Abstract
Actin and small heat shock proteins (sHsps) are ubiquitous and multifaceted proteins that exist in 2 reversible forms, monomers and multimers, ie, the microfilament of the cytoskeleton and oligomers of the sHsps, generally, supposed to be in a spherical and hollow form. Two situations are described in the literature, where the properties of actin are modulated by sHsps; the actin polymerization is inhibited in vitro by some sHsps acting as capping proteins, and the actin cytoskeleton is protected by some sHsps against the disruption induced by various stressful conditions. We propose that a direct actin-sHsp interaction occurs to inhibit actin polymerization and to participate in the in vivo regulation of actin filament dynamics. Protection of the actin cytoskeleton would result from an F-actin–sHsp interaction in which microfilaments would be coated by small oligomers of phosphorylated sHsps. Both proteins share common structural motives suggesting direct binding sites, but they remain to be demonstrated. Some sHsps would behave with the actin cytoskeleton as actin-binding proteins capable of either capping a microfilament when present as a nonphosphorylated monomer or stabilizing and protecting the microfilament when organized in small, phosphorylated oligomers.
INTRODUCTION
The cytoskeleton is an active framework of cytosolic filaments composed of 3 types of protein fibers, the microtubules, the intermediate filaments, and the microfilaments. Microfilaments made up essentially of actin are the thinnest and most flexible filaments. Heat shock proteins (Hsps) are synthesized by organisms or cells as a response to heat and any physiological, physical, or chemical stress. Mammalian cells contain 5 major subfamilies of stress proteins, Hsp100, 90, 70, and 60, and a large family of small Hsps (sHsps) having a molecular weight of 15–40 kDa.
When exposed to stress, cells respond by drastic modifications of the different cytoskeletal networks and by a rapid and selective increase in Hsp synthesis. Microtubules undergo disassembly, intermediate filaments collapse toward the perinuclear region, and actin microfilaments are disorganized. Although several Hsps were reported to interact with the different cytoskeletal components, large Hsps, such as Hsp90 and Hsp70, appear to bind mostly to the microtubule network and centrosome, whereas sHsps seem to play an important role in maintaining the integrity of actin and the intermediate filaments (Liang and MacRae 1997).
This review will focus on the relationships known to date between actin, microfilaments, and sHsps and will propose hypotheses on how actin and sHsps may interact and participate in the dynamics and stability of the actin cytoskeleton.
ACTIN CYTOSKELETON
Actin
Actin is an abundant protein present in all eucaryotic cells. There are in fact several actin proteins, for instance, 6 in mammals, whose names reflect their tissue expression and origin: the α-skeletal and α–cardiac muscle actins, the α- and γ–smooth muscle actins, the 2 nonmuscle actins, and the α- and β-cytoplasmic actins. Muscle actins are the main components of thin filaments in sarcomeres of the muscle cells, whereas cytoplasmic actins form microfilaments of the cytoskeleton of muscle and nonmuscle cells (Vandekerckhove and Weber 1978). Microfilaments have an essential role in many cellular processes, such as maintenance of shape, motility, endocytosis and exocytosis, cytokinesis, anchorage to other cells or substrates, and signal transduction.
Actin is remarkably conserved through evolution. The 6 mammalian actins have a very high degree of protein sequence identity from 99.5% to 93.5%, and each isoactin is strictly conserved between humans, rodents, and chicken. Actins from mammals and insects differ by only 3% to 7% (Vandekerckhove and Weber 1978; Mounier et al 1992).
In spite of their high similarity, isoactins are not interchangeable and rather appear to be functionally distinct. Muscle and nonmuscle actins present several structural and biochemical differences (Allen et al 1996; Mounier and Sparrow 1997) and are differentially sorted and utilized by the cellular machinery when expressed in transfected cells (Schevzov et al 1992; von Arx et al 1995; Mounier et al 1997, 1999). A mammalian actin does not fully substitute a Drosophila actin (Brault et al 1999). Most mice lacking the α–cardiac actin gene do not survive (Kumar et al 1997), and α–smooth muscle actin null mice present a highly compromised vascular contractility and blood flow (Schildmeyer et al 2000).
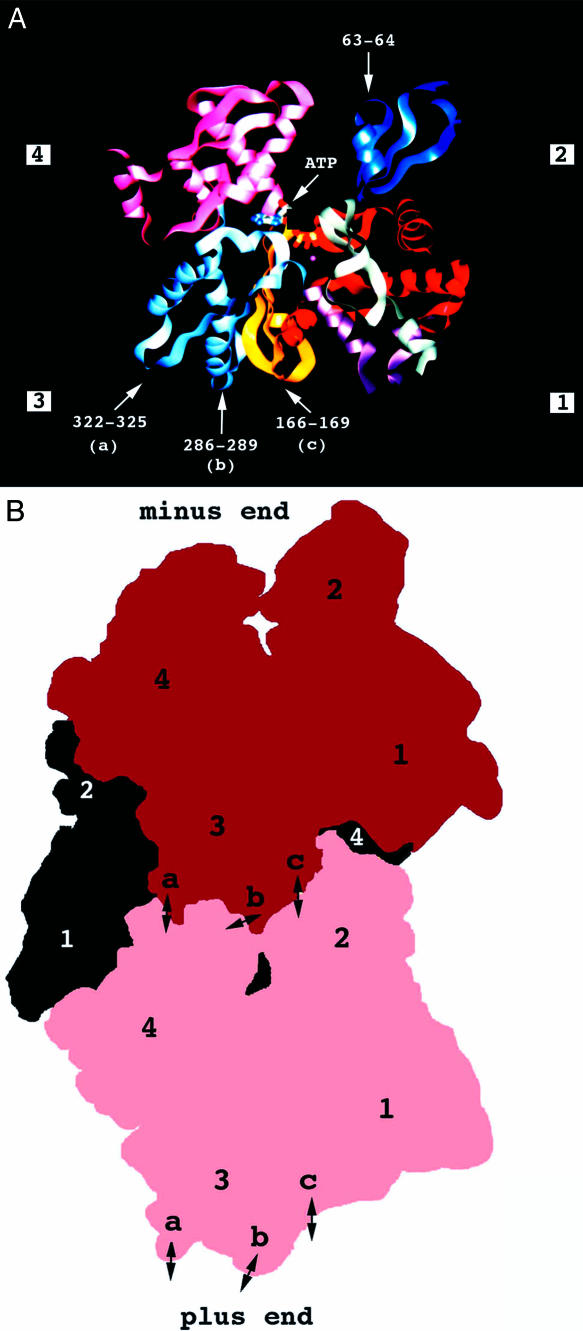
The actin monomer is a 43-kDa polypeptide with 375 amino acid residues. Its atomic structure was determined from the crystals of complexes of the rabbit skeletal actin with different proteins (Kabsch et al 1990; McLaughlin et al 1993; Schutt et al 1993). The actin protein is globular, fits into a cube of 55 × 55 × 35 Å, and consists of 2 domains, each subdivided into 2 subdomains (Fig 1A).
