Abstract
Technological advances often dictate progress in cancer research. Melanoma research has been considerably influenced by implementation of novel techniques and has contributed to our understanding of the mechanism of tumor progression. The three-dimensional (3D) human skin reconstruct is an ideal model to dissect each step of melanoma development and progression. Reconstructed human skin consists of fibroblast-contracted collagen gels as a dermal compartment and a stratified epidermal compartment. The epidermis comprises keratinocytes and normal melanocytes or melanoma cells from different stages. Normal melanocytes in skin reconstructs remain singly distributed at the basement membrane within the basal layer of keratinocytes. The radial growth phase (RGP) melanoma cells grow as cell clusters in the epidermis. The vertical growth phase (VGP) melanoma cells invade the dermis of reconstructs. Metastatic melanoma cells aggressively invade deep into the dermis. Grafting melanoma skin reconstructs onto mice induces local tumor formation and metastatic foci in distant organs such as lungs. The growth patterns and the range of metastases reflect proliferation and metastatic capacity of the original tumors. Skin reconstruct as xenografts enable us to observe to which organs melanoma cells spread. In this chapter, we describe the usefulness of the model in studying not only melanocyte physiology but also pathophysiological conditions such as melanocyte transformation and melanoma progression. A better understanding of these processes will benefit the entire melanoma field.
Keywords: Melanocytes, Radial growth phase melanoma, Vertical growth phase melanoma, Metastatic melanoma, 3D skin reconstruct, Grafting, Mouse model
1 Introduction
Melanoma is the cancer that shows most rapidly increasing incidence [1, 2]. Aggressive local growth and metastatic ability are common features of malignant melanoma, which accounts for 75 % of all deaths associated with skin cancer [1]. Despite that targeted therapies such as BRAF inhibitors and immunotherapy such as checkpoint inhibitors show promise in subsets of melanoma patients, most advanced melanomas remain refractory to those therapies. The steps of melanoma progression are defined by levels of invasion: the radial growth phase (RGP) melanoma; the vertical growth phase (VGP) melanoma and metastatic melanoma [3]. Our understanding of molecular pathogenesis that underlies melanoma progression remains incomplete, and this needs to be improved for the development of new therapeutic concepts. One of the solutions to this challenge is to establish an experimental model that recapitulates the disease development in a natural human tissue microenvironment. Accumulating evidence shows that cells grown within a three-dimensional (3D) extracellular matrix in contact with other cells behave differently from cells in 2D monolayer cultures [4–9].
Normal human melanocyte homeostasis studies allow us to better understand melanoma biology. In the state of homeostasis, keratinocytes control growth and behavior of melanocytes. Pathological changes, which lead to development of malignant melanoma, interrupt this delicate homeostatic balance between the two cell types and can alter expression of cell–cell adhesion and secreted signal molecules [10]. The escape of melanocytes from the control by keratinocytes may be a hallmark of melanocyte transformation, and the constitutive production of various growth factors and cytokines appears to be a major characteristic of melanoma cells [11]. Fibroblasts are the main cells in the dermis and the major source of extracellular matrix proteins. Among the fibroblast-producing matrix proteins, collagen is a major component of skin connective tissue and it comprises 70 % of the proteins in the dermis. Type I collagen is found in all human dermal layers [12], and its synthesis by fibroblasts can be modulated by a variety of growth factors and cytokines that are produced by melanoma and/or stroma cells [13].
