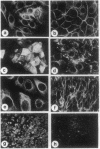
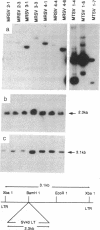
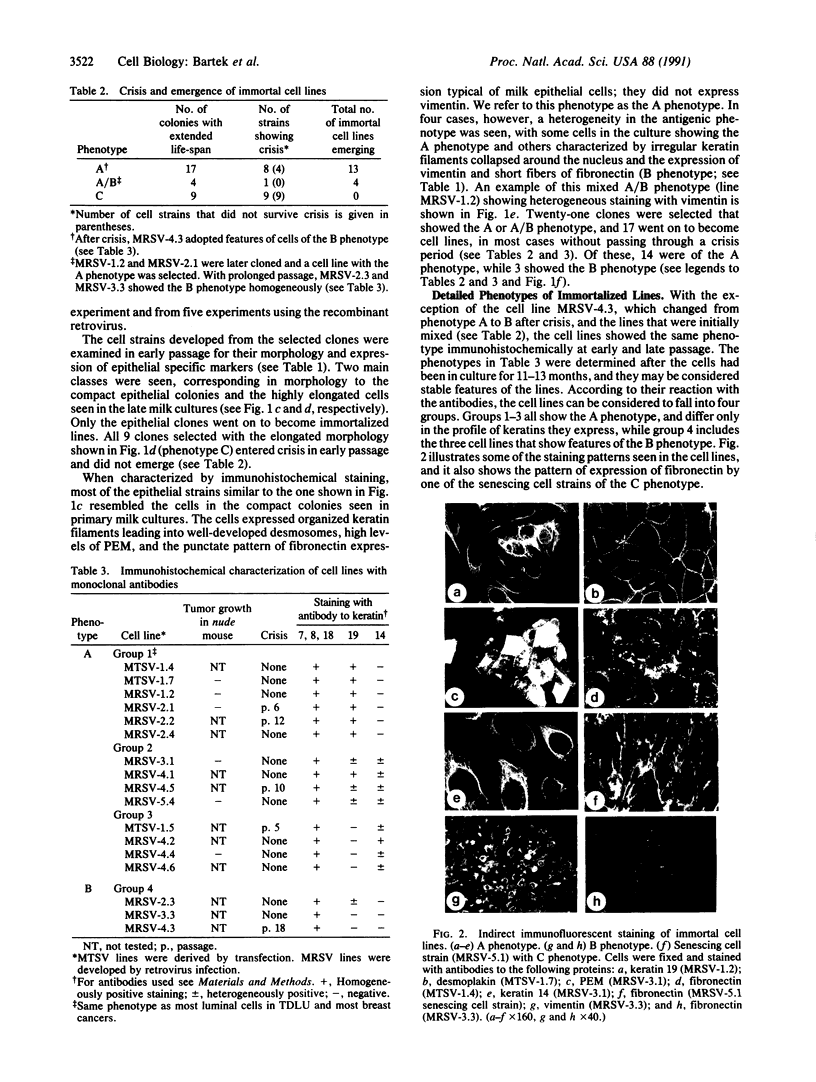
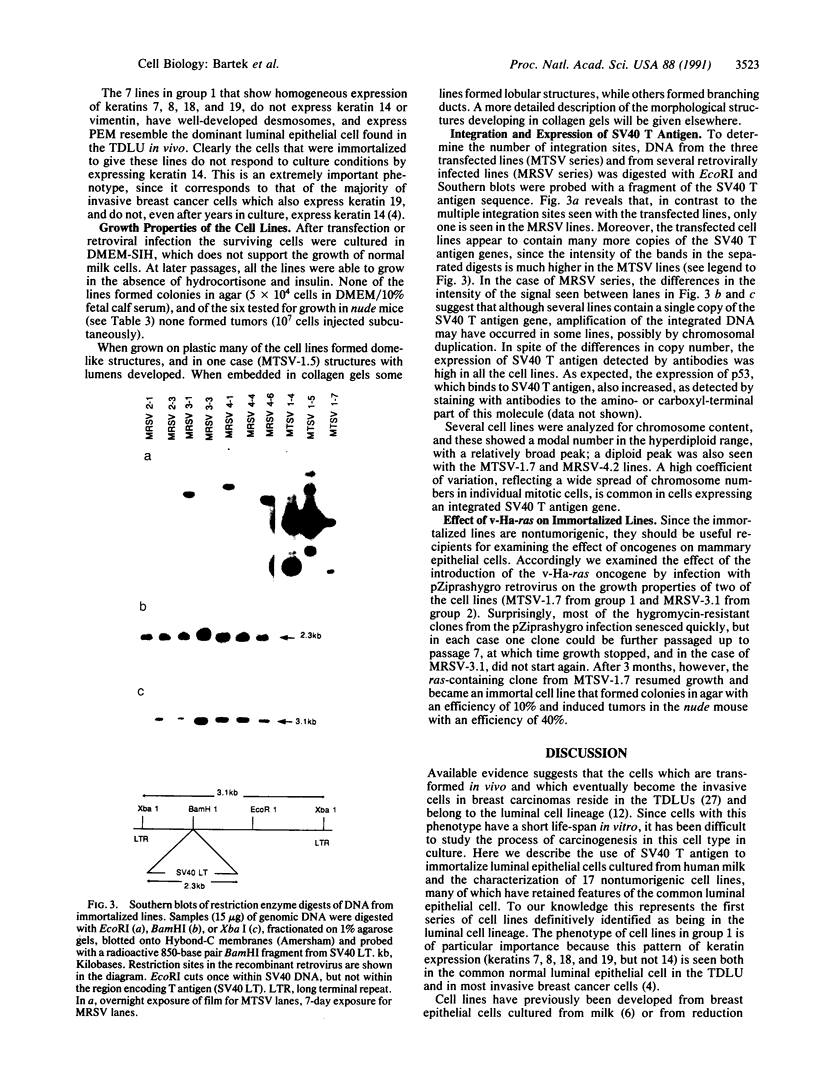
Abstract
When defined in terms of markers for normal cell lineages, most invasive breast cancer cells correspond to the phenotype of the common luminal epithelial cell found in the terminal ductal lobular units. Luminal epithelial cells cultured from milk, which have limited proliferative potential, have now been immortalized by introducing the gene encoding simian virus 40 large tumor (T) antigen. Infection with a recombinant retrovirus proved to be 50-100 times more efficient than calcium phosphate transfection, and of the 17 cell lines isolated, only 5 passed through a crisis period as characterized by cessation of growth. When characterized by immunohistochemical staining with monoclonal antibodies, 14 lines showed features of luminal epithelial cells and of these, 7 resembled the common luminal epithelial cell type in the profile of keratins expressed. These cells express keratins 7, 8, 18, and 19 homogeneously and do not express keratin 14 or vimentin; a polymorphic epithelial mucin produced in vivo by luminal cells is expressed heterogeneously and the pattern of fibronectin staining is punctate. Although the cell lines have a reduced requirement for added growth factors, they do not grow in agar or produce tumors in the nude mouse. When the v-Ha-ras oncogene was introduced into two of the cell lines by using a recombinant retrovirus, most of the selected clones senesced, but one entered crisis and emerged after 3 months as a tumorigenic cell line.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartek J., Bartkova J., Taylor-Papadimitriou J. Keratin 19 expression in the adult and developing human mammary gland. Histochem J. 1990 Oct;22(10):537–544. doi: 10.1007/BF01005976. [DOI] [PubMed] [Google Scholar]
- Bartek J., Durban E. M., Hallowes R. C., Taylor-Papadimitriou J. A subclass of luminal epithelial cells in the human mammary gland, defined by antibodies to cytokeratins. J Cell Sci. 1985 Apr;75:17–33. doi: 10.1242/jcs.75.1.17. [DOI] [PubMed] [Google Scholar]
- Buehring G. C. Culture of human mammary epithelial cells: keeping abreast with a new method. J Natl Cancer Inst. 1972 Nov;49(5):1433–1434. [PubMed] [Google Scholar]
