Abstract
We recently identified the genes encoding the guinea pig cytomegalovirus (GPCMV) homologs of the upper and lower matrix proteins of human CMV, pp71 (UL82) and pp65 (UL83), which we designated GP82 and GP83, respectively. Transient-expression studies with a GP82 plasmid demonstrated that the encoded protein targets the nucleus and that the infectivity and plaquing efficiency of cotransfected GPCMV viral DNA was enhanced by GP82. The transactivation function of GP82 was not limited to GPCMV, but was also observed for a heterologous virus, herpes simplex virus type 1 (HSV-1). This was confirmed by its ability to complement the growth of an HSV-1 VP16 transactivation-defective mutant virus in an HSV viral DNA cotransfection assay. Study of a GP82 “knockout” virus (and its attendant rescuant), generated on a GPCMV bacterial artificial chromosome construct, confirmed the essential nature of the gene. Conventional homologous recombination was used to generate a GP83 mutant to examine the role of GP83 in the viral life cycle. Comparison of the one-step growth kinetics of the GP83 mutant (vAM409) and wild-type GPCMV indicated that GP83 protein is not required for viral replication in tissue culture. The role of GP83 in vivo was examined by comparing the pathogenesis of wild-type GPCMV, vAM409, and a control virus, vAM403, in guinea pigs. The vAM409 mutant was significantly attenuated for dissemination in immunocompromised strain 2 guinea pigs, suggesting that the GP83 protein is essential for full pathogenicity in vivo.
Infection with human cytomegalovirus (HCMV), although ubiquitous in nature, can lead to severe disease manifestations in immunocompromised patients, including newborn infants (35). As the most common congenital viral infection in the developed world, HCMV acquisition in utero may cause significant neurodevelopmental handicap, including sensorineural deafness. Viral and host factors involved in the pathogenesis of CMV infection remain poorly understood. The species specificity of CMV infection necessitates the use of small-animal models and the study of the appropriate animal CMV for experimental investigation of pathogenesis. Among the CMVs of small mammals, the guinea pig CMV (GPCMV) is unique in its ability to cross the placenta and cause fetal infection, making this a useful model, particularly for the study of vaccines for congenital CMV infection (5, 19, 27, 54). Unfortunately, GPCMV research is hampered by a limited number of mutants to study pathogenicity and a poorly defined genome compared to the murine and rat CMVs (41, 56).
In an effort to facilitate investigation in the guinea pig model, we have identified a number of GPCMV homologs of important HCMV genes, including gB, gH, gL, and the pp65 homolog, UL83 (7, 48-50). More recently, our investigators reported the ability to generate recombinant GPCMV and successfully cloned the GPCMV genome as an infectious bacterial artificial chromosome (BAC) in Escherichia coli (33). In this report we extend these studies by the analysis and mutagenesis of the GPCMV homologs of HCMV proteins, UL82 and UL83. These two tegument phosphoproteins, also referred to as the upper (pp71 [UL82]) and lower (pp65 [UL83]) matrix proteins, are major components of the tegument layer and play important roles in the viral life cycle (17, 18). HCMV UL82 plays an essential role in viral replication by controlling expression of immediate-early (IE) genes to initiate the viral replication cascade (8). Transient expression of UL82 enhances transcription of reporter genes under the control of the major IE promoter, as well as up-regulating other cellular promoters (3). The mechanism of UL82 action is unclear, but it appears distinct from the herpes simplex virus (HSV) transactivator protein VP16, which interacts with cellular proteins HCF and Oct1 and with the TAATGARATT motifs of the HSV IE promoter to initiate transcription (45). Instead, the pp71 transactivation function appears to be mediated via interaction with cellular proteins, such as Daxx (22, 26, 30). UL83, in addition to being a major constituent of dense bodies (25, 39), plays a critical role in cell-mediated immunity to HCMV infection. The majority of HCMV-seropositive individuals possess CD8+ cytotoxic T-lymphocyte responses directed against pp65 (6, 21, 29), and the adoptive transfer of pp65-specific cytotoxic T lymphocytes isolated from HCMV-seropositive bone marrow donors to transplant recipients has been shown to confer protection against HCMV viremia and pneumonitis (43). These observations have provided support for the concept of development of pp65 vaccines for prevention of HCMV infection (4, 13).
Our group previously demonstrated that GP82 and GP83 are transcribed as a bicistronic message in a manner similar to that of HCMV (46, 49). In this report, we examine functional characteristics of these proteins. We demonstrate that the GP82 gene has an essential gene function, associated with viral replication, through the ability of the encoded protein in transient-expression assays to enhance the infectivity of GPCMV viral DNA and the efficiency of establishment of virus infection. We investigated the transactivation function of the GP82 protein by assaying for the growth complementation of an HSV VP16 mutant in an HSV viral DNA cotransfection. We established the essential nature of GP82 gene function by “knockout” mutagenesis of the GP82 gene on a GPCMV BAC, resulting in a clone unable to reconstitute virus when transfected onto guinea pig fibroblasts, a phenotype which we were able to rescue by expression of GP82 by transient-transfection methodology. We have extended our characterization of GP83 by investigating the ability of the protein to target the nucleus via multiple nuclear localization signals (NLS). Finally, to investigate the role of GP83 in pathogenesis, we generated a knockout virus via homologous recombination. The transcription and protein expression characteristics of this mutant were investigated, and growth comparisons against wild-type virus were carried out both in tissue culture and in an in vivo pathogenicity model, which was investigated in guinea pigs.
MATERIALS AND METHODS
Cells and viruses.
GPCMV (strain 22122, ATCC VR682) was propagated on guinea pig fibroblast lung cells (GPL; ATCC CCL 158) and maintained in F-12 medium supplemented with 10% fetal calf serum (HyClone Laboratories), 10,000 IU of penicillin/liter, 10 mg of streptomycin (Gibco-BRL)/liter, and 7.5% NaHCO3 (Gibco-BRL). Recombinant viruses were selected by the addition of mycophenolic acid (MPA) and xanthine (Gibco-BRL) into the medium at 200 and 10 μM, respectively (33). Virus titrations were carried out on six well-plates overlaid with 1.5% (wt/vol) methylcellulose followed either by staining of the cell monolayer with 10% Giemsa stain to enumerate plaques or by visualization under a fluorescence microscope for enhanced green fluorescent protein (EGFP)-expressing viruses.
Cloning and sequence analyses.
The sequence for the GP82 coding sequence was originally derived from BamHI subclone fragments from the large GPCMV EcoRI C fragment. A complete UL82 clone was generated by PCR from viral DNA and cloned as a HindIII fragment into pNEB193. The GP82 primers utilized for PCR cloning were GP82F (5′-CCGTATAAGCTTAGAACATGGCGTACGTGGGGCTG-3′) and GP82R (5′-CAGTCTAAGCTTACGGTCAACGTCCCGTATCCGGC-3′). The plasmid was designated pNEBGP82, and the GP82 gene was sequenced from two independent clones. For transient-expression assays, the GP82 coding sequence was subcloned into pCMVTAG2A as an EcoRI, HindIII fragment via PCR. Immunofluorescence expression assays were carried out as previously described (32, 44), using an anti-FLAG monoclonal antibody (Sigma), diluted 1:2,000, and a fluorescein isothiocyanate-conjugated anti-mouse Fab monoclonal antibody (Sigma), diluted 1:1,000.
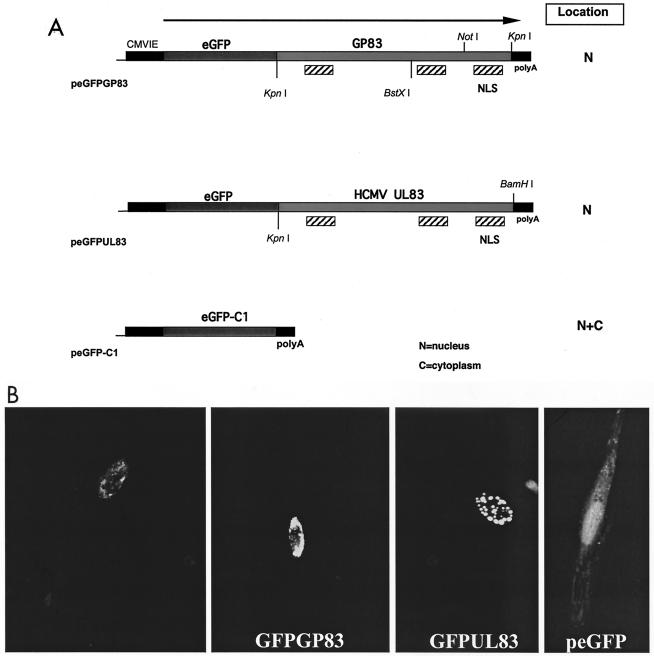
For studies of putative NLS encoded by GP83, a series of EGFP-GP83 fusion clones were constructed. Briefly, the full-length GP83 coding sequence was cloned in frame into the C-terminal domain of peGFP-C1 (Clontech) as a KpnI fragment, to generate peGFPGP83 (from amino acid [aa] 3 to 565 of GP83). Similarly, the HCMV pp65-EGFP fusion expression plasmid was constructed by PCR of the HCMV UL83 open reading frame (ORF) from AD169 viral DNA (peGFPUL83). A KpnI site was introduced at aa 3, similar to the GP83 ORF, and a BamHI site was introduced at the 3′ end of the ORF to allow the in-frame cloning of the HCMV pp65 coding sequence into the C-terminal polylinker in peGFP-C1 (see Fig. 7, below).