It is well known that the tissue microenvironment plays a major role in sustaining cellular homeostasis and can drive tumor initiation and tumor progression. Based on this knowledge we have established an in vitro 3D skin reconstruct culture system and a technique of grafting skin reconstructs onto mice. Skin reconstructs consist of a dermal compartment containing fibroblasts embedded in a collagen I matrix, and an epidermal compartment composed of human keratinocytes and melanocytic cells. Skin reconstructs are cultured for 18–21 days in vitro and then grafted to mice. In the skin reconstructs, normal human melanocytes are located at the junction between epidermis and dermis. RGP melanoma cells proliferate in reconstructs but still stay at the epidermal–dermal interface. VGP melanoma cells grow as cell clusters and invade the dermis of reconstructs. Metastatic melanoma cells proliferate rapidly, and aggressively invade deep into the dermis. After grafting the melanoma cell-containing skin reconstructs to the backs of mice, tumor formation can be observed within 4–8 weeks. Metastatic melanoma cells can be detected in mouse lung tissue 8 weeks after grafting. The growth patterns of melanoma cells in skin reconstruct xenografted to mice mimic the development and progression of the original tumors. This model provides the biological basis for melanoma research and therapy. Manipulation of gene expression is feasible in this model through overexpressing or knocking down genes in any cell types using adenoviral, retroviral or lentiviral vectors. BRAF inhibitors used to treat BRAF-mutant melanoma cells also showed promising results in this model [14, 15]; however, intrinsic and acquired resistance to BRAF inhibitors is increasingly well recognized. Although many questions still need to be answered, this model will help future research to find effective treatments for melanoma.
2 Materials
0.25 % trypsin–EDTA.
Minimal essential medium with Eagle salts (10× EMEM).
Fetal bovine serum (FBS).
Sodium bicarbonate.
l-glutamine.
Bovine tendon acid-extracted collagen I.
10 % buffered formalin.
Forceps.
Scissors.
Iris scissors.
Surgical blades.
CO2 incubator (CO2 at 5 % (vol/vol); humidified, T = 37 °C).
Tissue culture 6-well trays with inserts.
Compound benzoin tincture.
Alcohol prep swabs.
Gauze sponges (sterile).
Silk, black braided 5-0.
EZ anesthesia.
Meloxicam.
Keratinocyte serum free medium.
Bovine pituitary extract (BPE).
EGF human recombinant.
Medium 254CF.
Human melanocyte growth supplement-2, PMA-free.
Trypsin inhibitor, soybean.
Dialyzed fetal calf serum.
Recombinant human stem cell factor.
Basic FGF.
Endothelin-3, human.
MCDB153.
Leibovitz’s L15.
Insulin.
Calcium chloride.
DMEM.
F12 (HAM’s).
Hydrocortisone.
Insulin, transferrin, ethanolamine, selenium (ITES, 500×).
O-phosphorylethanolamine.
Adenine.
Progesterone.
Triiodothyronine.
Newborn calf serum.
Mouse: female hair or hairless immunodeficient mouse (e.g., SCID hairless outbred mouse, Charles River laboratories) around 6 weeks of age.
3 Methods
3.1 Human Skin Reconstruct Culture In Vitro: Dermal Layer
Preparation of acellular layer (6 ml): Prepare a sterile 50 ml falcon tube on ice, add the following reagents in the tube: 0.59 ml 10× EMEM, 50 µl 200 mM l-glutamine, 0.6 ml FBS, 120 µl 7.5 % sodium bicarbonate, and 4.6 ml bovine collagen I. Mix well and add 1 ml of the mixture into each insert of a tissue culture tray (see Note 1). Incubate for 30 min at 37 °C in a 5 % CO2 tissue culture incubator and allow the gel to solidify. Color of the mixture should be from straw-yellow to pink.
Preparation of human fibroblasts: Foreskin fibroblasts are grown in DMEM with 10 % FBS. Remove spent medium, wash cells with HBSS, and detach fibroblasts from the culture vessel with 0.25 % trypsin–EDTA (for 5 min). Add DMEM containing 10 % FBS to neutralize. Collect cells by centrifugation and resuspend 0.45 × 106 cells in 1.5 ml DMEM with 10 % FBS.
Preparation of cellular layer (18 ml): Set a sterile 50 ml falcon tube on ice, add the following reagents in the tube: 1.65 ml 10× EMEM, 150 µl 200 mM l-glutamine, 1.85ml FBS, 350 µl 7.5% Sodium bicarbonate, 14 ml bovine collagen I, and 1.5 ml fibroblast suspension. Mix well and add 3 ml of the mixture into each insert of a tissue culture tray on top of a solidified acellular layer (see Note 2). Incubate for 45 min at 37 °C in a 5 % CO2 tissue culture incubator and allow the gel to solidify. The color of the mixture should be from straw-yellow to pink. Add 2 ml fibroblasts growth medium to the inside of each insert (on top of the gel) and 10 ml to the outside of each insert. Incubate for 4–7 days and make sure that the gel contracts.