Fig. 1A.
Structure of the actin monomer. The peptide backbone is represented as a large ribbon. The molecule is subdivided into 4 subdomains, and the central cleft contains a nucleotide, adenosine triphosphate or adenosine diphosphate, and a divalent cation, Ca2+ or Mg2+. The different colors indicate parts of the polypeptide chain: residues 1–32 in light green (subdomain 1), 33–69 in blue (subdomain2), 70–144 in red (subdomain 1), 145–180 in yellow (subdomain 3), 181–269 in pink (subdomain 1), 270–337 in light blue (subdomain 3), and 338–372 in purple (subdomain 1). Arrows indicate the position of the loops containing residues involved in the actin-actin contacts mentioned in the text and visible on this side of the molecule (from Kabsch et al 1990 and adapted from Mounier and Sparrow 1997). Fig. 1B. Structure of F-actin showing the relative position of 3 monomers. The actin filament is a double-stranded helix in which subdomains 3 and 4 of each monomer are located near the central axis of the filament, each subdomain making several contacts with the neighboring molecules. The 2 facing monomers belong to 1 helix and the third, in black, to the other helix. Positions of the 4 subdomains are indicated. The plus end or barbed end is the fast-growing end of the filament. The positions of the actin-actin contacts, shown in A, mentioned in the text and visible on this side of the 2 facing monomers, are indicated by double arrows. They correspond to contacts between (a) residues 322–325 (subdomain 3) and 243–245 (subdomain 4), (b) residues 286–289 (subdomain 3) and 202–204 (subdomain 4), and (c) residues 166–169 (subdomain 3) and 40–45 (subdomain 2). Residues 63–64 (subdomain 2) of a monomer are involved in a trimeric contact, implying the residues of the (b) and (c) loops of the adjacent monomer and residues of the subdomain 4 of the monomer located on the opposite strand (not visible in this view) (from Holmes et al 1990 and adapted from Mounier and Sparrow 1997)
Actin filaments and dynamics
Actin is present within cells in a dynamic equilibrium between the globular monomeric form, G-actin, representing the soluble pool of actin, and the polymerized form, F-actin, forming microfilaments. Actin monomers self-polymerize in vitro in the presence of ions, and this process is reversible.
A model of the actin filament organization was proposed by Holmes et al (1990) and refined by Lorenz et al (1993). The filament is a double-stranded helix in which each monomer makes several contacts with the neighboring molecules. The actin filament has a structural and functional polarity, with a minus or pointed end and a plus or barbed or fast-growing end (Fig 1B). Once the polymerization process is initiated by the nucleation of 3–4 subunits, elongation occurs principally at the plus end, which grows 5–10 times faster than the minus end. At the steady state, subunits assemble at the plus end and disassemble at the minus end at an identical rate, maintaining a constant length in spite of the net flow of subunits (reviewed by Steinmetz et al 1997; Chen et al 2000).
A myriad of proteins that bind to G- and/or F-actin regulate actin polymerization and depolymerization as well as the organization of actin filaments in a 3-dimensional network. These actin-binding proteins make possible the large variety of microfilaments observed in the different cell types. They are classified according to their properties to promote or inhibit (or both) in vitro actin assembly. Some facilitate the nucleation or sequester the monomer, others bind to assembled actin filaments and act as capping proteins that block 1 or both ends of the filament, as severing proteins that break the filament into shorter fragments, or as cross-linking proteins that hold together the filaments. Others interact with actin filaments as side-binding and motor proteins. The mode of action of these proteins is complex because some of them were shown to have different and apparently opposite functions according to the intracellular environment (reviewed by McGough 1998; Borisy and Svitkina 2000; Cooper and Schafer 2000).
The Rho family of small guanosine triphosphatases (GTPases) regulates actin dynamics. These small GTPases act as molecular switches controlling signal transduction from membrane receptors to a variety of intracellular responses, particularly actin cytoskeleton assembly. Rho controls the formation of actin stress fibers and focal adhesions, whereas other members of this family, Rac and Cdc42, induce membrane ruffling and filopodia formation, respectively. These small GTPases activate numerous cascades, particularly the p21-activated kinase (PAK) and consequently the p38 mitogen–activated protein (MAP) kinases. Proteins involved in the actin cytoskeletal rearrangements, such as Wiskott-Aldrich syndrome protein (WASP) and Ezrin, radixin and moesin (ERM), can then also be activated (reviewed by Machesky and Hall 1996; Hall 1998; Galan and Zhou 2000; Hall and Nobes 2000). For instance, WASP stimulates the Arp2/3 complex, which greatly increases the nucleation of actin filaments (Borisy and Svitkina 2000; Cooper and Schafer 2000; Pantaloni et al 2001).
Another signaling pathway involves phosphatidyl inositol-4,5-biphosphate (PIP2) and the intracellular concentration of Ca2+. This signaling pathway is not completely independent of the Rho family because the PIP2 production can be regulated by Rac (Welch et al 1997; Hall 1998; Janmey 1998).