- Burchell J., Durbin H., Taylor-Papadimitriou J. Complexity of expression of antigenic determinants, recognized by monoclonal antibodies HMFG-1 and HMFG-2, in normal and malignant human mammary epithelial cells. J Immunol. 1983 Jul;131(1):508–513. [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chang S. E., Keen J., Lane E. B., Taylor-Papadimitriou J. Establishment and characterization of SV40-transformed human breast epithelial cell lines. Cancer Res. 1982 May;42(5):2040–2053. [PubMed] [Google Scholar]
- Cowin P., Kapprell H. P., Franke W. W. The complement of desmosomal plaque proteins in different cell types. J Cell Biol. 1985 Oct;101(4):1442–1454. doi: 10.1083/jcb.101.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Parada L. F., Weinberg R. A. Specific growth response of ras-transformed embryo fibroblasts to tumour promoters. Nature. 1985 Dec 5;318(6045):472–475. doi: 10.1038/318472a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa T., Ruley H. E. Rescue of cells from ras oncogene-induced growth arrest by a second, complementing, oncogene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1519–1523. doi: 10.1073/pnas.85.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P. S., Cepko C. L., Mulligan R. C., Sharp P. A. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986 Apr;6(4):1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen J., Chang S. E., Taylor-Papadimitriou J. Monoclonal antibodies that distinguish between human cellular and plasma fibronectin. Mol Biol Med. 1984 Feb;2(1):15–27. [PubMed] [Google Scholar]
- Lauerová L., Kovarik J., Bártek J., Rejthar A., Vojtesek B. Novel monoclonal antibodies defining epitope of human cytokeratin 18 molecule. Hybridoma. 1988 Oct;7(5):495–504. doi: 10.1089/hyb.1988.7.495. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McKay I. A., Taylor-Papadimitriou J. Junctional communication pattern of cells cultured from human milk. Exp Cell Res. 1981 Aug;134(2):465–470. doi: 10.1016/0014-4827(81)90447-x. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Schaart G., Moesker O., Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987 May;170(1):235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Noble M., Land H. Ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. EMBO J. 1988 Jun;7(6):1635–1645. doi: 10.1002/j.1460-2075.1988.tb02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Miller J. S., Kimelman D., Cepko C. L., Lemischka I. R., Mulligan R. C. Individual adenovirus type 5 early region 1A gene products elicit distinct alterations of cellular morphology and gene expression. J Virol. 