Abstract
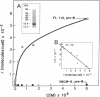
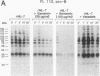
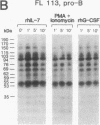
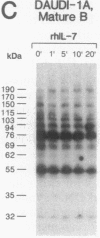
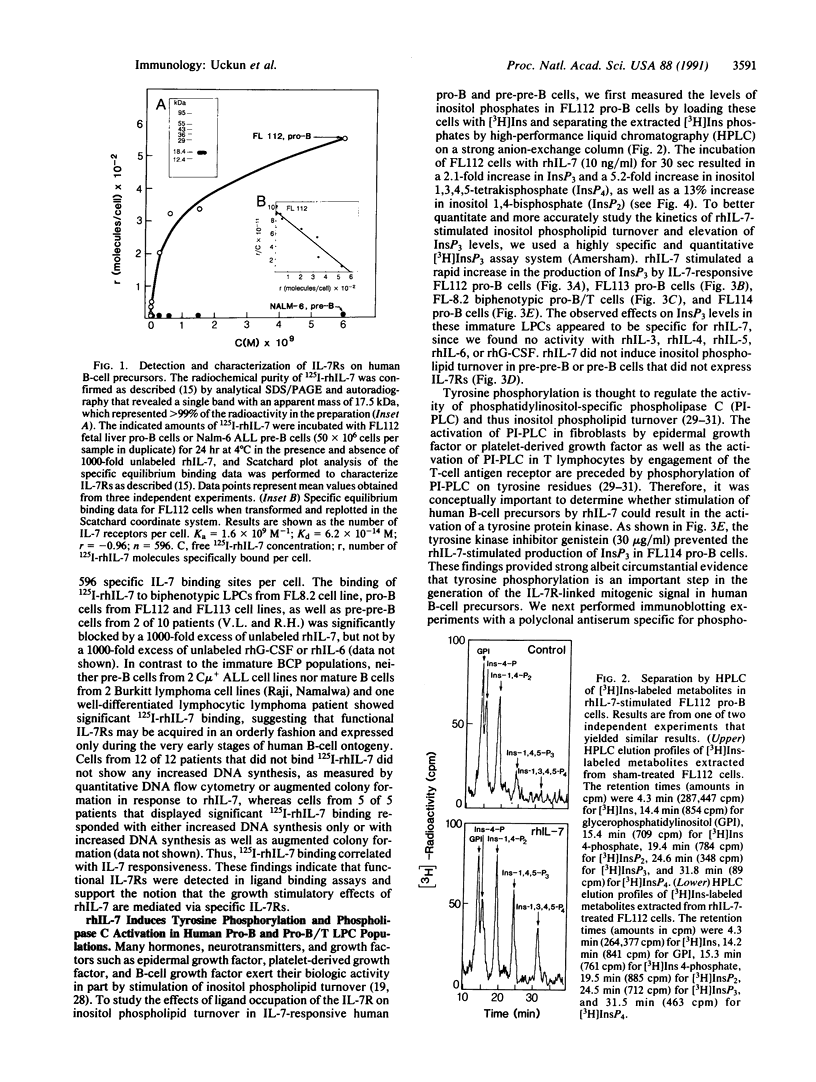
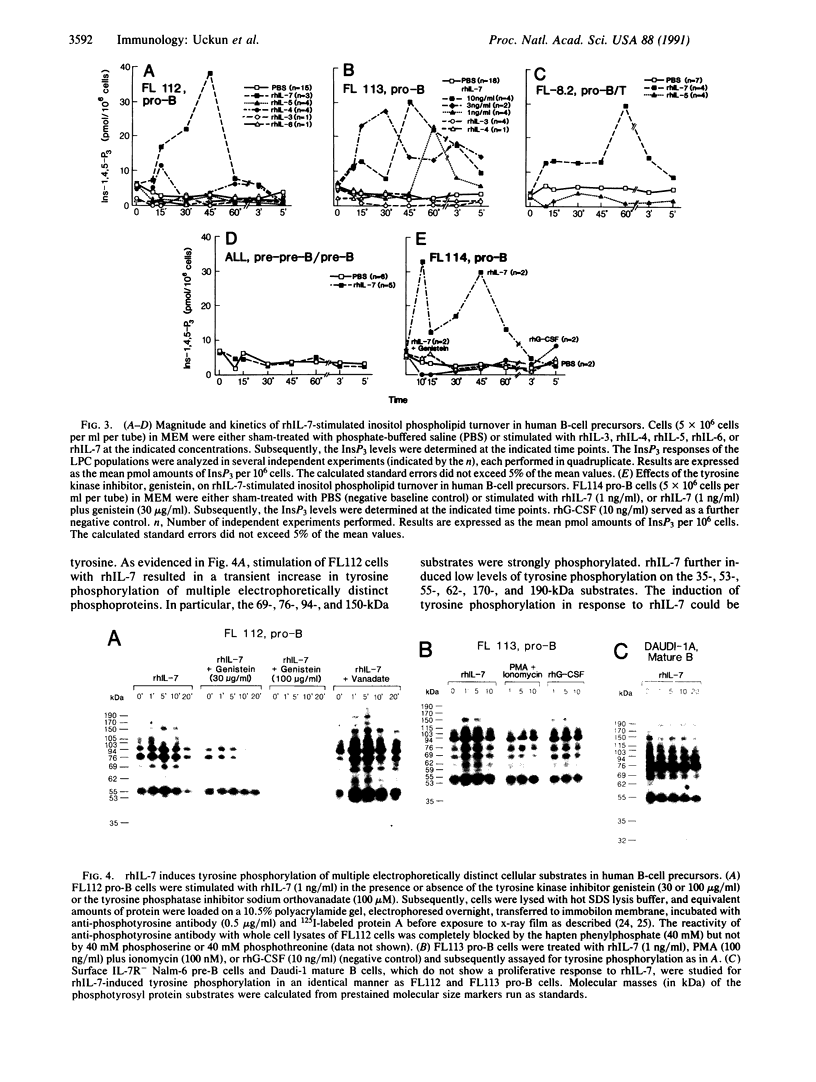
Functional interleukin 7 (IL-7) receptors are expressed on the surface of multiphenotypic, biphenotypic, and immature B-lineage human lymphoid precursor cells with germ-line immunoglobulin heavy-chain genes but not on more mature B-lineage lymphoid cells with rearranged and/or expressed immunoglobulin heavy-chain genes. Thus, IL-7 may have an important regulatory role during the earliest stages of human B-cell ontogeny. The engagement of the surface IL-7 receptors on immature B-cell precursor cells with recombinant human IL-7 (rhIL-7) results in enhanced tyrosine phosphorylation of multiple phosphoproteins, stimulates inositol phospholipid turnover and DNA synthesis, and promotes their clonal proliferation. These effects are (i) specific for rhIL-7, since rhIL-3, rhIL-4, rhIL-5, rhIL-6, and recombinant human granulocyte colony-stimulating factor do not elicit similar activities on IL-7 receptor-positive human pro-B cells; and (ii) mediated by IL-7 receptors, since they are not observed in IL-7 receptor-negative B-lineage lymphoid cell populations. rhIL-7-induced tyrosine phosphorylation on the 35-, 53-, 55-, 62-, 69-, 76-, 94-, 150-, 170-, and 190-kDa substrates as well as rhIL-7-induced stimulation of inositol phospholipid turnover are abrogated by the tyrosine kinase inhibitor genistein. These results demonstrate that the IL-7 receptor on immature human B-cell precursor populations is intimately linked to a functional tyrosine kinase pathway and tyrosine phosphorylation is an important and perhaps mandatory step in the generation of the IL-7 receptor-linked transmembrane signal.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage R. J., Namen A. E., Sassenfeld H. M., Grabstein K. H. Regulation of human T cell proliferation by IL-7. J Immunol. 1990 Feb 1;144(3):938–941. [PubMed] [Google Scholar]
- Balla T., Baukal A. J., Hunyady L., Catt K. J. Agonist-induced regulation of inositol tetrakisphosphate isomers and inositol pentakisphosphate in adrenal glomerulosa cells. J Biol Chem. 1989 Aug 15;264(23):13605–13611. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Cooper M. D. Current concepts. B lymphocytes. Normal development and function. N Engl J Med. 1987 Dec 3;317(23):1452–1456. doi: 10.1056/NEJM198712033172306. [DOI] [PubMed] [Google Scholar]
- Dean N. M., Beaven M. A. Methods for the analysis of inositol phosphates. Anal Biochem. 1989 Dec;183(2):199–209. doi: 10.1016/0003-2697(89)90468-5. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. L., Ferris D. K. Two-dimensional analysis of interleukin 2-regulated tyrosine kinase activation mediated by the p70-75 beta subunit of the interleukin 2 receptor. J Biol Chem. 1989 Jul 25;264(21):12562–12567. [PubMed] [Google Scholar]
