to map the H-2b-restricted immunodominant and subdominant CD8 T cell epitopes in SIVmac239 Env (Env233–241 and Env465–473, respectively). Following challenge with LCMV Cl-13, virus-specific CD8 T cell responses elicited by replication-incompetent rLCMV vectors expanded robustly (27.8-fold greater expansion relative to unvaccinated at day 5, p = 0.04), and resulted in viral control by day 15 post-challenge. Massive CD8 T cell recall was a feature of boosting with replicating LCMV Cl-13, consistent with some of our prior data showing that the replicative capacity of the boosting antigen can influence the recall potential of primed immune responses [21]. Note that an LCMV Cl-13 challenge in unimmunized mice normally results in chronic infection and exhaustion of primary immune responses [17,18,22–29], but our data show that when LCMV Cl-13 is administered as a boost, it results in an impressive expansion of anamnestic T cell responses. Therefore, the order of prime-boost immunizations is critical for rational vaccine design, and our results suggest the potential of live replicating agents as boosts. However, future studies should rigorously assess the safety of replicating vectors as boosting antigens.
Moreover, we tested the immunogenicity of rLCMV vectors in cynomolgus macaques in dose-escalating studies, and we observed that a 2 × 107 FFU dose resulted in higher SIV-specific T cell responses relative to lower doses. Importantly, for all dosage groups, the frequencies of antigen specific T-cell responses could be augmented after each homologous booster vaccination. It was interesting that higher doses of rLCMV vaccines induced biased effector memory CD8 T cell differentiation. Effector memory CD8 T cells are known to mediate rapid cytotoxic killing following antigen challenge [30–32], thus representing a potential advantage of increasing vaccine dose, especially in the context of SIV challenge, where immediate cytotoxic function may be critical even hours after initial mucosal exposure [33].
Although the level of virus neutralization by humoral responses is known to increase with time following SIV/HIV [34–41], and chronic LCMV infections [13], likely as a result of continuous antigen-driven germinal center reactions with concomitant affinity maturation, it is currently unclear whether multiple homologous boosting during vaccination could lead to the same result. Importantly, our studies suggest that, different from chronic replicating infection with wild type LCMV, repetitive homologous vaccination with rLCMV vectors does not lead to LCMV-neutralizing antibody titers, which would prevent vector re-administration. With respect to rLCMV-induced SIV-specific antibodies, it remains unclear why SIV-neutralizing antibodies peaked after the first rLCMV vaccination, but did not measurably increase after multiple rLCMV boosts, whereas binding antibodies (measured by ELISA) did. Whether the same may apply to heterologous prime-boost regimens, such as those involving poxvirus- or adenovirus-based vectors, remains to be determined. Our results demonstrate, however, that higher doses of rLCMV vaccine result in higher virus neutralization relative to lower doses. These findings are, therefore, novel and important for rational vaccine design.
The rLCMV vaccine platform offers various attractive features such as its robust induction of dendritic cell activation, and the fact that these vectors bypass LCMV (vector-specific, but not transgene-specific) neutralizing antibody responses [12]. Moreover, a prior study showed the high boosting potential of a mismatched Ad5 prime, rLCMV boost vaccine regimen in a model of SIVsmE660 challenge in rhesus macaques [14]. Our data extend these prior results by assessing the effect of a homologous, dose-escalating rLCMV prime-boost regimens in NHPs, focusing longitudinally on both the humoral and cellular immune responses. Importantly, we evaluate the effects of homologous vaccine dosing in determining the levels of neutralizing antibodies. Future studies will assess whether rLCMV vectors expressing SIV envelopes can induce additional neutralization capacity and provide immune protection against heterologous SIV challenges in rhesus macaques, as well as other viral and bacterial diseases.
Acknowledgments
The mouse work in this manuscript was supported by NIH grants AI007245 and AI07387 (P.P.M.) and AI078526 and AI096040 (D.H.B.); the Bill and Melinda Gates Foundation grant OPP1033091 (D.H.B.); the Ragon Institute of MGH, MIT, and Harvard (D.H.B.); and the Herchel Smith Graduate Fellowship (N.M.P.). The NHP work in this manuscript was supported by Hookipa Biotech and by the Austrian Research Promotion Agency (Die Österreichische Forschungsförderungsgesellschaft, FFG). We thank Michelle Lifton for technical advice and assistance.
Footnotes
The authors declare no financial conflicts of interest.