FIG. 7.
Nuclear localization of GP83 and HCMV pp65-EGFP fusion proteins. (A) Map of expression constructs. The peGFP-C1 and modified EGFP plasmids encoding C-terminal fusions of either GP83 ORF (peGFPGP83) or HCMV pp65 (peGFPUL83) are described in Materials and Methods. Restriction sites utilized for cloning purposes are indicated, as well as putative NLS predicted by coding sequence analysis. (B) Evaluation of fluorescence following transfection. Expression plasmids (5 μg) were transfected onto separate confluent monolayers of a six-well dish, and expression was allowed to proceed for 24 h prior to cell fixation with 4% paraformaldehyde and detection of autofluorescence as described in Materials and Methods. Transfection data are shown for peGFPGP83 (a and b, duplicate samples), peGFPUL83 (c), and the EGFP control plasmid, peGFP-C1 (d).
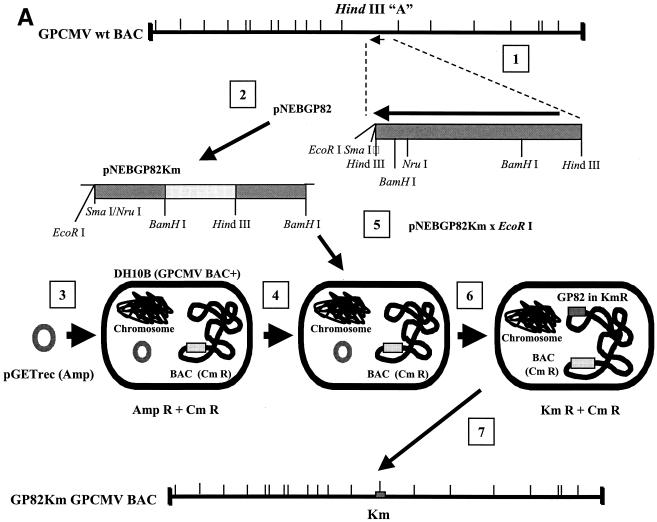
In order to disrupt the GPCMV GP82 coding sequence in a recombinant virus, a BamHI fragment of the GPCMV genome that contained GP83 and GP82 coding sequences, pKTS409 (49), was modified by the insertion of a gpt/EGFP cassette, isolated as a PvuII fragment from plasmid pQ106 (the generous gift of J. Vieira, Fred Hutchinson Cancer Research Center, Seattle, Wash.), and subsequently cloned into the EcoRV site on plasmid pKTS409, just downstream from the start of the GP82 coding sequence, to generate plasmid pKTS409EcVGG(see Fig. 3). For generation of GP83 deletion virus the same plasmid, pKTS409, was modified by XmnI collapse, yielding pKTS409Xm. This resulted in a 250-bp out-of-frame NH-terminal deletion of coding sequences of GP83. Subsequently, a BamHI cassette containing the gpt/EGFP cassette (55) was subcloned from plasmid pQ106 into a unique BglII site within the carboxy-terminal coding sequence of GP83, yielding plasmid pKTS409XmGG (see Fig. 3). Both plasmids were used separately in the generation of recombinant gpt/EGFP+ virus under metabolic selection with MPA and xanthine (33).
FIG. 3.
Strategy for the generation of GPCMV GP83 and GP82 mutant viruses. (A) The BamHI subfragment of the HindIII A fragment of the GPCMV genome, encoding the entire GP83 ORF and part of the coding sequences of GP82 and GP84 homolog genes, was cloned into pBluescript to generate pKTS409. To generate the GP83 knockout virus, a shuttle plasmid was generated by collapsing the internal XmnI sites towards the start of the GP83 coding sequence to create an out-of-frame, approximately 250-bp deletion. A BamHI cassette encoding a gpt/EGFP cassette was then introduced into a unique BglII site towards the 3′ end of the ORF. The modified vector was designated pKTS409XmGG and was cotransfected with GPCMV viral DNA onto GPL cells to generate GP83 null virus (vAM409) under gpt+ selection conditions. (B) To generate a GP82 knockout virus, a shuttle vector was generated by introducing a blunt-ended gpt/EGFP cassette into a unique EcoRV site at the start of the GP82 coding sequence to disrupt the GP82 coding sequence. The modified plasmid (pGP82GG) was cotransfected with viral DNA onto GPL cells to generate progeny virus, and mutant virus was selected under gpt+ selection.
Isolation of recombinant viruses and virus one-step growth kinetics.
Viral DNA and plasmid DNA were cotransfected onto confluent monolayers of GPL cells in six-well dishes by using Lipofectin (Gibco-BRL) following the manufacturer's specifications. Transfection-quality viral DNA was prepared as previously described (33). Plasmids were linearized to maximize the frequency of recombination with viral DNA. After an overnight incubation at 37°C, the cells were washed with F-12 medium and incubated for a further 30 days posttransfection, or until significant cytopathic effect (CPE) was observed. At this stage the cell monolayer and supernatant were harvested as primary progeny stock virus. This was used to infect a small flask of GPL cells under continued positive selection in the presence of xanthine and MPA. Two additional passages were performed under continuous selection. Following the third passage, supernatant titers were determined in 24-well plates and monitored for green fluorescence using a fluorescence microscope. The end point of the viral titration was replated on a further series of plates under limiting dilution conditions, such that only 1 in 3 to 1 in 10 wells was infected. Once significant CPE was observed, a number of these gpt/EGFP+ cells were used to generate a clonal viral stock.
Tissue culture-passaged GPCMV (ATCC 21222) and the GP83 deletion virus (designated vAM409) were used to infect confluent monolayers at a multiplicity of infection (MOI) of 0.5 PFU/cell. After adsorption for 1 h at 37°C, the cells were washed twice with F-12 medium and overlaid with 2 ml of medium. At the zero time point samples were harvested, and the remaining cells were incubated at 37°C with additional samplings taken at 12, 24, 36, 48, 60, and 72 h postinoculation. Samples were stored at −70°C until required, and the viral titer at each time point was subsequently determined by plaque titration assay on GPL cells.
GPCMV BAC mutagenesis.
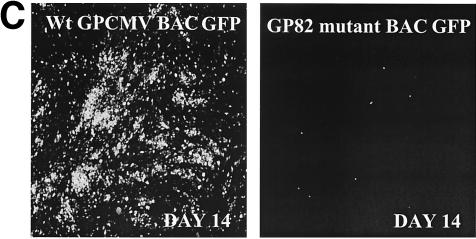
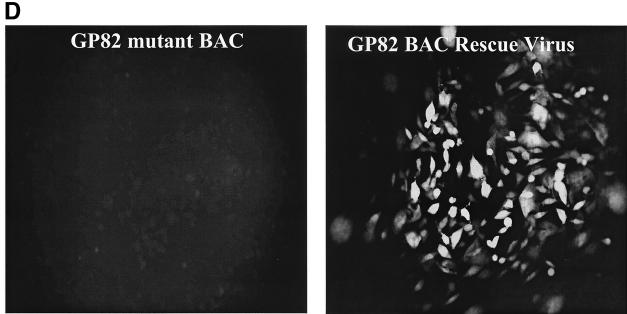
An inducible recombination system, referred to as the ET system, was introduced into DH10B bacterial cells containing the GPCMV BAC plasmid (see Fig. 6) (33). The protocol for generation of electrocompetent cells and for recombination was as described elsewhere (38). The ET recombination system encoded by plasmid pGETrec (the generous gift of P. Ioannou) was introduced into the GPCMV BAC-positive strain with ampicillin selection (100 μg/ml) for the pGETrec plasmid and with chloramphenicol (12.5 μg/ml) to maintain selection for the GPCMV BAC. The recombination system was induced as described elsewhere (38), except that the induction time was reduced to 15 min after the bacterial culture reached an optical density at 600 nm of 0.5 by addition of l-arabinose (Sigma) to a final concentration of 0.2% (wt/vol) at 37°C. To generate a knockout of the GP82 gene against the GPCMV BAC background, a modified GP82 gene initially created on a shuttle plasmid was utilized. Part of the GP82 gene was subcloned as an NruI/HindIII fragment from pNEBGP82HindIII into pNEB193 digested with HindIII and SmaI, to generate pNEBGPNru. A kanamycin resistance cassette encoded by plasmid pACYC177 was then PCR amplified as a BamHI fragment by utilizing the oligonucleotide primer pair Km1 (5′-GGATCCCGATTTATTCAACAAAGCCACG-3′) and Km2 (5′-GCCAGTGTTACAACCAATTAACC-3′). The cassette was cloned into pNEBGP82Nru digested with BamHI to introduce the Km cassette into the GP82 gene (at codon 168) to create an insertional knockout of the GP82 ORF. This plasmid was designated pNEBGP82Km. Approximately 30 μg of pNEBGP82Km was linearized by digestion with EcoRI, which linearized the vector in the polylinker sequence. The linearized plasmid was band isolated by agarose gel electrophoresis and purified by using GeneClean II (Qbiogene). The linearized pNEBGP82Km was resuspended at a concentration of 1 μg/μl and, to carry out recombination, 10 μl was mixed with 50 μl of electrocompetent cells in a 1-mm gapped cuvette and electroporation was carried out at 1.4 kV, 100 Ω, and 25 μF. After incubation in 1 ml of SOC medium for 2 h at 37°C, GP82-mutated GPCMV BAC colonies were isolated by chloramphenicol (12.5 μg/μl) and kanamycin (20 μg/μl) double selection on Luria-Bertani agar plates, and the ET plasmid was lost by patching onto duplicate chloramphenicol and kanamycin, or ampicillin, plates. Large-scale DNA preps of GPCMV BAC GP82 mutants were prepared as previously described (33). EcoRI restriction digestion was performed to verify that the GPCMV genome in the mutant BAC was intact. To verify correct insertion of the Km cassette into the GP82 locus, HindIII- and EcoRI-restricted mutant and wild-type GPCMV BAC DNA were analyzed by agarose gel electrophoresis and Southern blotting, using a GP82-specific probe (see Fig. 6). To generate mutant GP82 knockout virus, wild-type and mutant GPCMV BAC DNA preps were transfected onto guinea pig fibroblasts (GPL cells) as previously described (33). In order to confirm that the results observed with the GP82 deletion virus were generated by BAC mutagenesis, a rescue virus was generated by transient transfection in GPL cells, utilizing a plasmid construct expressing the GP82 ORF, as described below (see Fig. 6D).