SMALL HEAT SHOCK PROTEINS
sHsps are more largely spread in living cells than in actin because they are present in procaryotes and eucaryotes. In mammals, 9 sHsps have been identified to date, with a molecular mass ranging from 15 kDa to 30 kDa: Hsp27 (denoted Hsp25 in mice), αA- and αB-crystallins, Hsp20, HspB2, HspB3, cvHsp or HspB7, Hsp22 or HspB8, and HspB9. In fact, only the expression of Hsp27 and αB-crystallin is induced by heat shock. All except αA-crystallin are abundantly found in the skeletal muscle and the heart, whereas αA- and αB-crystallins are highly expressed in the eye lens. Hsp27, Hsp20, and αB-crystallin are also present in numerous other tissues, such as lens, kidney, bladder, lung, stomach, and skin (de Jong et al 1998; Krief et al 1999; Sugiyama et al 2000; Benndorf et al 2001; Kappe et al 2001).
sHsps are involved in several apparently unrelated cellular processes, such as response to stress from various origins, modulation of the actin cytoskeleton and the intermediate filaments, cell growth, differentiation, apoptosis, tumorigenesis, and signal transduction. It is generally assumed that sHsps exert their pleiotropic effects using at least 3 different pathways characterized by a chaperone-like activity (Jakob et al 1993; Beissinger and Buchner 1998), a control of the redox status (reviewed by Arrigo 1998), and a modulation of the cytoskeleton dynamics as analyzed subsequently.
The protein sequence of sHsps is weakly conserved through evolution when compared with that of actin. For instance, human sHsps that consist of 150–205 amino acids share a homologous sequence of about 80 residues, the α-crystallin domain (Ingolia and Craig 1982). This domain presents a percentage of amino acid identity ranging from 38% to 60% and short consensus sequences that are highly conserved from procaryotes to eucaryotes. Surrounding this central conserved domain, the N- and C-terminal regions have a variable length and are weakly conserved between the sHsp family members (de Jong et al 1998; Sugiyama et al 2000).
It also appears that, as is the case for actins, sHsps are probably not functionally equivalent. For instance, muscle cells appear to have 2 independent chaperone systems, one consisting of HspB2 and HspB3 and the other of Hsp27, Hsp20, and αB-crystallin (Sugiyama et al 2000). αA-crystallin knockout mice develop cataract, whereas αB-crystallin knockout mice develop muscle abnormalities but no cataract (Brady et al 1997; Wawrousek and Brady 1998). Lens cells lacking the αA- or αB-crystallin gene exhibits a decreased or increased proliferative activity, respectively (Andley et al 1998, 2001). Furthermore, αA-crystallin has an antiapoptotic activity weaker than that of αB-crystallin (Andley et al 2000).
Knowledge of the atomic structures of sHsps is less extensive than that of actins. The crystal structure of sHsps was only determined for the 16.5-kDa sHsp from the hyperthermophylic archaeon Methanococcus jannaschii. The protein is rather compact with an overall dimension of 25 × 25 × 75 Å and contains a high number of β-sheets: the core domain of the monomer is composed of 9 β-strands arranged into 2 sheets (Kim et al 1998).
Oligomerization and phosphorylation
Oligomerization and phosphorylation are 2 essential characteristics of sHsps. These proteins can form large cytosolic aggregates ranging from 150 kDa to 1000 kDa and consisting of up to 40 monomers. Oligomerization is a highly dynamic process depending on several signaling pathways and results in homo- or hetero-oligomers of 2 or more sHsps (Bova et al 2000; Sugiyama et al 2000).
The oligomeric organization of sHsps is poorly understood. It was determined only for the M. jannaschii 16.5-kDa Hsp: the oligomer forms a hollow spherical complex of 24 monomers, and the building block is a dimer in which a β-sheet forms an important interaction with another β-sheet from the neighboring molecule (Kim et al 1998). Several models of the αA-crystallin quaternary structure were proposed, as for instance, an open micelle-like arrangement (Farnsworth et al 1998; Smulders et al 1998). The oligomeric arrangement of sHsps is still a matter of discussion in the literature and appears to be variable, shaped by a continuous exchange of subunits (Ehrnsperger et al 1999; Lambert et al 1999; Haley et al 2000).
sHsps can be phosphorylated at several serine residues by different kinases, and this process is reversible. For instance, Hsp27 phosphorylation is achieved by the activation of (at least) the p38 MAP kinase cascade and subsequent activation of the MAP kinase–activated protein (MAPKAP) kinase-2 and -3, which directly phosphorylate sHsps (Welch 1985; Stokoe et al 1992; Arrigo and Landry 1994; Landry and Huot 1995; Guay et al 1997). The p38 MAP kinase is one of the downstream targets of Rac and Cdc42, suggesting a possible connection between actin cytoskeleton dynamics and sHsp phosphorylation (Zhang et al 1995; Guay et al 1997).
Phosphorylation results in a change in the oligomerization status of the several sHsps. It provokes an Hsp27 shift from multimers to small oligomers (Mehlen and Arrigo 1994; Lambert et al 1999; Rogalla et al 1999). A similar dissociation of large oligomers is induced by Hsp20 and αB-crystallin phosphorylation (Brophy et al 1999; Ito et al 2001).
RELATIONSHIPS BETWEEN ACTIN AND sHSPS
Actin polymerization is inhibited in vitro by some sHsps
An inhibitor of actin polymerization (IAP), the 25-kDa IAP, was first identified in vitro in turkey gizzard and was then recognized as Hsp25. It was able to cap the barbed end of the actin filament, thus preventing monomer addition and, subsequently, filament growth. The 25-kDa IAP presents a much more pronounced inhibitory effect when the protein is added to the preformed actin filaments (Miron et al 1988, 1991).
The activity of actin polymerization inhibition depends on the degree of sHsp phosphorylation and the structural organization. Only monomeric and nonphosphorylated murine Hsp25 inhibits actin polymerization with a 1:1 ratio of Hsp25 monomer to actin, whereas phosphorylated monomers and nonphosphorylated multimers have no effect (Benndorf et al 1994).
The Hsp25-mediated inhibitory effect on actin polymerization is lost with disulfide-linked dimers but is restored by reduction (Miron et al 1988). Hsp25 can indeed form dimeric structures based on an intermolecular disulfide bond formed under intracellular oxidizing conditions. The involved cysteine residue is located in a buried position in the region of intermolecular contact sites, and a disulfide bond probably changes the final conformation of the dimer (Zavialov et al 1998).