References
- 1.Dudek T, Knipe DM. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006;344:230. doi: 10.1016/j.virol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Barouch DH, Picker LJ. Novel vaccine vectors for HIV-1. Nat Rev Microbiol. 2014;12:765. doi: 10.1038/nrmicro3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbink P, et al. Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors. J Virol. 2015;89:1512. doi: 10.1128/JVI.02950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen SG, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen SG, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouch DH, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elbers AR, et al. Low prevalence of antibodies against the zoonotic agents Brucella abortus, Leptospira spp., Streptococcus suis serotype II, hantavirus, and lymphocytic choriomeningitis virus among veterinarians and pig farmers in the southern part of The Netherlands. Vet Q. 1999;21:50. doi: 10.1080/01652176.1999.9694991. [DOI] [PubMed] [Google Scholar]
- 8.Lledo L, Gegundez MI, Saz JV, Bahamontes N, Beltran M. Lymphocytic choriomeningitis virus infection in a province of Spain: analysis of sera from the general population and wild rodents. J Med Virol. 2003;70:273. doi: 10.1002/jmv.10389. [DOI] [PubMed] [Google Scholar]
- 9.Fischer SA, et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. New Engl J Med. 2006;354:2235. doi: 10.1056/NEJMoa053240. [DOI] [PubMed] [Google Scholar]
- 10.Welsh RM, et al. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J Virol. 2000;74:2210. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams MA, et al. Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance. J Immunol. 2001;167:4987. doi: 10.4049/jimmunol.167.9.4987. [DOI] [PubMed] [Google Scholar]
- 12.Flatz L, et al. Development of replication-defective lymphocytic choriomeningitis virus vectors for the induction of potent CD8+ T cell immunity. Nat Med. 2010;16:339. doi: 10.1038/nm.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommerstein R, et al. Arenavirus glycan shield promotes neutralizing antibody evasion and protracted infection. PLoS Pathog. 2015;11:e1005276. doi: 10.1371/journal.ppat.1005276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flatz L, et al. Gene-based vaccination with a mismatched envelope protects against simian immunodeficiency virus infection in nonhuman primates. J Virol. 2012;86:7760. doi: 10.1128/JVI.00599-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penaloza-MacMaster P, et al. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J Virol. 2013;87:1373. doi: 10.1128/JVI.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seaman MS, et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol. 2010;84:1439. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penaloza-MacMaster P, et al. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med. 2014;211:1905. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukashevich IS, et al. LCMV-mediated hepatitis in rhesus macaques: WE but not ARM strain activates hepatocytes and induces liver regeneration. Arch Virol. 2004;149:2319. doi: 10.1007/s00705-004-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penaloza-MacMaster P, et al. Augmented replicative capacity of the boosting antigen improves the protective efficacy of heterologous prime-boost vaccine regimens. J Virol. 2014;88:6243. doi: 10.1128/JVI.00406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79:8960. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 25.Stelekati E, Wherry EJ. Chronic bystander infections and immunity to unrelated antigens. Cell Host Microbe. 2012;12:458. doi: 10.1016/j.chom.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stelekati E, et al. Bystander chronic infection negatively impacts development of CD8(+) T cell memory. Immunity. 2014;40:801. doi: 10.1016/j.immuni.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawford A, et al. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity. 2014;40:289. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed R, Oldstone MB. Organ-specific selection of viral variants during chronic infection. J Exp Med. 1988;167:1719. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 31.Gubser PM, et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14:1064. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- 32.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitney JB, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong R, et al. Broad and potent neutralizing antibody responses elicited in natural HIV-2 infection. J Virol. 2012;86:947. doi: 10.1128/JVI.06155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malherbe DC, et al. Envelope variants circulating as initial neutralization breadth developed in two HIV-infected subjects stimulate multiclade neutralizing antibodies in rabbits. J Virol. 2014;88:12949. doi: 10.1128/JVI.01812-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao HX, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doria-Rose NA, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 39.Moir S, Malaspina A, Fauci AS. Prospects for an HIV vaccine: leading B cells down the right path. Nat Struct Mol Biol. 2011;18:1317. doi: 10.1038/nsmb.2194. [DOI] [PubMed] [Google Scholar]
- 40.Scheid JF, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 41.Alpert MD, et al. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac) 251 challenge. PLoS Pathog. 2012;8:e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]