3.2 Preparation of Skin Reconstruct Medium
Melanocyte skin reconstruct medium (500 ml): Basic medium including 490 ml keratinocyte serum-free medium, 10 ml dialyzed fetal bovine serum, 60 µg/ml bovine pituitary extract, 10 ng/ml stem cell factor, 100 nM Endothelin-3, 1.1 ng/ml basic FGF. Medium I (for day 1–2): add 1 ng/ml EGF to basic medium. Medium II (for day 3–4): add 0.2 ng/ml EGF to basic medium. Medium III (for day 5–14): add 2.4 mM calcium chloride to basic medium.
Melanoma skin reconstruct medium: Medium I (for day 1–2, 100 ml): 75 ml DMEM, 25 ml F12, 4 mM l-glutamine, 1.48 µM hydrocortisone, 200 µl ITES of 500×, 100 µM O-phosphorylethanolamine, 180 µM adenine, 4 pM progesterone, 20 pM triiodothyronine, 2.4mMcalcium chloride, and 100 µl of chelexed newborn calf serum. Medium II (for day 3–4, 100 ml): 75 ml DMEM, 25 ml F12, 4 mM l-glutamine, 1.48 µM hydrocortisone, 200 µl ITES of 500×, 100 µM O-phosphorylethanolamine, 180 µM adenine, 4 pM progesterone, 20 pM triiodothyronine, 2.4 mM calcium chloride, and 100 µl of newborn calf serum. Medium III (for day 5–14, 300 ml): 142.5 ml DMEM, 142.5 ml F12, 4 mM l-glutamine, 1.48 µM hydrocortisone, 200 µl ITES of 500×, 100 µM O-phosphorylethanolamine, 180 µM adenine, 20 pM triiodothyronine, 2.4 mM calcium chloride, and 6 ml of newborn calf serum.
3.3 Preparation of Human Keratinocytes, Melanocytes and Melanoma Cells
Human foreskin keratinocytes are grown in keratinocyte serum-free medium with BPE and EGF (see Note 3). Remove spent medium, wash cells with HBSS, and detach keratinocytes with 0.05 % trypsin–EDTA (for 5 min at 37 °C), neutralize with a soybean trypsin inhibitor, resuspend cells in reconstruct medium I at 4.17 × 106 cells/ml.
Human melanocytes are grown in 254CF medium with melanocyte growth supplement-2. Remove spent medium, wash cells with HBSS, detach melanocytes with 0.05 % trypsin–EDTA (for 1 min at room temperature), neutralize with a soybean trypsin inhibitor, resuspend cells in reconstruct medium I at 0.83 × 106 cells/ml.
Human melanoma cells are grown in Tu 2 % medium (MCDB153 with 20 % Leibovitz’s L15, 2 % FBS, 5 µg/ml insulin and 1.68 mM calcium chloride). Remove spent medium, wash cells with HBSS, and detach cells with 0.25 % trypsin–EDTA (for 1 min at room temperature), neutralize with a soybean trypsin inhibitor, resuspend cells in melanoma reconstruct medium I at 0.83 × 106 cells/ml.
3.4 Preparation of Epidermal Layer
Wash the mature dermal reconstruct with HBSS containing 1 % dialyzed FBS (2 ml to the inside and 10 ml to the outside of each insert), incubate for 60 min at 37 °C.
Melanocyte skin reconstruct: Aspirate off washing medium and add melanocyte reconstruct medium I (1.5 ml to the inside and 10 ml to the outside of each insert). Mix 100 µl melanocyte suspension with 100 µl keratinocyte suspension and drop on top of each dermal reconstruct (500,000 cells total/reconstruct). Incubate for 2 days at 37 °C.