FIG. 6.
Generation of GP82-GPCMV BAC mutant. (A) Overall GPCMV BAC GP82 knockout strategy (steps 1 to 7). GP82 coding plasmid was modified to introduce a Km drug resistance cassette to disrupt the GP82 coding sequence (pNEBGP82Km). Modified GP82 plasmid was linearized by restriction digestion and band purified by gel electrophoresis. Linearized GP82Km plasmid (pNEBGP82Km) was introduced into ET recombination-induced GPCMV BAC-containing DH10B cells. GP82 GPCMV BAC mutant colonies were selected on chloramphenicol-kanamycin LB agar plates grown overnight at 37°C. Mutant BACs were verified by restriction profile analysis (EcoRI and HindIII) and by Southern blotting. (B) Restriction profile and Southern analyses of GPCMV GP82 BAC mutant versus wild-type GPCMV BAC. Left panel, HindIII profile. Lane 1, wild-type BAC; lane 2, GP82 mutant. Mutagenesis as predicted introduced a novel HindIII restriction site into a HindIII A region of the genome, resulting in a shift in the band by gel electrophoresis and Southern analysis (arrows). Right panel, Southern blot analyses of the GP82 BAC mutant. Blot of HindIII restriction digest of wild-type and GP82 GPCMV BAC DNA. Lane 1, wild-type BAC DNA; lane 2, GP82 mutant. Probing of blot with GP82 probe confirmed polymorphism introduced by pGETrec-mediated mutagenesis. Position of molecular weight markers is indicated. (C) Confirmation of essential nature of the GP82 gene. GP82 BAC mutant DNA and wild-type GPCMV BAC DNA were transfected onto GPL cells in separate experiments, and virus production was monitored by EGFP expression. Absence of EGFP-positive CPE and absence of virus from culture supernatants in GP82-transfected cells confirmed the essential nature of the GP82 gene. (D) Rescue of the GP82 BAC mutant. Cotransfection of GP82 BAC DNA with GP82 expression plasmid restored replication competence (right panel), compared to GP82 BAC DNA alone (left panel). Monolayers were photographed 10 days posttransfection and visualized for EGFP fluorescence.
Southern blotting and PCR analyses of recombinant GP83 mutant virus.
Southern blot analyses of viral DNA isolated from recombinant viruses were carried out using standard techniques previously described (32). DNA probes corresponding to the GP83 coding region of the viral genome were labeled with [32P]dCTP by using a High Prime kit (Boehringer Mannheim) according to the manufacturer's specifications. Viral DNA from both wild-type GPCMV and vAM409 was digested with BamHI, subjected to agarose gel electrophoresis, and blotted to nitrocellulose membranes. Hybridization was performed using a GP83-specific probe, and bands were detected by autoradiography. PCR was performed with wild-type or mutant viral DNA template in a 50-μl total reaction volume using Vent polymerase (New England BioLabs) and standard conditions for 30 cycles of 97.5°C (30 s), 60°C (30 s), and 72°C (30 s), as described elsewhere (51). PCR samples were analyzed by agarose gel electrophoresis.
RT-PCR analyses of GP82 RNA expression in the GP83 mutant virus.
Monolayers of GPL cells were inoculated (MOI of 5), and total RNA was purified at 96 h postinfection as previously described (49). To confirm that insertion of the gpt/EGFP cassette in the vAM409 virus did not result in an inability of the adjacent GP82 region to be transcribed, RNA transcripts from wild-type and mutant virus were examined by reverse transcription-PCR (RT-PCR) analysis. RNA was extracted from uninfected GPL cells, or cells infected with either wild-type or the vAM409 deletion virus, by using the TRIzol method (Invitrogen, Carlsbad, Calif.). Two micrograms of RNA was reverse transcribed and PCR amplified using a OneStep RT-PCR kit (QIAGEN, Valencia, Calif.). The primers (specific for GP82 mRNA) used were GP82 forward (5′-GTTGCGAATCTTGACCGTCAGC-5′) and GP82 reverse (5′-GACTGTACGGTTTCACTCAC-3′). The reaction mixtures consisted of a 0.6 μM concentration (200 ng) of each primer, 400 μg of each deoxynucleoside triphosphate, 10 U of RNase inhibitor, 1× RT-PCR buffer containing 2.5 mM MgCl2, and 2 μl of the QIAGEN enzyme mix. OneStep RT-PCR enzyme mix contained optimized concentrations of Omniscript and Sensiscript reverse transcriptases and HotStar Taq DNA polymerase. RT was performed at 50°C for 30 min. HotStar Taq was activated, and cDNA was denatured by heating at 95°C for 15 min. Thirty-five cycles were performed as follows: denaturation at 95°C for 45 s, annealing at 63°C for 45 s, and extension at 72°C for 45 s, followed by a final extension of 2 min at 72°C. To control for DNA contamination, a sample was added at the start of the 95°C PCR activation step to inactivate the reverse transcriptases. As a control for the amplification procedure using endogenous mRNA, amplification of guinea pig 18S rRNA was performed (2). All conditions were the same as above and included the following primers: 18S forward (5′-TGCATGGCCGTTCTTAGTTG-3′) and 18S reverse (5′-AGTTAGCATGCCAGAGTCTCGTT-3′). PCR products were analyzed on a 1% agarose gel stained with ethidium bromide. Amplification was also performed under identical conditions, but with no reverse transcriptase, to control for amplification of contaminating DNA.
Western blot analysis of recombinant virus.
Wild-type and recombinant viruses were used to infect GPL cells, and virus particles were purified as described elsewhere (49). Proteins were solubilized with 2% sodium dodecyl sulfate (SDS), 62.5 mM Tris base (pH 6.8), 10% glycerol, 0.001% bromophenol blue, and 5% β-mercaptoethanol. Proteins were subjected to SDS-polyacrylamide gel electrophoresis and then were electroblotted to Nytran membranes (Schleicher & Schuell). Membranes were blocked with Blotto (10 mM Tris HCl [pH 7.5], 0.9% NaCl, 5% nonfat dry milk, and 0.5% NP-40), washed in Tris-buffered saline (TBS; 0.2 M Tris HCl [pH 7.5] and 0.5 M NaCl), and then incubated for 2 h with polyclonal anti-GPCMV antiserum or a monospecific guinea pig serum raised against a GP83-glutathione S-transferase fusion protein (1:1,000 dilutions) in TBS at 25°C (49). After washing in TTBS (TBS containing 0.5% Tween 20), blots were incubated with a horseradish peroxidase-conjugated rabbit anti-guinea pig antiserum (1:20,000 dilution; Accurate Chemical and Scientific Co.) for 2 h at 25°C. Antibody binding was then detected using the enhanced chemiluminescence detection system, followed by autoradiography, using the manufacturer's specifications (SuperSignal West Pico; Pierce, Rockford, Ill.).
Analysis of GP82 effect on enhancement of viral DNA infectivity.
GP82 was cloned in frame as an EcoRI/HindIII fragment into the expression vector pCMVTAG2A (Promega) to generate pFLAGGP82, and a range of 2 to 20 μg of plasmid DNA was cotransfected with viral GPCMV DNA (1 to 2 μg) onto GPL cells with Lipofectamine 2000 in Opti-MEM, using the manufacturer's protocol for six-well dishes. After overnight incubation, the transfection reaction was replaced with F-12 medium, and plaques were allowed to develop over a 2-week period. Transfection-quality viral DNA was generated as previously described (33). Assays were performed in the presence or absence of GP82-expressing plasmid. In addition, in separate experiments GPCMV viral DNA was cotransfected with an expression plasmid (2 to 20 μg) that expresses pGP83 (50) or with an expression plasmid expressing HSV transactivation protein VP16, pVP16+ (a gift from Rick Thompson), or with empty pCMVTAG2A vector. Cell monolayers were fixed with 4% paraformaldehyde, and plaques were identified by microscopy following staining with crystal violet.
Analysis of the transactivation function of GP82 by complementation of viral growth of an HSV VP16-impaired mutant.