However, Hsp27 is devoid of nucleating activity, ie, unable to initiate a new polymerization (Miron et al 1991), and is not an actin-severing protein because it has no effect on actin stress fibers when microinjected into cells, independently of its phosphorylation status (Schneider et al 1998).
α-Crystallins also affect actin polymerization in vitro but to a lower extent. They stabilize microfilaments and prevent their depolymerization induced by cytochalasin D and their aggregation induced by heat (Wang and Spector 1996).
Actin cytoskeleton dynamics is regulated by Hsp27
In cultured cells, overexpression of Hsp27 by transfection experiments increases the amount of F-actin at the cell cortex with membrane ruffling, pinocytosis, cell migration, and accumulation of stress fibers. All these processes are related to an increased dynamics of the actin filaments (Lavoie et al 1993a; Landry and Huot 1995; Mairesse et al 1998; Piotrowicz et al 1998).
The survival rate of cells overexpressing Hsp27 after a heat shock or an oxidative stress is enhanced by a stronger stability of the actin microfilaments and their accelerated recovery after disruption (Lavoie et al 1993a, 1993b, 1995; Huot et al 1996). Actin cytoskeleton protection by Hsp27 is also observed when cells are submitted to mitogens, cytochalasin D, carcinogenic, or anticancer drugs (reviewed by Arrigo and Préville 1999; Arrigo 2000).
A diminution of the Hsp27 intracellular level by antisense cDNA transfection experiments leads to growth inhibition and, in some cells, to actin cytoskeleton disorganization (Mairesse et al 1996; Horman et al 1999). When nonphosphorylatable mutants of Hsp27 are transfected into mammalian cells, there is no subsequent increase in the microfilament stability and cell migration (Lavoie et al 1993a, 1993b, 1995; Huot et al 1996; Piotrowicz and Levin 1997; Piotrowicz et al 1998).
Inhibition of actin polymerization in vitro appears to require nonphosphorylated monomers, whereas actin cytoskeleton dynamics and protection seem to require large amounts of phosphorylatable sHsps.
HOW DO ACTIN AND sHSPS INTERACT?
For the inhibition of actin polymerization
The in vitro experiments on the inhibition of actin polymerization by Hsp27 indicate a direct interaction between the 2 proteins. To date no sequence homology between sHsps and actin, suggestive of any putative binding site, has ever been described, except a structural motif shared by both proteins (Rahman et al 1995 and shown in Fig. 2). Interestingly, this motif contains in actin the residues G63 and I64 involved in a direct contact between 3 monomers in the actin filament (Holmes et al 1990). A part of this trimeric contact involves G63, I64, and some other residues of the subdomain 2 of one actin monomer and residues located in subdomain 3 of the adjacent monomer, particularly residues of the b and c loops shown in Fig 1A. The following 4 residues, L65 to K68, form a β-sheet (Kabsch et al 1990). A motif relatively well conserved between actin and sHsps is located at the C-terminal end of the α-crystallin domain and is also predicted to form a β-sheet from residues T162 to A167 (de Jong et al 1998; Kim et al 1998).
Another actin-actin contact occurring between 2 adjacent monomers of the same strand binds the loop containing residues 322–325 of the subdomain 3 (loop a in Fig 1A) and the loop containing residues P243, D244, and G245 of the subdomain 4 of the following monomer (Holmes et al 1990). A P_E_G motif is present in Hsp27, Hsp20, and Hsp22, just upstream of the previous motif (Fig 2).
Fig. 2.
Putative binding sites between actin and sHsps. This figure shows the multiple alignment of 2 regions of actin involved in actin-actin contacts and a region relatively well conserved between the 8 sHsps (Krief et al 1999; Benndorf et al 2001) and their corresponding position in the molecule. The actin residues G63 and I64 located in subdomain 2, and P243, D244, G245 located in subdomain 4, indicated in bold, are involved in actin-actin contacts (Holmes et al 1990). L65 to K68 form a β-sheet (Kabsch et al 1990). In Hsp27, T162 to A167 is also predicted to form a β-sheet (de Jong et al 1998; Kim et al 1998)
The motif P-D/E-G-(X)-L-T/S-(X)3–4-P is relatively well conserved between sHsps (Fig 2). It is thus tempting to speculate that this motif containing residues similar to those involved in 2 known actin-actin contacts could mimic an actin monomer and, therefore, bind to actin at the corresponding actin-actin contacts located in subdomain 3. The P_E_G motif is present in Hsp27 and Hsp20, and both proteins were shown to interact directly with actin (Miron et al 1988; Brophy et al 1999). The homologous region in the crystal structure of the archaebacterium sHsp (Kim et al 1998) forms a loop, which seems to be accessible at the outer surface of the monomer, it being maintained by the β9-sheet, well-conserved between Hsp16.5 and Hsp27.
This interaction between actin and sHsps, such as Hsp27 and Hsp20, would cap the plus end of the actin filament and thus inhibit filament polymerization. Slight conformational changes occurring in phosphorylated sHsps, disulfide-linked dimers, and oligomers would prevent this motif from interacting.
However, a synthetic 15-mer peptide containing this motif was recently shown to have no effect on in vitro actin polymerization, contrary to 2 other 15-mer peptides that present no obvious similarity with any known actin-actin–binding sites (Wieske et al 2001). It remains to demonstrate whether the conformation of these synthetic peptides is comparable with that of the whole protein. Data on the 3-dimensional organization of sHsps will be likely available in the near future and will give insights into the structural organization of the protein and the binding site localization.
Whatever be the location of the binding site, a direct actin-sHsp interaction probably also occurs in vivo and may participate in the regulation of the actin filament assembly. Nearly 30% of the Hsp27 fractionates with plasma membrane components, and the capping activity of Hsp27 on F-actin may be effective in the submembrane regions, where ruffling and lamelipodia formation continuously occur (Piotrowicz and Levin 1997). This capping activity of Hsp27 would protect the plus end and might favor the depolymerization of the minus end, allowing growth of the uncapped filaments. This process would be abolished when the sHsp is phosphorylated and released from the actin microfilament.