Melanoma skin reconstruct: Aspirate off washing medium and add melanoma reconstruct medium I (1.5 ml to the inside and 10 ml to the outside of each insert). Mix 100 µl melanoma cell suspension with 100 µl keratinocyte suspension and drop on top of each dermal reconstruct (500,000 cells total/reconstruct). Incubate for 2 days at 37 °C.
On day 3, aspirate spent medium and add melanocyte reconstruct medium II and melanoma reconstruct medium II for melanocyte reconstructs and melanoma reconstructs, respectively (2 ml to the inside and 10 ml to the outside of each insert).
On day 5, aspirate spent medium and add 7.5 ml medium III to only the outside of each insert, so that the surface of the skin reconstruct is lifted to the air-liquid interface. Change medium III every other day until day 14 (see Note 4).
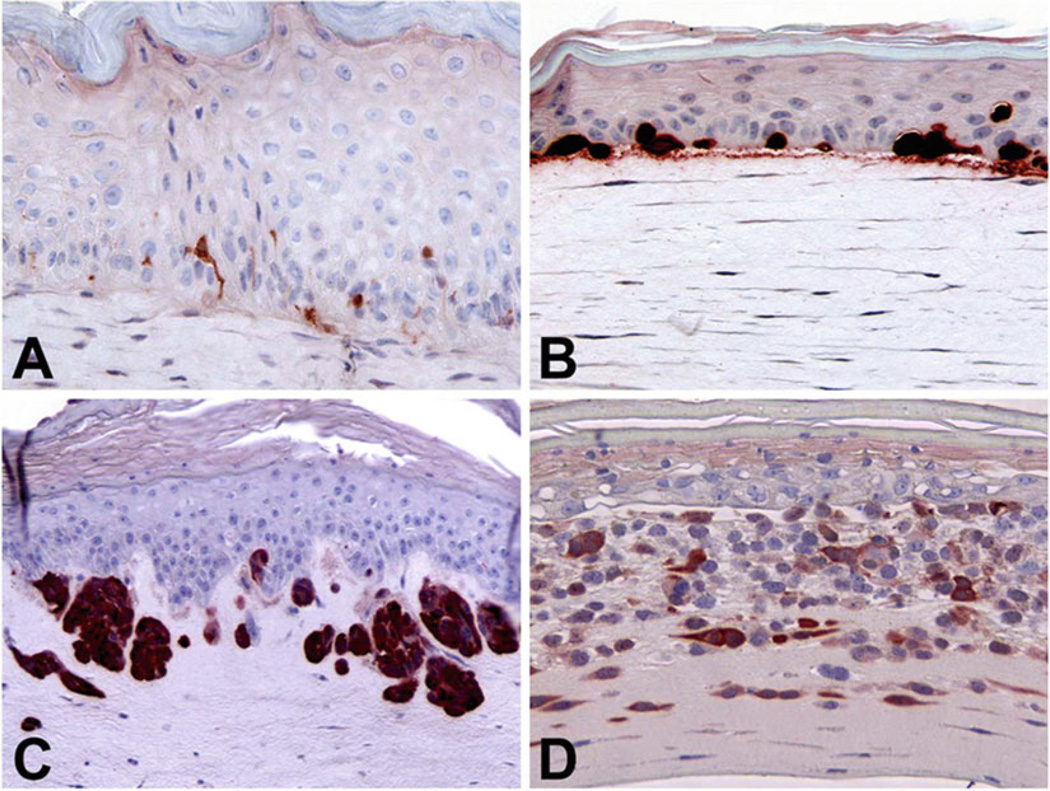
Representative results of in vitro skin reconstructs: When cultured in 3D skin reconstructs, normal melanocytes and melanoma cells from different stages display the steps of melanoma development and progression by level of invasion (Fig. 1).
Fig. 1.