- Goodwin R. G., Friend D., Ziegler S. F., Jerzy R., Falk B. A., Gimpel S., Cosman D., Dower S. K., March C. J., Namen A. E. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990 Mar 23;60(6):941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- Goodwin R. G., Lupton S., Schmierer A., Hjerrild K. J., Jerzy R., Clevenger W., Gillis S., Cosman D., Namen A. E. Human interleukin 7: molecular cloning and growth factor activity on human and murine B-lineage cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):302–306. doi: 10.1073/pnas.86.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S. Interleukin 7: effects on early events in lymphopoiesis. Immunol Today. 1989 May;10(5):170–173. doi: 10.1016/0167-5699(89)90175-8. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. B-cell stimulatory factors (BSFs): molecular structure, biological function, and regulation of expression. J Clin Immunol. 1987 Sep;7(5):343–355. doi: 10.1007/BF00917012. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rabinovitch P. S., June C. H., Song C. W., Clark E. A., Uckun F. M. Antigen-independent regulation of cytoplasmic calcium in B cells with a 12-kDa B-cell growth factor and anti-CD19. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1897–1901. doi: 10.1073/pnas.85.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Schieven G. L., Kuebelbeck V. M., Uckun F. M. Accessory receptors regulate coupling of the T-cell receptor complex to tyrosine kinase activation and mobilization of cytoplasmic calcium in T-lineage acute lymphoblastic leukemia. Blood. 1991 Mar 15;77(6):1271–1282. [PubMed] [Google Scholar]
- Lee G., Namen A. E., Gillis S., Ellingsworth L. R., Kincade P. W. Normal B cell precursors responsive to recombinant murine IL-7 and inhibition of IL-7 activity by transforming growth factor-beta. J Immunol. 1989 Jun 1;142(11):3875–3883. [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Goodwin R. G., Nordan R. P., Anderson D., Grabstein K. H., Cosman D., Sims J., Lupton S., Acres B., Reed S. G. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J Exp Med. 1989 Mar 1;169(3):707–716. doi: 10.1084/jem.169.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Schmierer A. E., March C. J., Overell R. W., Park L. S., Urdal D. L., Mochizuki D. Y. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988 Mar 1;167(3):988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Rhee S. G., Carpenter G. Tyrosine phosphorylation of phospholipase C-II in vitro by the epidermal growth factor receptor. J Biol Chem. 1989 Jun 25;264(18):10335–10338. [PubMed] [Google Scholar]
- Okazaki H., Ito M., Sudo T., Hattori M., Kano S., Katsura Y., Minato N. IL-7 promotes thymocyte proliferation and maintains immunocompetent thymocytes bearing alpha beta or gamma delta T-cell receptors in vitro: synergism with IL-2. J Immunol. 1989 Nov 1;143(9):2917–2922. [PubMed] [Google Scholar]