For the protection of the actin cytoskeleton
Disorganization of the cytoskeleton and phosphorylation of the sHsps are the earliest events induced by a stress. In some cases, however, for instance, during the response to oxidative stress in mammalian cells, 2 concomitant events occur, leading to a transient formation of larger Hsp27 oligomers and the phosphorylation of Hsp27; the latter event then triggers the subsequent disruption of the aggregates and the accumulation of small oligomers (Mehlen et al 1996; Préville et al 1998; Rogalla et al 1999).
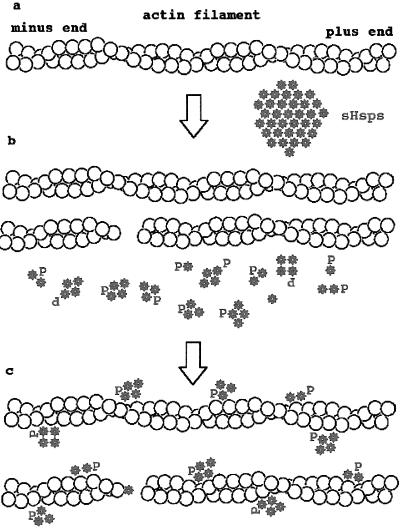
We propose a model for the protection of the actin microfilaments by sHsps once they are released from the large aggregates (Fig 3). sHsps organized in small oligomers, with a variable amount and level of phosphorylated monomers, would interact with actin filaments. These phosphorylated small oligomers, such as possibly the rod-like tetramers described by Rogalla et al (1999), would coat the microfilaments, protect them against further disruption by preventing or neutralizing the action of actin-severing proteins activated by the stress response, and then participate in promoting their subsequent recovery. This model would explain why, when present in large amounts as in cells overexpressing an sHsp, this sHsp would protect and stabilize the actin cytoskeleton by preventing its disruption by stress-induced signals. It is also in agreement with the requirement of the sHsps to be phosphorylatable for an efficient actin cytoskeleton protection and with the sHsp localization in stressed cells bound to cytoskeletal elements in the insoluble fraction.
Fig. 3.
Model of the protection of actin microfilaments by sHsps. (a) In unstressed cells sHsps form large aggregates of nonphosphorylated monomers. (b) The earliest responses to heat shock or other stresses are phosphorylation of sHsps, disruption of sHsp large aggregates after, in some cases, a transient hyperoligomerization, and disorganization of the actin cytoskeleton. (c) Phosphorylated sHsps organized in small oligomers would interact directly or indirectly with F-actin, protect the actin filament against breakage by actin-severing proteins, and promote its subsequent reorganization. Nonphosphorylated monomers may cap the plus end of the actin filament and participate in the regulation of the microfilament assembly
In addition to this protective activity, nonphosphorylated monomeric sHsps might cap the plus end of the actin filament and exert the capping activity as described earlier.
It remains to determine whether this protective activity results from a direct or indirect interaction between F-actin and sHsps. Coimmunoprecipitation and cosedimentation experiments failed until now to demonstrate a direct interaction, except for the turkey Hsp25 and the bovine Hsp20 (Miron et al 1988; Brophy et al 1999), which likely corresponds to the capping activity of these 2 proteins as described previously.
The yeast 2-hybrid system identified to date only a limited number of partners interacting with the sHsps. Hsp27 interacts with other sHsps, αB-crystallin, Hsp20, and Hsp22 (Liu and Welsh 1999; Benndorf et al 2001), and other proteins, Ubc9 (Joanisse et al 1998), PASS1 (Liu et al 2000), and Daxx (Charette et al 2000). αB-crystallin interacts with the subunit C8/α7 of the 20S proteasome (Boelens et al 2001). HspB2 binds and activates the myotonic dystrophy protein kinase (DMPK) (Suzuki et al 1998), and cvHsp interacts with α-filamin, an actin–cross-linking protein that also connects proteins from the cell membrane to the cytoskeleton (Krief et al 1999).
Several hypotheses can explain why such a limited number of proteins related to the cytoskeleton were demonstrated to interact directly with the sHsps. sHsps may interact only with polymerized actin, or sHsps require to be only in small oligomeric forms containing some phosphorylated monomers, or both conditions are needed. Phosphorylation of Ser15 in Hsp27, located upstream of a conserved region predicted to be an α-helix, was proposed to be involved in the interaction with other proteins such as F-actin (de Jong et al 1998; Lambert et al 1999). Interestingly, the F-actin–binding site of gelsolin, a multifunctional actin-binding protein that binds to the actin filament, is an α-helix, the gelsolin fold, common to other actin-binding proteins such as the ADF-cofilin family (McGough 1998; Van Troys et al 1999). When phosphorylated, Ser15 may confer to the adjacent α-helix in Hsp27 a conformation compatible with a lateral F-actin–binding site, allowing a direct contact between the phosphorylated sHsp and the actin filament.
Another possibility could be that the F-actin–sHsp interaction is mediated by a third and still unknown partner that would specifically bind to both the sHsp and the actin microfilament or a microfilament-associated protein. Such an interaction was observed in the smooth muscle. Hsp20 has a domain that is reasonably well conserved between sHsps and troponin I (TnI), a subunit of the troponin complex, which binds to the actin filament in association with tropomyosin and regulates muscle contraction. This Hsp20 domain was shown to bind specifically to the actin filaments only when tropomyosin was present, thus mimicking TnI (Rembold et al 2000).
CONCLUDING REMARKS
Actin and sHsps are multifaceted proteins and exist as 2 reversible forms, monomers and multimers. Actin polymerization and sHsp oligomerization are highly dynamic processes depending on the different signaling pathways, which could have some common steps. sHsps appear to form a family of proteins more heterogeneous in structure and function than the actin family.
Some sHsps would behave with the actin cytoskeleton as actin-binding proteins, with 2 different functions. They would act either as a capping protein when present in a nonphosphorylated and monomeric form or as a stabilizing filament protein when organized in a phosphorylated and small oligomeric form. These functions of the sHsps on the actin cytoskeleton seem to be dissociated from the well-characterized chaperone-like activity, which is rather correlated to a multimeric organization.