Melanoma development and progression in 3D skin reconstructs. Normal human foreskin melanocytes distribute to the basement membrane among basal keratinocytes (a). WM35 RGP melanoma cells grow with forming clusters but still stay in the epidermis (b). WM3248 VGP melanoma cells break the basement membrane, grow inside the dermis, and form nests (c). The metastatic melanoma cell line 1205Lu invades deeply into the dermis (d). Skin reconstructs were fixed with 10 % formalin, embedded in paraffin, sectioned, and stained with anti-S100 antibodies
3.5 Grafting Skin Reconstructs in Mice
Prepare 5 ml of DMEM in a 50 ml tube (for freezing and defrosting mouse skin).
Use a female mouse around 6 weeks of age.
Set up a beaker with 600 ml water on top of a hot plate to keep water temperature between 40 and 60 °C.
Anesthetizing mouse with isoflurane (EZ anesthesia system): The anesthesia is performed by using the induction chamber with 4 % isoflurane and maintained through a nose cone breather during procedure with 1.5–2 % isoflurane.
Mouse skin preparation: Remove hair by using clippers if a mouse with hair is used. Prepare an area approximately twice the size of the surgical area. Clean mouse skin with compound benzoin tincture first then use alcohol prep swabs.
Move mouse with a nose cone breather on the surgical area covered with a sterile paper pad.
A heat pad is used under each mouse as heat supports to prevents hypothermia.
Wound bed preparation: On the mouse back, cut a 1.2 cm diameter round wound bed by using Iris scissors and forceps. Cover the wound bed with a sterile gauze sponge.
Transfer the round mouse skin into 5 ml of DMEM tube, leave the tube in a liquid nitrogen container until it is totally frozen. Move the frozen tube in a beaker containing hot water until totally thawed (see Note 5). Repeat this step three times.