Transfection-quality HSV viral DNA was generated from cells infected with an HSV type 1 (HSV-1) 17+ viral mutant, 1780, which is devoid of the VP16 transactivation function (32). This virus is capable of normal growth when supplemented with the chemical compound N,N′- hexamethylene-bisacetamide (HMBA; Sigma) in the medium to up-regulate viral transcription (32) in a manner similar to that for the HSV VP16 mutant in1814 (31). HSV viral DNA (1 to 2 μg) was transfected in the presence or absence of the GP82 expression plasmid (2 to 20 μg) and also, in separate experiments, it was cotransfected with plasmids expressing GPCMV GP83 (2 to 20 μg) or HSV-1 VP16 (2 to 20 μg) as described above. GPL cells were transfected using Lipofectamine 2000 according to the manufacturer's protocol. After an overnight incubation, the transfection reaction mixture was replaced with F-12 medium and plaques were allowed to develop over a 2-week period. Cell monolayers were fixed with 4% paraformaldehyde and stained with crystal violet, and plaques were identified by microscopy.
Analyses of viral replication and pathogenicity in guinea pigs.
To analyze the in vivo replication of recombinant viruses, inbred strain 2 guinea pigs were purchased from the Children's Hospital Research Foundation (Cincinnati, Ohio). Animals were housed under conditions approved by the American Association for Accreditation of Laboratory Animal Care, in accordance with rules and regulations established by the Children's Hospital Research Foundation animal use committee. Weanling guinea pigs (approximately 200 to 250 g) were pretreated with cyclophosphamide, 200 mg/kg of body weight intraperitoneally, 24 h prior to viral challenge. Groups of animals (n = 8) were injected via the intraperitoneal route with 107 PFU of either wild-type GPCMV (ATCC), a mutant virus (vAM403) with an insertion in the nonessential HindIII N region of the genome (33), or with the GP83 deletion mutant, vAM409. Animals were monitored for weight loss (see Fig. 11) and observed for signs of illness (e.g., ruffled fur, listlessness). At either day 10 or 21 postinoculation, animals were humanely sacrificed in accordance with Children's Hospital Research Foundation institutional animal use policies, and organs were removed for viral culture and DNA extraction for PCR. Organ homogenates were prepared (0.1 g/ml [wt/vol]), and serial dilutions were set up in tissue culture in quadruplicate. The dilution which resulted in CPE in ≥50% of the wells was defined as the 50% tissue culture infective dose. Additionally, for selected samples, DNA was extracted from 100 μl of organ homogenate and eluted using the QIamp DNA purification system, according to the manufacturer's specifications. A total of 2 μl of eluent (2% of sample) was then amplified in a quantitative competitive PCR (qcPCR) assay (51) to assess viral load in infected organs.
FIG. 11.
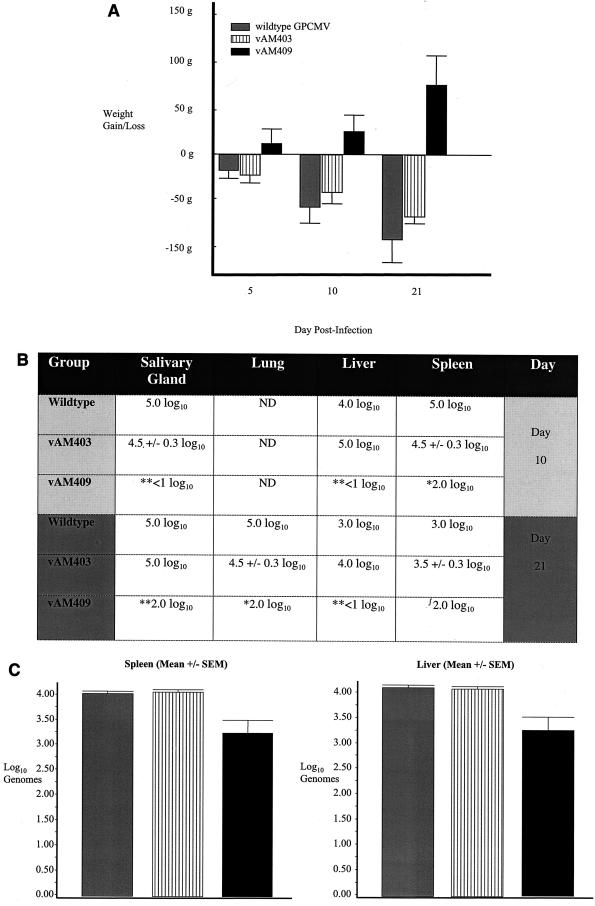
Analysis of phenotype of GP83 deletion virus in infected guinea pigs. (A) Patterns of weight gain or loss following inoculation of wild-type GPCMV and GP83 deletion virus in immunocompromised strain 2 guinea pigs. Cumulative weight gain or loss is indicated over the course of the study (mean ± standard error of the mean). Attenuation of the GP83 deletion virus was evident, based on weight gain in these animals compared to weight loss in wild-type-inoculated animals (P < 0.05, vAM409 versus control and vAM403, one-way ANOVA with Bonferroni correction). (B) Viral culture data. Animals were sacrificed at day 10 or day 21 postviral inoculation, and organ homogenates were prepared for culture. The dilution at which ≥50% of the wells were positive for viral CPE was determined (50% tissue culture infective dose). *, P < 0.05 versus ATCC, Kruskal-Wallis test; **, P < 0.05 versus ATCC and vAM403, Kruskal-Wallis test; , P < 0.05 versus vAM403, Kruskal-Wallis test. (C) qcPCR was performed on liver and spleen homogenates at 21 days postinoculation to compare animals inoculated with wild-type (gray bars), vAM403 (striped bars), and vAM409 (black bars). Tissues from vAM409-infected animals had significantly less viral DNA (P < 0.05, one-way ANOVA with Bonferroni correction), indicating impairment of replication of this mutant in vivo.
RESULTS
Comparison of GPCMV GP82 amino acid sequence with other HCMV pp71 homologs.
Full-length GP82 was generated by PCR from viral DNA and cloned into the pNEB193 vector, using unique HindIII sites incorporated into the amplifying oligos. The GP82 gene was sequenced from two independent clones, and the predicted ORF was 521 aa in length (predicted Mr of 57.5), which is shorter than HCMV pp71 (UL82), which has a predicted length of 559 aa. Phylogenetic comparison and sequence alignment of the GP82 homolog to other UL82 homologs from other sequenced animal CMVs are indicated in Fig. 1. The length of the positional UL82 homologs from other animal CMVs so far sequenced ranged from 638 aa (tupiaia herpesvirus [THV]) to 532 aa (baboon CMV [BCMV]). In all, five positional HCMV UL82 homolog sequences are currently available in the GenBank database (Fig. 1, BCMV, chimpanzee CMV [CCMV], murine CMV [MCMV], rat CMV [RCMV], rhesus CMV [RhCMV], and THV). CCMV is the closest-evolved CMV to HCMV. CCMV UL82 is 558 aa in length (12) with 63% identity with HCMV pp71, whereas GPCMV has significantly lower identity (19%) with HCMV pp71, instead showing a stronger phylogenetic relationship to rodent CMV UL82 genes. A comparison of the predicted ORFs of all the UL82 homologs was made by using an evolutionary tree homology program (MacVector 5.0) (Fig. 1) to illustrate that these homologs share only modest homology, despite presumably fulfilling similar functions in their respective animal hosts. Two functional motifs have recently been identified in the HCMV UL82 predicted coding sequence, DIDs (Daxx interaction domains) for interaction with Daxx and ND10 bodies (23) and LXCXD for modification of the cell cycle (28). Although the DID motifs are positionally conserved in primate pp71 homologs (CCMV, BCMV, and RhCMV), their sequence conservation with HCMV pp71 DIDs (Fig. 1B) and human CENP-C, which is a human cellular protein that interacts with Daxx (24; A. McGregor, unpublished data), is relatively poor. However, both position and sequence conservation are lost in any potential DID sequences in all nonprimate UL82 homologs sequenced so far (MCMV, RCMV, THV, and GPCMV), where possibly Daxx interaction is apparently mediated via an alternative motif. The cell cycle alteration LXCXD motif (28, 30) would appear to be conserved in human and primate CMV UL82 homologs (CCMV, BCMV, and RhCMV), but even in this case the conserved motif in RhCMV is changed to LXCXN. Presumably, the poor conservation of these motifs reflects adaptation by the animal CMV strain to its respective species.
FIG. 1.
Molecular comparison of CMV UL82 homologs. (A) Phylogenetic comparison of GP82 and other CMV UL82 (pp71) homologs. GP82 diverges down the same pathway as rodent UL82 homologs (MCMV and RCMV). Neighbor-joining tree methodology used the following Clustal multiple alignment parameters: open gap penalty, 10; extend gap penalty, 0.1; delay divergent, 40%; gap distance, 8; similarity matrix, blosum. (B) Alignment of GPCMV GP82 protein coding sequence with primate UL82 homologs. Amino acid residues conserved across species are indicated: double dot, conserved residue; asterisk, identical residue. Conserved DIDs (shaded regions marked I and II) (23) and LXCXD motifs (shaded regions marked III) (29) important in primate UL82 function are shaded.