Under normal conditions these sHsps would act in addition to or in competition with other actin-binding proteins. In stressed cells a part of them would function as specialized chaperones for the actin cytoskeleton, participating in its protection and recovery, while the remaining would be implied in other cellular rescue processes.
Actin cytoskeleton–sHsp relationships are far from being fully listed and understood. It is likely that each sHsp has specific characteristics and properties and does not behave in a manner similar to other sHsps in various cell contexts, increasing the complexity of the interactions between the actin and the sHsps.
Acknowledgments
This work was supported by grants from the Association de la Recherche contre le Cancer (grant 5204), the Région Rhône-Alpes, and the Association Française contre les Myopathies.
REFERENCES
- Allen PG, Shuster CB, Kas J, Chaponnier C, Janmey PA, Herman IM. Phalloidin binding and rheological differences among actin isoforms. Biochemistry. 1996;35:14062–14069. doi: 10.1021/bi961326g. [DOI] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Bassnett S. The molecular chaperone alphaA-crystallin enhances lens epithelial cell growth and resistance to UVA stress. J Biol Chem. 1998;273:31252–31261. doi: 10.1074/jbc.273.47.31252. [DOI] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Brady JP, Bassnett S, Fleming TP. Lens epithelial cells derived from alphaB-crystallin knockout mice demonstrate hyperproliferation and genomic instability. FASEB J. 2001;15:221–229. doi: 10.1096/fj.00-0296com. [DOI] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of alpha A- and alpha B-crystallin in lens epithelial cells. J Biol Chem. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- Arrigo AP. sHsp as novel regulators of programmed cell death and tumorigenicity. Pathol Biol (Paris) 2000;48:280–288. [PubMed] [Google Scholar]
- Arrigo AP, Landry J 1994 Expression and function of the low–molecular-weight heat shock proteins. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto R, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 335–373. [Google Scholar]
- Arrigo AP, Préville X 1999 Role of hsp27 and related proteins. In: Stress Proteins. Handbook of Experimental Pharmacology Series, ed Latchman DS. Springer Verlag, Berlin, 136: 101–132. [Google Scholar]
- Beissinger M, Buchner J. How chaperones fold proteins. Biol Chem. 1998;379:245–259. [PubMed] [Google Scholar]
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- Benndorf R, Sun X, and Gilmont RR. et al. 2001 HSP22, a new member of the small heat shock protein superfamily, interacts with mimic of phosphorylated HSP27 ((3D)HSP27). J Biol Chem. 276:26753–26761. [DOI] [PubMed] [Google Scholar]
- Boelens WC, Croes Y, de Jong WW. Interaction between aB-crystallin and the human 20S proteasomal subunit C8/α7. Biochim Biophys Acta. 2001;1544:311–319. doi: 10.1016/s0167-4838(00)00243-0. [DOI] [PubMed] [Google Scholar]
- Borisy GG, Svitkina TM. Actin machinery: pushing the envelope. Curr Opin Cell Biol. 2000;12:104–112. doi: 10.1016/s0955-0674(99)00063-0. [DOI] [PubMed] [Google Scholar]
- Bova MP, McHaourab HS, Han Y, Fung BK. Subunit exchange of small heat shock proteins. Analysis of oligomer formation of alphaA-crystallin and Hsp27 by fluorescence resonance energy transfer and site-directed truncations. J Biol Chem. 2000;275:1035–1042. doi: 10.1074/jbc.275.2.1035. [DOI] [PubMed] [Google Scholar]
- Brady JP, Garland D, Duglas-Tabor Y, Robison WG Jr,, Groome A, Wawrousek EF. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci U S A. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Reedy MC, Sauder U, Kammerer RA, Aebi U, Schoenenberger C. Substitution of flight muscle-specific actin by human (beta)-cytoplasmic actin in the indirect flight muscle of Drosophila. J Cell Sci. 1999;112:3627–3639. doi: 10.1242/jcs.112.21.3627. [DOI] [PubMed] [Google Scholar]
- Brophy CM, Lamb S, Graham A. The small heat shock-related protein-20 is an actin-associated protein. J Vasc Surg. 1999;29:326–333. doi: 10.1016/s0741-5214(99)70385-x. [DOI] [PubMed] [Google Scholar]
- Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Bernstein BW, Bamburg JR. Regulating actin-filament dynamics in vivo. Trends Biochem Sci. 2000;25:19–23. doi: 10.1016/s0968-0004(99)01511-x. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Schafer DA. Control of actin assembly and disassembly at filament ends. Curr Opin Cell Biol. 2000;12:97–103. doi: 10.1016/s0955-0674(99)00062-9. [DOI] [PubMed] [Google Scholar]
- de Jong WW, Caspers GJ, Leunissen JA. Genealogy of the alpha-crystallin-small heat-shock protein superfamily. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Lilie H, Gaestel M, Buchner J. The dynamics of Hsp25 quaternary structure. Structure and function of different oligomeric species. J Biol Chem. 1999;274:14867–14874. doi: 10.1074/jbc.274.21.14867. [DOI] [PubMed] [Google Scholar]
- Farnsworth PN, Frauwirth H, Groth-Vasselli B, Singh K. Refinement of 3D structure of bovine lens alpha A-crystallin. Int J Biol Macromol. 1998;22:175–185. doi: 10.1016/s0141-8130(98)00015-4. [DOI] [PubMed] [Google Scholar]