Prepare grafting skin reconstruct: Detach the skin reconstruct from the tray insert using a surgical blade, leave the skin reconstruct on top of a sterile dish (epidermis side up), and carefully remove the plastic membrane under the skin reconstruct.
Transfer the skin reconstruct (epidermis side up) to the wound bed of the mouse back, lift mouse skin around wound bed and let the skin reconstruct edge embed under the mouse skin.
Stretch the defrosted mouse skin and place on top of the skin reconstruct as a biological cover.
Suturing the wound: make the first suture on top of the wound area, and the second on the bottom, then on the sides. Twelve stitches are needed at least for the whole wound area (see Note 6).
Post-Operative management: Clean the mouse skin after surgery and remove the nosecone. The mouse should stay on the heat pad until recovered. Monitor closely the recovery from anesthesia and be prepared to provide respiratory support.
Transfer the mouse into a new cage.
Pain management: Subcutaneously inject 1 mg/kg Meloxicam immediately after grafting, 24 h and 48 h after surgery.
Monitor the mouse every day for the first week after surgery.
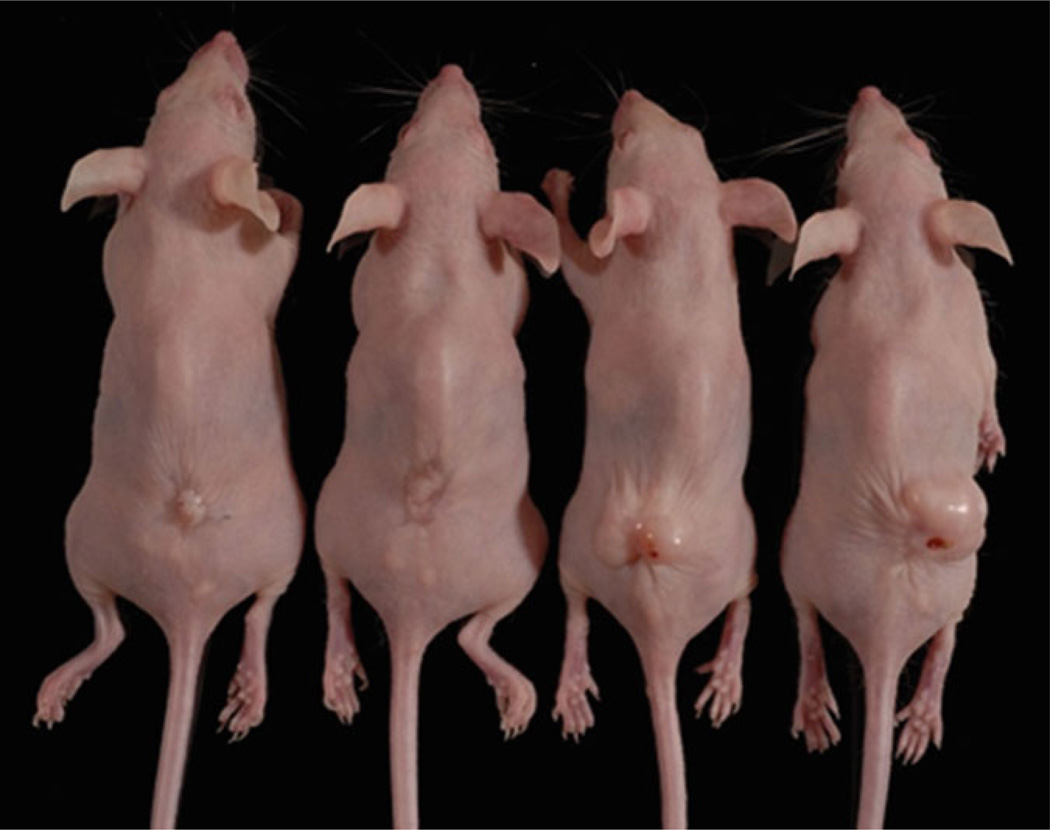
Local tumor formation is observed on the mouse back in 8 weeks after grafting. The RGP melanoma cell line WM35 and the VGP melanoma cell line WM793 form smaller tumors, whereas the two mice for metastatic melanoma cell line 1205Lu skin reconstruct forms larger tumors (Fig. 2).
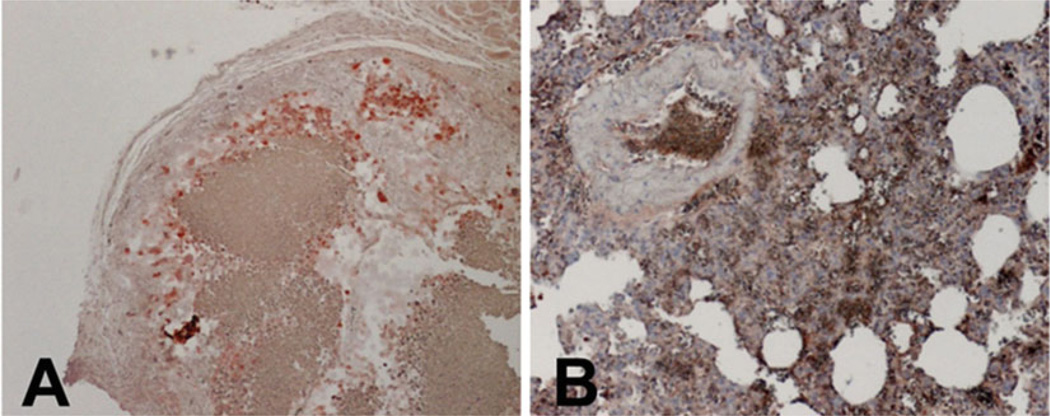
Tumors and lungs from mice, which were grafted with a metastatic melanoma skin reconstruct, are harvested 8 weeks after grafting. Fix the tissues and perform immunostaining using the standard protocols. Anti-human vimentin staining shows the positive human melanoma cells in the tumor and the lung tissue (Fig. 3a and b, respectively).
Fig. 2.
Different sizes of melanoma tumors form on the mouse back 8 weeks after grafting. From left to right: RGP melanoma cell line WM35, VGP melanoma cell line WM793, and two mice with metastatic melanoma cell line 1205Lu
Fig. 3.