The GPCMV GP82 homolog enhances GPCMV viral DNA infectivity and plaquing efficiency and complements VP16 transactivation-deficient HSV mutants.
Previous studies of the UL82 homologs of CMVs identified a transactivation function associated with HCMV UL82. However, to date this transactivation function has not been confirmed for any nonhuman CMV. Although a number of UL82 positional homologs have been identified in other CMVs, the functional activity of HCMV pp71 has only recently been investigated. Hence, there is no evidence to indicate that any of the positional homologs of UL82 in other species CMVs have equivalent functional properties. The functional properties of the GPCMV UL82 homolog were therefore investigated. GP82 was PCR amplified and subcloned in frame into the FLAG tag vector (pTAG2A) under the control of the CMV IE promoter. Examination of transient protein expression detected distinct nuclear fluorescence (Fig. 2), verifying nuclear targeting similar to that of HCMV pp71 (22). However, more study is required to examine interactions of the GPCMV pp71 homolog with components of the ND10 bodies as described recently for HCMV pp71 (23, 26, 30).
FIG. 2.
Immunofluorescence assay of the transient expression of FLAG-tagged GP82. Expression plasmid FLAG82GP (5 μg) was transfected onto subconfluent monolayers on glass coverslips in individual wells of a six-well plate, as described in Materials and Methods. At 36 h posttransfection, cells were fixed and GP82 expression was assayed by immunofluorescence assay using anti-FLAG mouse monoclonal and secondary anti-mouse-fluorescein isothiocyanate conjugate as described in Materials and Methods. (A) FLAG82GP plasmid; (B) mock-transfected cells.
Initially, to determine if the GP82 protein was the UL82 HCMV functional homolog, a series of transient GP82 protein expression-GPCMV viral DNA transfection assays were performed (Fig. 3). In these experiments GPCMV viral DNA was cotransfected with expression plasmids encoding either the GP83 tegument protein, GP82 tegument protein, or HSV VP16 transactivating protein. As expected, only cells transfected with GP82 and GPCMV viral DNA produced CPE rapidly (Fig. 4). Plaques began to appear within 7 days posttransfection in GP82 plasmid-cotransfected wells, in comparison to the normal time of 14 to 30 days when viral DNA is transfected into cells. The 14-to-30-day range is reflected in the passage number of the GPL cells. Above passage 30, there is a progressive slowing of virus development from transfected DNA (McGregor, unpublished). All of our experiments were performed with cells below passage number 20, to eliminate any confounding influence associated with the use of higher-passage cells. The GP83 protein, as expected, had no effect on enhancing viral plaque formation. Similarly transfected wells expressing the HSV VP16 protein had no effect on GPCMV viral DNA plaque formation, as the mechanism of transactivation by VP16 is specific to TAATGARAT motifs in HSV promoters, which are absent in CMV. In a separate experiment, progressively higher concentrations of GP82 plasmid (range, 2 to 20 μg) were cotransfected with a fixed amount of viral DNA (2 μg), and in this experiment the efficiency or infectivity of viral DNA was directly enhanced by increasing levels of input GP82 plasmid DNA by up to 50-fold (data not shown). Not only was an increased number of plaques observed, but also the inclusion of GP82 accelerated virus production was noted. These results are similar to those obtained for HCMV pp71-expressing plasmid cotransfected with HCMV viral DNA, which enhanced viral DNA plaque formation efficiency and infectivity of viral DNA (3). However, it was not possible to make a direct comparison with HCMV studies, as the transfection efficiency onto human foreskin fibroblasts is much lower (∼1%) than that obtainable in GPL cells (∼30% [McGregor, unpublished]). Additionally, as both GP82 and UL82 (pp71) are species specific, it was not possible to quantify results for GP82 and pp71 against the same cellular background. Hence, these results suggest that GP82 is the functional homolog of HCMV pp71, although species specificity constraints preclude definitive comparisons.
FIG. 4.
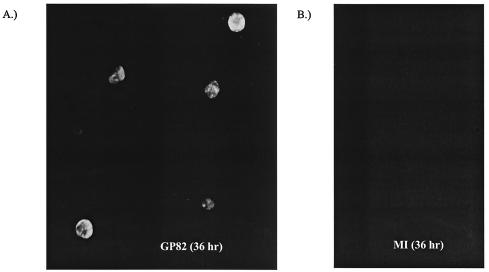
GP82 enhancement of generation of GPCMV from viral DNA transfections. A fixed amount of GPCMV viral DNA (2 μg) was cotransfected onto one well of a six-well dish of GPL cells (106 cells) in the presence of expression plasmids (5 μg) encoding GP82 (A), GP83 (B), VP16 (C), or mock transfected (D). Virus was allowed to develop over a 2-week time period and then stained for viral CPE as described in Materials and Methods. During this time period, viral CPE was only detected in GP82-cotransfected cells, indicating the ability of GP82 to accelerate the infectivity of GPCMV DNA. These results demonstrate the transactivation function of the GP82 ORF, the first demonstration of a transactivation function for a nonhuman CMV.
In order to further evaluate the transactivation function of GP82, we next investigated the ability of GP82 to complement the growth of an HSV VP16 mutant virus. A GP82 transactivation assay was designed that employed an HSV VP16 transactivation-deficient mutant 1780, previously described (32). The 1780 mutant incorporates a similar mutation to in1814 in the VP16 transactivation domain by insertion of a 12-bp BamHI linker to disrupt the zinc finger transactivation domain (1). Additionally, this virus carries a lacZ cassette insertion into the nonessential UL43 locus (32). Although HSV can grow in the absence of VP16 transactivating function, the mutant viral DNA, when transfected, is greatly impaired in its ability to form plaques and produce infectious virus, especially in primary cell lines (McGregor, unpublished). The VP16 transactivation deficiency can be complemented by the addition of chemical transcriptional up-regulators, such as HMBA, into the medium (31) or by supplying VP16 in trans (58). However, it has recently been demonstrated that HCMV pp71 is capable of complementing VP16 transactivation function in human cells in a recombinant VP16 mutant HSV virus expressing HCMV pp71 (24). We therefore tested GP82 transactivating function in an HSV transactivation complementation assay. HSV viral DNA was cotransfected with plasmids expressing GPCMV GP82, GP83, or HSV VP16 in separate wells. Cells in six-well plates were overlayed with 2 ml of medium and incubated at 37°C (5% CO2) for 14 days posttransfection, at which time all plates were fixed and stained. The supernatant was used to determine virus released into the supernatant by titrating it on fresh cells in the presence of HMBA. Cells transiently expressing GP82 or VP16 produced similar viral CPE, and the viral titer was also similar (ranging between 1 × 106 and 3 × 106 PFU/ml), whereas cells expressing GP83 failed to generate CPE or virus (Fig. 5A). This supports the conclusion that the GP82 protein is the functional homolog of HCMV UL82, with similar transactivation functions.
FIG. 5.
GP82 enhancement of growth of the HSV-1 VP16 transactivation-deficient mutant. HSV viral DNA (1780 VP16 mutant), 2 μg, was cotransfected onto one well of a six-well dish of GPL cells (106 cells) in the presence of expression plasmids (5 μg) encoding GP82 (A), GP83 (B), or VP16 (C). Virus was allowed to develop over a 2-week time period and then stained for viral CPE as described in Materials and Methods. Viral CPE was only detected in VP16 and GP82-transfected cells. Additional control wells were MI (D) and HSV 1780 DNA, cotransfected in the presence of 3 mM HMBA (E). Demonstration of CPE in the presence of GP82 expression for the VP16 transactivation-deficient mutant indicates the transactivation function of the GP82 ORF, but not GP83.
Mutagenesis and rescue of the GPCMV GP82 ORF.
The HCMV UL82 has been demonstrated to be an essential gene, and viruses devoid of this protein can only be isolated on complementing cells (8). We sought to verify the essential nature of the GPCMV UL82-encoded protein by the generation of a GP82 knockout virus. This approach has been successful in the isolation of previous GPCMV mutants (33). A plasmid, pKTS409 (49), encoding part of the GP82 gene was modified to introduce a gpt/EGFP selection cassette into a unique EcoRV site just downstream from the start of the UL82 ORF, thereby disrupting the GP82 gene (Fig. 3). Cotransfection of this plasmid with viral DNA resulted in the generation of EGFP-expressing virus. However, despite repeated attempts, we found it impossible to isolate GP82 mutant virus to complete homogeneity by plaque purification. Therefore, to corroborate the results observed with the gpt mutagenesis approach, an alternative strategy, using a GPCMV BAC plasmid, was devised to generate a GP82 mutant. DH10B containing the GPCMV BAC plasmid was made recombination positive by introducing the ET recombination system into this strain through transformation of the pGETrec plasmid (33). We next generated a knockout of GP82 by insertion of a Km cassette into the GP82 locus on the GPCMV BAC in E. coli, as described in Materials and Methods. Although this strategy was successful in generation of the predicted mutated GPCMV BAC genome in E. coli, transfection of GP82 mutant GPCMV BAC plasmid DNA into fibroblasts could not reconstitute EGFP-positive virus, unlike transfected wild-type GPCMV BAC DNA (Fig. 6) . We therefore concluded, based on two independent mutagenesis strategies, that GP82 is an essential gene.