1985 Nov;56(2):404–413. doi: 10.1128/jvi.56.2.404-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlitz C. F., Scott G. K., Dodson J. M., Liu E., Dollbaum C., Smith H. S., Benz C. C. Incidence of activating ras oncogene mutations associated with primary and metastatic human breast cancer. Cancer Res. 1989 Jan 15;49(2):357–360. [PubMed] [Google Scholar]
- Stampfer M. R., Bartley J. C. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M., Perryman M., Eeles R. Clonal analysis of morphological phenotype in cultured mammary epithelial cells from human milk. Proc R Soc Lond B Biol Sci. 1982 May 22;215(1199):231–240. doi: 10.1098/rspb.1982.0039. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Burchell J., Hurst J. Production of fibronectin by normal and malignant human mammary epithelial cells. Cancer Res. 1981 Jun;41(6):2491–2500. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Purkis P., Fentiman I. S. Cholera toxin and analogues of cyclic AMP stimulate the growth of cultured human mammary epithelial cells. J Cell Physiol. 1980 Mar;102(3):317–321. doi: 10.1002/jcp.1041020306. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Shearer M., Tilly R. Some properties of cells cultured from early-lactation human milk. J Natl Cancer Inst. 1977 Jun;58(6):1563–1571. doi: 10.1093/jnci/58.6.1563. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Stampfer M., Bartek J., Lewis A., Boshell M., Lane E. B., Leigh I. M. Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J Cell Sci. 1989 Nov;94(Pt 3):403–413. doi: 10.1242/jcs.94.3.403. [DOI] [PubMed] [Google Scholar]
- Thompson T. C., Southgate J., Kitchener G., Land H. Multistage carcinogenesis induced by ras and myc oncogenes in a reconstituted organ. Cell. 1989 Mar 24;56(6):917–930. doi: 10.1016/0092-8674(89)90625-9. [DOI] [PubMed] [Google Scholar]
- Van Muijen G. N., Warnaar S. O., Ponec M. Differentiation-related changes of cytokeratin expression in cultured keratinocytes and in fetal, newborn, and adult epidermis. Exp Cell Res. 1987 Aug;171(2):331–345. doi: 10.1016/0014-4827(87)90166-2. [DOI] [PubMed] [Google Scholar]
- Wellings S. R., Jensen H. M., Marcum R. G. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975 Aug;55(2):231–273. [PubMed] [Google Scholar]