- Park L. S., Friend D. J., Schmierer A. E., Dower S. K., Namen A. E. Murine interleukin 7 (IL-7) receptor. Characterization on an IL-7-dependent cell line. J Exp Med. 1990 Apr 1;171(4):1073–1089. doi: 10.1084/jem.171.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seishima M., Yada Y., Nagao S., Mori S., Nozawa Y. Defective formation of inositol 1,4,5-trisphosphate in bradykinin-stimulated fibroblasts from progressive systemic sclerotic patients. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1077–1082. doi: 10.1016/s0006-291x(88)80742-3. [DOI] [PubMed] [Google Scholar]
- Suda T., Okada S., Suda J., Miura Y., Ito M., Sudo T., Hayashi S., Nishikawa S., Nakauchi H. A stimulatory effect of recombinant murine interleukin-7 (IL-7) on B-cell colony formation and an inhibitory effect of IL-1 alpha. Blood. 1989 Nov 1;74(6):1936–1941. [PubMed] [Google Scholar]
- Takeda S., Gillis S., Palacios R. In vitro effects of recombinant interleukin 7 on growth and differentiation of bone marrow pro-B- and pro-T-lymphocyte clones and fetal thymocyte clones. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1634–1638. doi: 10.1073/pnas.86.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Fauci A. S., Chandan-Langlie M., Myers D. E., Ambrus J. L. Detection and characterization of human high molecular weight B cell growth factor receptors on leukemic B cells in chronic lymphocytic leukemia. J Clin Invest. 1989 Nov;84(5):1595–1608. doi: 10.1172/JCI114337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Fauci A. S., Heerema N. A., Song C. W., Mehta S. R., Gajl-Peczalska K., Chandan M., Ambrus J. L. B-cell growth factor receptor expression and B-cell growth factor response of leukemic B cell precursors and B lineage lymphoid progenitor cells. Blood. 1987 Oct;70(4):1020–1034. [PubMed] [Google Scholar]
- Uckun F. M., Gajl-Peczalska K. J., Kersey J. H., Houston L. L., Vallera D. A. Use of a novel colony assay to evaluate the cytotoxicity of an immunotoxin containing pokeweed antiviral protein against blast progenitor cells freshly obtained from patients with common B-lineage acute lymphoblastic leukemia. J Exp Med. 1986 Feb 1;163(2):347–368. doi: 10.1084/jem.163.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Gesner T. G., Song C. W., Myers D. E., Mufson A. Leukemic B-cell precursors express functional receptors for human interleukin-3. Blood. 1989 Feb;73(2):533–542. [PubMed] [Google Scholar]
- Uckun F. M., Ledbetter J. A. Immunobiologic differences between normal and leukemic human B-cell precursors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8603–8607. doi: 10.1073/pnas.85.22.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M. Regulation of human B-cell ontogeny. Blood. 1990 Nov 15;76(10):1908–1923. [PubMed] [Google Scholar]