In order to confirm the results observed with the GP82 deletion virus generated by BAC mutagenesis, a rescue virus was next generated by transient transfection in GPL cells, utilizing a plasmid construct expressing the GP82 ORF (50). Plasmid was expressed by transient transfection, and GPL cells were subsequently transfected with viral DNA purified from E. coli encoding the GP82 BAC deletion virus. These studies (Fig. 6D) indicated that cells expressing the GP82 plasmid, but not controls, were now capable of rescuing growth of the GP82 knockout virus.
The GPCMV GP83 homolog contains nuclear targeting signals.
HCMV UL83 is a nuclear targeting protein with three independent functional NLS (11, 14, 22, 47, 52). To authenticate the relevance of the GP83 ORF and further characterize the encoded protein, functional analysis of the GP83 protein (which contains potential nuclear targeting signals) was performed in transient-expression assays. The GP83 ORF was cloned in frame into the C-terminal domain of the EGFP coding sequence of the vector peGFP-C1. As a positive control to verify previous studies, the HCMV UL83 ORF was PCR amplified from viral DNA and cloned in frame into the C-terminal domain of peGFPC-1, to generate peGFPUL83. Fluorescence of the chimeric plasmids was then monitored 24 h posttransfection of GPL cells. These analyses revealed that the EGFP fusion constructs for both UL83 and GP83 generated a protein that targets to the nucleus of the cell (Fig. 7). The fluorescence pattern of the eGFPGP83 expression was more general than that obtained for the positive control construct (HCMV peGFPUL83), which as well as giving a general nuclear fluorescence also produced spotted nuclear fluorescence. The eGFPGP83 fluorescence was of variable intensity and nuclear distribution and varied between cells for the same construct (Fig. 7). The eGFPGP83 fluorescence may be potentially obscured in the nucleus by a similar tight integration with the nuclear chromatin structure, as observed with HCMV UL83 (11). The eGFPGP83 chimeric construct was further modified by a series of in-frame deletions to the NH2 and COOH domains of GP83. Transient expression of the various N- and C-terminal collapsed versions of the eGFPGP83 construct resulted in similar nuclear fluorescence, indicating that the GP83 protein has multiple putative NLS, similar to HCMV UL83 (data not shown). Therefore, it was concluded that nuclear targeting of GP83 is of a complexity similar to that of the HCMV UL83 (11, 14, 22, 47, 52).
The GP83 ORF is dispensable for GPCMV replication in tissue culture.
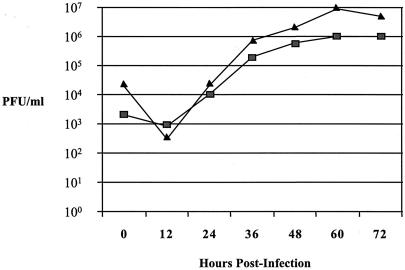
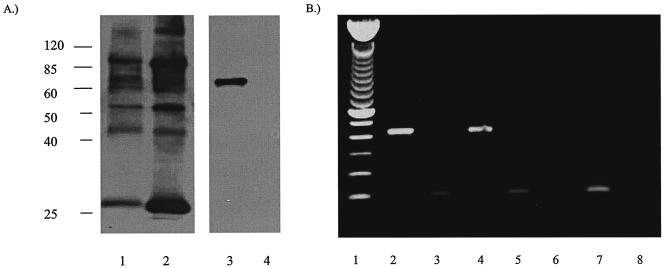
In order to assess the role of GP83 in viral replication, a GP83 deletion mutant was generated via homologous recombination, using a shuttle plasmid containing an amino-terminal out-of-frame deletion of the GP83 ORF, and the insertion of a gpt/EGFP cassette, as described in Materials and Methods and shown in Fig. 3. The isolated GP83 mutant was designated vAM409. The genomic structure of this recombinant virus was compared to that of wild-type GPCMV by agarose gel electrophoresis and Southern blot analysis of BamHI-digested wild-type and mutant viral DNA. The prediction for the genomic configuration of vAM409 was that a shift in the BamHI fragment encoding the GP83 homolog gene from 3.4 to 5.7 kb would be observed. This prediction was correct, based on the Southern analysis (Fig. 8A). To further confirm that the recombinant contained the amino-terminal out-of-frame deletion, PCR was performed using primers spanning the XmnI deletion site. PCR analysis revealed a shift of approximately 250 bp in vAM409, confirming that the predicted deletion was present in the genome of the recombinant virus (Fig. 8B). The ability of vAM409 recombinant virus to replicate in cell culture was analyzed by one-step growth curve analysis. Wild-type (ATCC) GPCMV and vAM409 virus were used to infect confluent monolayers of GPL cells at an MOI of 0.5 PFU/cell. Samples were harvested at multiple time points postinfection, and plaque titers were determined. A comparison of replication kinetics of vAM409 against wild-type virus revealed a slight diminution of viral replication, although the virus was still capable of growing to a high titer (Fig. 9). Finally, to verify that the vAM409 recombinant failed to express GP83 protein, Western blot analysis was performed using a GP83-specific antiserum. As indicated in Fig. 10, the vAM409 deletion mutant did not express GP83, as evidenced by absence of a signal at Mr ∼70 in virus particles prepared from vAM409-infected cells, in comparison to wild-type GPCMV-infected cells.
FIG. 8.
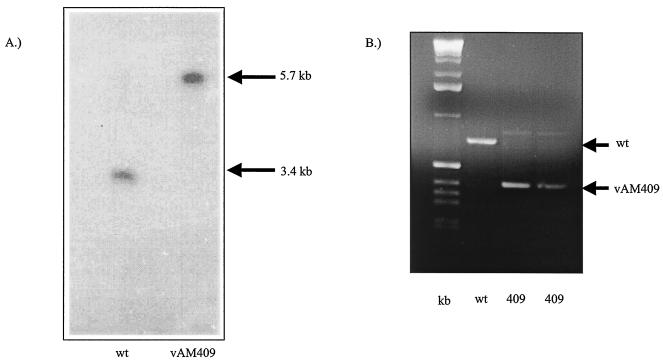
Analysis of the genomic configuration of the GP83 deletion virus. (A) Southern blot analysis of the vAM409 GPCMV recombinant. Viral DNA digested with BamHI was run on an agarose gel (0.7%), transferred to nylon membrane, and hybridized with a GP83 probe (pKTS409 [49]). Lane 1, wild type; lane 2, vAM409 DNA. The probe hybridized with a 3.4-kb BamHI subfragment of EcoRI C in wild-type DNA, but it hybridized with a larger 5.7-kb fragment in vAM409, indicating generation of recombinant virus containing a 2.3-kb gpt/EGFP cassette. (B) PCR analysis of recombinant genome and wild-type virus to verify incorporation of out-of-frame deletion (XmnI internal GP83 collapse). PCR of the region flanking the internal XmnI sites on GPCMV GP83 in both vAM409 and wild-type GPCMV demonstrated a deletion of ∼250 bp introduced into the GP83 mutant (vAM409). Lane 1, kilobase ladder; lane 2, wild-type CMV DNA; lanes 3 and 4, duplicate PCR analyses of vAM409 DNA.
FIG. 9.
One-step growth curve of the vAM409 mutant (▪) versus wild-type virus (▴). Wild-type GPCMV and the GP83 mutant were used to infect separate confluent cell culture monolayers at an MOI of 0.5 PFU/cell. After adsorption for 1 h at 37°C, the cells were washed and overlaid with medium and a zero time point sample was harvested. The remainder of the sample was then incubated at 37°C, with additional samples taken at the indicated times (12, 24, 36, 60, and 72 h postinfection). The viral titer of each time point was determined by plaque titration assay on GPL cells. Data shown are reproducible, representative results of several experiments.
FIG. 10.
Expression analyses of vAM409 and wild-type GPCMV. (A) Western blot analysis of wild-type and vAM409 (GP83 deletion) virus. Virus particles were purified from infected cell supernatants (49) and subjected to SDS-12% polyacrylamide gel electrophoresis, transferred onto nylon membrane, and probed with either anti-GPCMV antiserum or monospecific anti-GP83 polyclonal antisera (49). Anti-GPCMV antiserum was immunoreactive with multiple protein species in both wild-type and vAM409 particles (lanes 1 and 2). The band corresponding to GP83 protein (∼70 kDa) was detected only in the wild-type-infected virus particles (lane 3) by using the monospecific antiserum and was missing from vAM409 particles (lane 4). Lysates prepared from mock-infected cells were not immunoreactive with this antibody (49). Positions of molecular mass markers (kilodalton ladder) are indicated. (B) RT-PCR analysis of GP82 transcription. GP82-specific primers as described in Materials and Methods were used to amplify RNA (2 μg) from uninfected, wild-type GPCMV-infected, or vAM409-infected cells at 72 h postinfection. Guinea pig rRNA primers (2) were used as a positive control. Lane 1, kilobase ladder; lane 2, GP82 RT-PCR product, wild-type-infected cells; lane 3, rRNA amplification product, wild-type-infected cells; lane 4, GP82 RT-PCR product, vAM409-infected cells; lane 5, rRNA amplification product, vAM-409-infected cells; lane 6, GP82 RT-PCR product, uninfected cell RNA; lane 7, rRNA amplification product, uninfected cell RNA; lane 8, GP82 RT-PCR amplification product, no-reverse transcriptase control, wild-type-infected cells. RNA from both wild-type- and vAM-infected cells was also negative to rRNA RT-PCR amplification in the absence of reverse transcriptase (data not shown).