- Galan JE, Zhou D. Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc Natl Acad Sci U S A. 2000;97:8754–8761. doi: 10.1073/pnas.97.16.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase–mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Haley DA, Bova MP, Huang QL, McHaourab HS, Stewart PL. Small heat-shock protein structures reveal a continuum from symmetric to variable assemblies. J Mol Biol. 2000;298:261–272. doi: 10.1006/jmbi.2000.3657. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 2000;355:965–970. doi: 10.1098/rstb.2000.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Horman S, Fokan D, Mosselmans R, Mairesse N, Galand P. Anti-sense inhibition of small-heat-shock-protein (HSP27) expression in MCF-7 mammary-carcinoma cells induces their spontaneous acquisition of a secretory phenotype. Int J Cancer. 1999;82:574–582. doi: 10.1002/(sici)1097-0215(19990812)82:4<574::aid-ijc17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Drosophila gene related to the major heat shock–induced gene is transcribed at normal temperatures and not induced by heat shock. Proc Natl Acad Sci U S A. 1982;79:525–529. doi: 10.1073/pnas.79.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Kamei K, Iwamoto I, Inaguma Y, Nohara D, Kato K. Phosphorylation-induced change of the oligomerization state of alpha B-crystallin. J Biol Chem. 2001;276:5346–5352. doi: 10.1074/jbc.M009004200. [DOI] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- Joanisse DR, Inaguma Y, Tanguay RM. Cloning and developmental expression of a nuclear ubiquitin-conjugating enzyme (DmUbc9) that interacts with small heat shock proteins in Drosophila melanogaster. Biochem Biophys Res Commun. 1998;244:102–109. doi: 10.1006/bbrc.1998.8214. [DOI] [PubMed] [Google Scholar]
- Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC. Atomic structure of the actin:DNase I complex. Nature. 1990;347:37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kappe G, Verschuure P, Philipsen RL, Staalduinen AA, Van de Boogaart P, Boelens WC, de Jong WW. Characterization of two novel human small heat shock proteins: protein kinase–related HspB8 and testis-specific HspB9. Biochim Biophys Acta. 2001;1520:1–6. doi: 10.1016/s0167-4781(01)00237-8. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Krief S, Faivre JF, and Robert P. et al. 1999 Identification and characterization of cvHsp. A novel human small stress protein selectively expressed in cardiovascular and insulin-sensitive tissues. J Biol Chem. 274:36592–36600. [DOI] [PubMed] [Google Scholar]
- Kumar A, Crawford K, and Close L. et al. 1997 Rescue of cardiac alpha-actin–deficient mice by enteric smooth muscle gamma-actin. Proc Natl Acad Sci U S A. 94:4406–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H, Charette SJ, Bernier AF, Guimond A, Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J Biol Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- Landry J, Huot J. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochem Cell Biol. 1995;73:703–707. doi: 10.1139/o95-078. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993a;268:3420–3429. [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993b;268:24210–24214. [PubMed] [Google Scholar]
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, MacRae TH. Molecular chaperones and the cytoskeleton. J Cell Sci. 1997;110:1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- Liu C, Gilmont RR, Benndorf R, Welsh MJ. Identification and characterization of a novel protein from Sertoli cells, PASS1, that associates with mammalian small stress protein hsp27. J Biol Chem. 2000;275:18724–18731. doi: 10.1074/jbc.M001981200. [DOI] [PubMed] [Google Scholar]
- Liu C, Welsh MJ. Identification of a site of Hsp27 binding with Hsp27 and alpha B-crystallin as indicated by the yeast two-hybrid system. Biochem Biophys Res Commun. 1999;255:256–261. doi: 10.1006/bbrc.1999.0174. [DOI] [PubMed] [Google Scholar]
- Lorenz M, Popp D, Holmes KC. Refinement of the F-actin model against X-ray fiber diffraction data by the use of a directed mutation algorithm. J Mol Biol. 1993;234:826–836. doi: 10.1006/jmbi.1993.1628. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Rho: a connection between membrane receptor signalling and the cytoskeleton. Trends Cell Biol. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- Mairesse N, Bernaert D, Del Bino G, Horman S, Mosselmans R, Robaye B, Galand P. Expression of HSP27 results in increased sensitivity to tumor necrosis factor, etoposide, and H2O2 in an oxidative stress-resistant cell line. J Cell Physiol. 1998;177:606–617. doi: 10.1002/(SICI)1097-4652(199812)177:4<606::AID-JCP11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Mairesse N, Horman S, Mosselmans R, Galand P. Antisense inhibition of the 27 kDa heat shock protein production affects growth rate and cytoskeletal organization in MCF-7 cells. Cell Biol Int. 1996;20:205–212. doi: 10.1006/cbir.1996.0025. [DOI] [PubMed] [Google Scholar]
- McGough A. F-actin–binding proteins. Curr Opin Struct Biol. 1998;8:166–176. doi: 10.1016/s0959-440x(98)80034-1. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Gooch JT, Mannherz HG, Weeds AG. Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature. 1993;364:685–692. doi: 10.1038/364685a0. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Arrigo AP. The serum-induced phosphorylation of mammalian hsp27 correlates with changes in its intracellular localization and levels of oligomerization. Eur J Biochem. 1994;221:327–334. doi: 10.1111/j.1432-1033.1994.tb18744.x. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Kretz-Remy C, Préville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron T, Wilchek M, Geiger B. Characterization of an inhibitor of actin polymerization in vinculin-rich fraction of turkey gizzard smooth muscle. Eur J Biochem. 1988;178:543–553. doi: 10.1111/j.1432-1033.1988.tb14481.x. [DOI] [PubMed] [Google Scholar]
- Mounier N, Desmouliere A, Gabbiani G. Subcutaneous tissue fibroblasts transfected with muscle and nonmuscle actins: a good in vitro model to study fibroblastic cell plasticity. Wound Repair Regen. 1999;7:45–52. doi: 10.1046/j.1524-475x.1999.00045.x. [DOI] [PubMed] [Google Scholar]
- Mounier N, Gouy M, Mouchiroud D, Prudhomme JC. Insect muscle actins differ distinctly from invertebrate and vertebrate cytoplasmic actins. J Mol Evol. 1992;34:406–415. doi: 10.1007/BF00162997. [DOI] [PubMed] [Google Scholar]
- Mounier N, Perriard JC, Gabbiani G, Chaponnier C. Transfected muscle and non-muscle actins are differentially sorted by cultured smooth muscle and non-muscle cells. J Cell Sci. 1997;110:839–846. doi: 10.1242/jcs.110.7.839. [DOI] [PubMed] [Google Scholar]