Metastatic melanoma 1205Lu growths in a grafted skin reconstruct and lung metastases. Tumors and lungs were harvested 8 weeks after grafting on mice. Paraffin sections were stained with anti-human vimentin antibodies. (a) Melanoma tumors and (b) Metastatic melanoma lesions in mouse lungs stained positive for human vimentin
Footnotes
Hold the plate while whirling it around to make sure that the collagen mixture evenly covers the bottom of each insert.
Avoid making bubbles. If bubbles are generated, use a sterile needle to break them before the gel solidifies.
Grow keratinocytes to 60–70 % confluency without differentiated cells. It is better to use low-passage keratinocytes. Keratinocyte quality determines the success of the epidermal layer formation.
Air-lift the epidermis: Add skin reconstruct medium III to only the outside of each insert to expose the epidermis to air. This step induces keratinocyte differentiation to form a thick epidermis.
The temperature of water in the beaker should not be higher than 60 °C. Do not overheat the mouse skin. Overheated mouse skin cannot be stitched as a biological cover.
Carefully lift the mouse skin using forceps and avoid stitching the skin reconstruct.
References
- 1.Jerant AF, Johnson JT, Sheridan CD, et al. Early detection and treatment of skin cancer. Am Fam Physician. 2000;62(2):357–368. [PubMed] [Google Scholar]
- 2.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer—the role of sunlight. Adv Exp Med Biol. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 3.Clark WH, Jr, Elder DE, Guerry D, 4th, et al. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984;15(12):1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- 4.Sun T, Jackson S, Haycock JW, et al. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol. 2006;122(3):372–381. doi: 10.1016/j.jbiotec.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Smalley KS, Lioni M, Herlyn M. Life isn’t flat: taking cancer biology to the next dimension. In Vitro Cell Dev Biol Anim. 2006;42(8–9):242–247. doi: 10.1290/0604027.1. [DOI] [PubMed] [Google Scholar]
- 6.Sun T, Norton D, McKean RJ, et al. Development of a 3D cell culture system for investigating cell interactions with electrospun fibers. Biotechnol Bioeng. 2007;97(5):1318–1328. doi: 10.1002/bit.21309. [DOI] [PubMed] [Google Scholar]
- 7.Kremer M, Lang E, Berger A. Organotypical engineering of differentiated composite-skin equivalents of human keratinocytes in a collagen-GAG matrix (INTEGRA Artificial Skin) in a perfusion culture system. Langenbecks Arch Surg. 2001;386(5):357–363. doi: 10.1007/s004230100227. [DOI] [PubMed] [Google Scholar]
- 8.Escámez MJ, García M, Larcher F, et al. An in vivo model of wound healing in genetically modified skin-humanized mice. J Invest Dermatol. 2004;123(6):1182–1191. doi: 10.1111/j.0022-202X.2004.23473.x. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Fukunaga-Kalabis M, Herlyn M. The three-dimensional human skin reconstruct model: a tool to study normal skin and melanoma progression. J Vis Exp. 2011;3(54):Pii:2937. doi: 10.3791/2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haass NK, Smalley KS, Herlyn M. The role of altered cell-cell communication in melanoma progression. J Mol Histol. 2004;35(3):309–318. doi: 10.1023/b:hijo.0000032362.35354.bb. [DOI] [PubMed] [Google Scholar]
- 11.Herlyn M, Shih IM. Interactions of melanocytes and melanoma cells with the microenvironment. Pigment Cell Res. 1994;7(2):81–88. doi: 10.1111/j.1600-0749.1994.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 12.Meigel WN, Gay S, Weber L. Dermal architecture and collagen type distribution. Arch Dermatol Res. 1977;259(1):1–10. doi: 10.1007/BF00562732. [DOI] [PubMed] [Google Scholar]
- 13.van Kempen LC, Ruiter DJ, van Muijen GN, et al. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol. 2003;82(11):539–548. doi: 10.1078/0171-9335-00346. [DOI] [PubMed] [Google Scholar]
- 14.Lee JT, Li L, Brafford PA, et al. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanma Res. 2010;23(6):820–827. doi: 10.1111/j.1755-148X.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105(8):3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]