Because of the importance of the GP82 protein in the virus life cycle (as evidenced by inability to propagate a deletion mutant), we next examined whether GP82 mRNA synthesis was perturbed in the GP83 deletion virus, vAM409. Since GP82 is synthesized from a bicistronic mRNA initiated from the upstream GP83 promoter (49), it was important to examine whether insertion of the gpt/EGFP cassette in the GP83 knockout virus impaired the ability of GP82 mRNA to be generated. We examined this in wild-type and vAM409-infected cells using RT-PCR (Fig. 10). To this end, RT-PCR (Fig. 10) demonstrated that there were not global alterations in the ability of GP82-specific transcript to be synthesized, thus implying that the viral phenotype was due to loss of GP83. Successful detection of GP82 transcripts in vAM409-infected cells suggested that the phenotype of GP83 deletion virus was related to the loss of the GP83 gene and was not due to impairment in the ability of the mutant virus to generate GP82 transcript. To further confirm that the growth phenotype was due to deletion of GP83, a rescue virus was generated against the background of the vAM409 virus. This rescue virus replicated in tissue culture with kinetics identical to that of wild-type virus (McGregor, unpublished). Thus, GP83, like HCMV UL83, is dispensable (53). A GP83 deletion results in a modest growth impairment in vitro, which does not appear to be secondary to any alteration in the ability of GP82 transcripts to be generated, and this growth phenotype can be rescued by generation of a revertant virus.
The vAM409 recombinant is impaired in its ability to produce disease in immunocompromised guinea pigs.
To assess if the function of the pp65 protein had a role to play in the pathogenicity of the virus in the guinea pig model, analyses were performed in strain 2 guinea pigs with either wild-type GPCMV, vAM409 virus, or another mutant virus, vAM403, which was previously described (33). The vAM403 mutant carries the gpt/EGFP cassette in the HindIII N locus of the virus and behaves like wild-type virus in vivo; hence, it is a good control for the insertion of the gpt/EGFP cassette into the viral genome. Cyclophosphamide-treated animals were inoculated intraperitoneally with 107 PFU of viral stock. Animals were monitored for weight loss and other signs of illness following viral inoculation. Guinea pigs were sacrificed at 10 and 21 days postinoculation, and virus load was quantified by coculture of organ homogenate in tissue culture in GPL cells (spleen, liver, and salivary glands). Viral titers were examined in spleen, liver, and salivary glands and lung homogenates from day 21 samples. In addition, qcPCR was performed on selected tissue homogenates (liver and spleen) at 21 days postinoculation, in order to quantify viral load and compare viral load among animals inoculated with the wild type, vAM403, and vAM409. Animals which were inoculated with either wild-type virus or vAM403 had significant weight loss, with a mean weight losses of 58 g for wild-type animals and 42 g for vAM403 animals at day 10 and losses of 94 and 68 g, respectively, at day 21 postinoculation (Fig. 11). In contrast, animals infected with an identical inoculum of vAM409 had weight gains of 25 and 76 g at days 10 and 21 postchallenge, respectively (P < 0.05 versus wild-type and vAM403, one-way analysis of variance [ANOVA] with Bonferroni correction). The vAM409-infected animals also had impairment in viral dissemination, evidenced by a reduction in overall GPCMV viral load in tissue. Viral recovery by tissue culture was compared in tissues (salivary glands, liver, spleen, and lung) collected from infected animals sacrificed at 10 or 21 days postinoculation (Fig. 11). These analyses indicated statistically significant reductions of viral titers of vAM409 compared to wild-type and vAM403 virus in both salivary gland and liver at days 10 and 21 (Fig. 11) (P < 0.05, Kruskal-Wallis test). Significant reductions in lung titer in vAM409-infected animals compared to wild type were also noted at day 21, in spleen at day 10, and in spleen (compared to vAM403) at day 21. Determination of viral load by qcPCR of spleen and liver tissues harvested at day 21 was undertaken to corroborate the tissue culture findings. Although DNA could be detected by qcPCR in vAM409-infected animals, viral load was approximately ∼0.75 log10 genome lower than for the equivalent homogenates from vAM403- or ATCC-infected animals. In liver, mean viral load was 3.3 ± 0.5 log10 genomes for vAM409, compared to 4.1 ± 0.1 log10 genomes for vAM403 and ATCC (P = 0.06, Kruskal-Wallis test). In spleen similar levels were observed, with a mean viral load of 3.25 ± 0.5 log10 genomes observed for vAM409, compared to a 4.1 ± 0.1 log10 genome viral load for vAM403 and a 4.0 ± 0.1 log10 genome viral load observed for the ATCC-inoculated animals (P < 0.05, Kruskal-Wallis test).
DISCUSSION
Since CMV is species specific, HCMV cannot be studied in animal models. Hence, animal CMVs must be used to study the in vivo pathogenesis of infection. Among the small-animal models of CMV infection, GPCMV has unique advantages, as it is the only small-animal model of congenital CMV infection. However, the GPCMV model does suffer from the lack of characterization at the molecular level (15), and only a limited number of mutants are available to study gene function (33, 34). We have attempted to rectify this failing by identification of homolog genes and the generation of mutant viruses to study gene function. Here we further develop the GPCMV model by analysis of the genes encoding the GPCMV homologs of the upper and lower matrix proteins of HCMV, pp71 (UL82) and pp65 (UL83).
In both GPCMV and HCMV, these proteins are components of the viral tegument. In HCMV, UL82 has an essential role that relates to its ability to function as a transactivating protein, which enables transcription of the IE proteins for the initiation of viral replication. However, to date there has not been any detailed description of a transactivating function associated with UL82 homologs of any other related CMVs. We found that GP82 possesses functions similar to those of HCMV pp71 in that it is capable of enhancing the infectivity and plaque formation efficiency of CMV viral DNA. The striking acceleration of plaque development (following viral DNA transfection) in the presence of pGP82 is similar to findings obtained in HCMV (3). The inability to generate a viable GP82 mutant by conventional strategy or BAC mutagenesis, in contrast to GP83, emphasizes the essential function of GP82, as is the case with HCMV UL82 (8; McGregor, unpublished). In an effort to demonstrate the transactivating function of the GPCMV pp71 homolog, we developed a transactivation assay based on the ability of pp71 to complement the growth of an HSV VP16 transactivating mutant via cotransfection of HSV viral DNA and a pGP82-expressing plasmid. This assay was based on the initial finding by Homer et al. (24), who described the ability of HCMV pp71 to complement HSV in the context of a recombinant HSV devoid of VP16 transactivation function carrying the pp71 gene product. We found, using HSV viral DNA in cotransfection assays, that wells cotransfected with HSV DNA and expression plasmid encoding GP82 HSV virus grew in a manner similar to those wells where the HSV VP16 protein was coexpressed. In other wells, no virus was detected. This approach has the potential to enable characterization and functional study of pp71 homologs in their appropriate species backgrounds, since HSV has a wide host range, unlike the CMVs. This effect may be important in the initial stages of GPCMV viral replication, during which the GP82 tegument protein, similar to HCMV UL82, likely plays a critical role in the viral life cycle (8). The CMV UL82 protein family, in addition to providing a transactivation function similar to HSV VP16, also plays a role in the interaction with ND10 bodies in infected cells and in formation of viral transcription centers (23, 26). Recently, a number of cellular proteins were identified which interact with UL82 via the yeast two-hybrid system (23). These results, combined with transient-expression assays of an EGFP-UL82 fusion in human cells, demonstrated that pp71 interaction with the Daxx protein, a component of ND10 domains, is the most important interaction for the recruitment of pp71 into ND10 domains and the subsequent formation of viral transcription centers (23). Further evidence in support of the importance of pp71 interaction with Daxx has been provided by Ishov et al. (26), who used Daxx-negative cell lines, where pp71 could not interact with ND10 bodies in the absence of Daxx. We are currently investigating the ability of GPCMV GP82 and MCMV M82 proteins to interact with components of ND10 bodies.
Similar to HCMV UL83, we found that GP83 was dispensable for growth in tissue culture (53). Deletion of GP83 had a slight effect on replication in tissue culture (overall reduction of between 0.5 and 1 log10 viral recovery in one-step growth curve analyses). However, the recombinant virus was severely attenuated for in vivo dissemination and disease in guinea pigs, in comparison to both wild-type virus and another mutant virus without a structural gene knockout. Although the reason for the high degree of attenuation of the vAM409 mutant for in vivo replication is unclear, the virus is undoubtedly attenuated due to the deletion of the GP83 protein, since GP82 transcript was detected in infected cells (Fig. 10). It is of note in this context that we have observed that a recently generated UL83 knockout virus derived from a clinical HCMV BAC isolate is similarly impaired in tissue culture, unlike mutants generated against attenuated strains such as AD169 (53; McGregor, unpublished). Furthermore, we were able to rescue this growth deficit by generation of a revertant virus against the background of vAM409 (McGregor, unpublished). The fact that another recombinant virus, vAM403, was able to replicate in vivo with a phenotype identical to wild-type virus suggests that attenuation does not occur simply secondary to insertion of a gpt/EGFP cassette into the viral genome. The reason for the severe attenuation of vAM409 in vivo is not clear. The most straightforward explanation is that the virus disseminates less efficiently because of its impairment in replication. However, the in vitro impairment in replication was modest, making it likely that the severe impairment in dissemination was due to functions played by the GP83 in vivo. Since interactions between endothelial cells and leukocytes involving trafficking of HCMV pp65 appear important in viral tropism (20), deletion of GP83 may modify the ability of the virus to disseminate in these cell types.