- Mounier N, Sparrow JC. Structural comparisons of muscle and nonmuscle actins give insights into the evolution of their functional differences. J Mol Evol. 1997;44:89–97. doi: 10.1007/pl00006125. [DOI] [PubMed] [Google Scholar]
- Pantaloni D, Le Clainche C, Carlier MF. Mechanism of actin-based motility. Science. 2001;292:1502–1506. doi: 10.1126/science.1059975. [DOI] [PubMed] [Google Scholar]
- Piotrowicz RS, Hickey E, Levin EG. Heat shock protein 27 kDa expression and phosphorylation regulates endothelial cell migration. FASEB J. 1998;12:1481–1490. doi: 10.1096/fasebj.12.14.1481. [DOI] [PubMed] [Google Scholar]
- Piotrowicz RS, Levin EG. Basolateral membrane-associated 27-kDa heat shock protein and microfilament polymerization. J Biol Chem. 1997;272:25920–25927. doi: 10.1074/jbc.272.41.25920. [DOI] [PubMed] [Google Scholar]
- Préville X, Schultz H, Knauf U, Gaestel M, Arrigo AP. Analysis of the role of Hsp25 phosphorylation reveals the importance of the oligomerization state of this small heat shock protein in its protective function against TNFalpha- and hydrogen peroxide–induced cell death. J Cell Biochem. 1998;69:436–452. [PubMed] [Google Scholar]
- Rahman DR, Bentley NJ, and Tuite MF 1995 The Saccharomyces cerevisiae small heat shock protein Hsp26 inhibits actin polymerisation. Biochem Soc Trans. 23:77. S. [DOI] [PubMed] [Google Scholar]
- Rembold CM, Foster DB, Strauss JD, Wingard CJ, Eyk JE. cGMP-mediated phosphorylation of heat shock protein 20 may cause smooth muscle relaxation without myosin light chain dephosphorylation in swine carotid artery. J Physiol. 2000;524:865–878. doi: 10.1111/j.1469-7793.2000.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, and Préville X. et al. 1999 Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 274:18947–18956. [DOI] [PubMed] [Google Scholar]
- Schevzov G, Lloyd C, Gunning P. High level expression of transfected beta- and gamma-actin genes differentially impacts on myoblast cytoarchitecture. J Cell Biol. 1992;117:775–785. doi: 10.1083/jcb.117.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, Bradley A, Schwartz RJ. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle alpha-actin null mouse. FASEB J. 2000;14:2213–2220. doi: 10.1096/fj.99-0927com. [DOI] [PubMed] [Google Scholar]
- Schneider GB, Hamano H, Cooper LF. In vivo evaluation of hsp27 as an inhibitor of actin polymerization: hsp27 limits actin stress fiber and focal adhesion formation after heat shock. J Cell Physiol. 1998;177:575–584. doi: 10.1002/(SICI)1097-4652(199812)177:4<575::AID-JCP8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Schutt CE, Myslik JC, Rozycki MD, Goonesekere NC, Lindberg U. The structure of crystalline profilin-beta-actin. Nature. 1993;365:810–816. doi: 10.1038/365810a0. [DOI] [PubMed] [Google Scholar]
- Smulders RH, van Boekel MA, de Jong WW. Mutations and modifications support a ‘pitted-flexiball’ model for alpha-crystallin. Int J Biol Macromol. 1998;22:187–196. doi: 10.1016/s0141-8130(98)00016-6. [DOI] [PubMed] [Google Scholar]
- Steinmetz MO, Stoffler D, Hoenger A, Bremer A, Aebi U. Actin: from cell biology to atomic detail. J Struct Biol. 1997;119:295–320. doi: 10.1006/jsbi.1997.3873. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Suzuki A, and Kishikawa M. et al. 2000 Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J Biol Chem. 275:1095–1104. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Sugiyama Y, and Hayashi Y. et al. 1998 MKBP, a novel member of the small heat shock protein family, binds and activates the myotonic dystrophy protein kinase. J Cell Biol. 140:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Vandekerckhove J, Ampe C. Structural modules in actin-binding proteins: towards a new classification. Biochim Biophys Acta. 1999;1448:323–348. doi: 10.1016/s0167-4889(98)00152-9. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J, Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978;126:783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- von Arx P, Bantle S, Soldati T, Perriard JC. Dominant negative effect of cytoplasmic actin isoproteins on cardiomyocyte cytoarchitecture and function. J Cell Biol. 1995;131:1759–1773. doi: 10.1083/jcb.131.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Spector A. Alpha-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur J Biochem. 1996;242:56–66. doi: 10.1111/j.1432-1033.1996.0056r.x. [DOI] [PubMed] [Google Scholar]
- Wawrousek EF, Brady JP 1998 AlphaB-crystallin gene knockout mice develop a severe fatal phenotype late in life. Invest Ophthalmol Vis Sci. 39 S. 523. [Google Scholar]
- Welch WJ. Phorbol ester, calcium ionophore, or serum added to quiescent rat embryo fibroblast cells all result in the elevated phosphorylation of two 28,000-dalton mammalian stress proteins. J Biol Chem. 1985;260:3058–3062. [PubMed] [Google Scholar]
- Welch MD, Mallavarapu A, Rosenblatt J, Mitchison TJ. Actin dynamics in vivo. Curr Opin Cell Biol. 1997;9:54–61. doi: 10.1016/s0955-0674(97)80152-4. [DOI] [PubMed] [Google Scholar]
- Wieske M, Benndorf R, Behlke J, Dolling R, Grelle G, Bielka H, Lutsch G. Defined sequence segments of the small heat shock proteins HSP25 and alphaB-crystallin inhibit actin polymerization. J Biochem Eur. 2001;268:2083–2090. doi: 10.1046/j.1432-1327.2001.02082.x. [DOI] [PubMed] [Google Scholar]
- Zavialov A, Benndorf R, Ehrnsperger M, Zav'yalov V, Dudich I, Buchner J, Gaestel M. The effect of the intersubunit disulfide bond on the structural and functional properties of the small heat shock protein Hsp25. Int J Biol Macromol. 1998;22:163–173. doi: 10.1016/s0141-8130(98)00014-2. [DOI] [PubMed] [Google Scholar]
- Zhang S, Han J, Sells MA, Chernoff J, Knaus UG, Ulevitch RJ, Bokoch GM. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]