Alternatively, impaired replication of vAM409 virus in vivo could be consequent to a modified virus-host interaction conferred by deletion of the GP83 protein. Potentially, deletion of GP83 may confer improved innate immune clearance of virus. The initial interaction of HCMV with fibroblasts triggers innate immune responses which lead to the induction of interferon (IFN)-responsive genes (40). However, this effect is rapidly modulated in part by UL83 (pp65), which is capable of inhibiting IFN-mediated induction (9). DNA microarray analysis demonstrated that infection with an HCMV pp65-deficient mutant virus caused a much stronger induction of many IFN-response RNAs than infection with wild-type virus (9). Similar mechanisms could account for the phenotype of the more rapid clearance and diminished disease produced by the GPCMV GP83 deletion mutant, compared to wild-type GPCMV, following in vivo inoculation. Alternatively, the GP83 deletion virus may be more efficiently cleared by the immune system through other mechanisms. HCMV UL83 facilitates immune evasion of cytotoxic T-cell recognition through blockade of IE antigen processing and presentation (16, 42). Since adaptive immune clearance mechanisms would not be expected to play a role in the immediate postinfection time window, further studies assessing clearance of the vAM409 mutant at later time points may provide insights into any putative immunoevasive function of GP83.
As already noted, one factor which could play a role in modification of the replication ability of the vAM409 mutant would be if insertion of the gpt/EGFP cassette in this virus modified the transcription of the adjacent GP82 gene (HCMV UL82 homolog). Synthesis of GP82 protein depends upon translation of the GP82 ORF as the second cistron of a bicistronic RNA in GPCMV-infected cells (49). We therefore examined transcription of GP82 message in vAM409-infected cells, using RT-PCR. These studies revealed that, in spite of insertion of the gpt/EGFP cassette into the mutant viral genome, a GP82-specific mRNA was detectable (Fig. 10). These observations are compatible with the essential role of GP82 we identified in using BAC mutagenesis to attempt to generate a GP82 deletion virus (Fig. 6), since transfection of a GP82 BAC mutant generated in E. coli could not reconstitute virus unless complemented by a GP82 expression plasmid in trans (McGregor, unpublished). We therefore conclude that GP82 is essential for viral replication and that deletion of the adjacent GP83 gene does not grossly impair the ability of the resulting mutant to express GP82 mRNA (Fig. 10). It should be noted that these analyses do not exclude the possibility that insertion of the gpt/EGFP cassette into the GP83 coding region could impact the secondary structure of the GP82-specific mRNA. Indeed, primer extension and/or RNase protection assays will be necessary to definitively identify the structure of the GP82 transcription unit in the vAM409 mutant virus, and efforts are currently in progress to develop a GP82-specific antibody to authenticate the expression of GP82 protein in the vAM409 deletion mutant.
It is of interest to compare our results with those observed in mice following inoculation of mutant knockouts of the two MCMV UL83 homologs, M83 and M84 (10, 36). In these studies, when deletion mutants in either M83 or M84 (ΔM83 and ΔM84) were inoculated intraperitoneally into BALB/c mice, both viruses showed moderate attenuation of growth in the spleen and liver, with reductions in titers of 6- to 13-fold, depending upon the organ analyzed and the viral mutant being evaluated. When salivary gland tropism was evaluated, only ΔM83 was severely growth restricted in the salivary glands (36). In a separate study of an M83 mutant in both BALB/c and SCID mice, a more widespread attenuation of the M83 mutant in the target organs assayed (salivary gland, lung, spleen, liver, and kidneys) was observed (57). These studies also indicated that the M83 mutant was attenuated in its ability to kill SCID mice in a 50% lethal dose assay. As noted, the GP83 deletion virus exhibited similar global replication defects in vivo. Overall, viral load, as quantified by qcPCR, was lower in solid organs at day 21 (liver and spleen) in vAM409-inoculated animals compared to that in animals inoculated with the wild-type (ATCC) GPCMV. The vAM409-inoculated animals did not lose weight in the same pattern exhibited by wild-type and vAM403-infected animals. A rescue virus of the vAM409 mutant had normal, wild-type genome structure by restriction profile analysis and normal replication kinetics in cell culture (data not shown). To control for the possibility that the vAM409 mutant had an altered replication and dissemination phenotype in vivo due solely to the insertion of the gpt/EGFP cassette into the viral genome, an additional control virus, vAM403, was utilized. This virus contained the gpt/EGFP cassette inserted into a noncoding region of the virus, within the HindIII N locus of the viral genome. vAM403 was not attenuated for replication or dissemination, suggesting that the attenuation phenotype of the vAM409 virus was due to deletion of the GP83 gene and not some adventitious effect attributable to insertion of the gpt/EGFP cassette into the viral genome. Additional in vivo studies comparing vAM409 with rescue (wild-type) virus will be required before we can definitively conclude that the phenotype of this virus in animals is solely due to deletion of the GP83 protein, but comparison with vAM403 excludes the possibility that the altered phenotype is related to insertion of the gpt/EGFP cassette.
Although the results of the GPCMV studies with the vAM409 virus were similar to those noted for the MCMV homologs, there were significant differences between the MCMV M83 homolog and GP83. The GP83 protein has a calculated Mr of ∼62, much smaller than the M83 homolog, which is ∼90, but comparable to the HCMV UL83, which is calculated to be ∼63 (10, 49). Furthermore, monospecific antiserum which targets M83 is immunoreactive with three different size classes of proteins, identifying species of 125, 105, and 70 kDa by Western blotting, whereas GP83- and UL83-specific antisera identify single polypeptide species. Additionally, the GP83 homolog is a constituent of dense bodies, which are present in both HCMV and GPCMV but have not been identified for MCMV (49). Therefore, the characterization of the GP83 homolog described in these studies provides information which may be of greater relevance to the biology of the HCMV UL83 protein than those results reported for MCMV.
The use of recombinant GPCMVs targeting these proteins should prove to be of value in ongoing vaccine studies in this animal model. It is of note that in MCMV vaccine studies, mice immunized with either M83 or M84 deletion viruses were noted to have protection against subsequent high-dose challenge with wild-type MCMV, indicating that immune responses against these proteins are not essential for protective immunity (36). However, in other experiments using DNA vaccines or vaccinia viruses expressing M83 or M84, it was demonstrated that M84 was capable of protecting against subsequent MCMV viral challenge, indicating that immunity to this protein is sufficient to confer some level of protection (37). Interestingly, although the M84 immune responses were protective, M83 responses were not protective (37), a surprising observation in light of the known importance of HCMV UL83 in protective immunity (6, 21, 29). The evolutionary divergence of M83 compared to HCMV UL83 may indicate that these proteins are not functional homologs, and it justifies further study of other CMV UL83 homologs, such as GP83. More will need to be learned about the targets of the cell-mediated immune response to GPCMV before the relevance of these proteins to human vaccine studies can be fully defined. In light of the impaired ability of the GP83 knockout to cause disease, this deletion mutant might be a candidate for study as a live, attenuated CMV vaccine in the GPCMV model. Study of GPCMV vaccines generated in this fashion may have implications for live, attenuated HCMV vaccine trials.
Acknowledgments
We acknowledge the gift of plasmids pQ106 (Jeff Vieira, Seattle, Wash.), pVP16+ (Richard Thompson, Cincinnati, Ohio), and pGETrec(P. Ioannou, Melbourne, Australia). We thank Greg Stroup and Nanette Huey for technical assistance and Juan Lacayo for a critical manuscript review.
This work was supported by NIH grants HD38416-01, HD44864-01, and AI65289 and March of Dimes grant 6-FY98-0416.
REFERENCES
- 1.Ace, C. I., T. A. McKee, J. M. Ryan, J. M. Cameron, and C. M. Preston. 1989. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J. Virol. 63:2260-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, S. S., and D. N. McMurray. 2003. Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs. Infect. Immun. 71:7035-7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldick, C. J., Jr., A. Marchini, C. E. Patterson, and T. Shenk. 1997. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J. Virol. 71:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berencsi, K., Z. Gyulai, E. Gonczol, S. Pincus, W. I. Cox, S. Michelson, L. Kari, C. Meric, M. Cadoz, J. Zahradnik, S. Starr, and S. Plotkin. 2001. A canarypox vector-expressing cytomegalovirus (CMV) phosphoprotein 65 induces long-lasting cytotoxic T cell responses in human CMV-seronegative subjects. J. Infect. Dis. 183:1171-1179. [DOI] [PubMed] [Google Scholar]
- 5.Bia, F. J., B. P. Griffith, C. K. Fong, and G. D. Hsiung. 1983. Cytomegaloviral infections in the guinea pig: experimental models for human disease. Rev. Infect. Dis. 5:177-195. [DOI] [PubMed] [Google Scholar]
- 6.Boppana, S. B., and W. J. Britt. 1996. Recognition of human cytomegalovirus gene products by HCMV-specific cytotoxic T cells. Virology 222:293-296. [DOI] [PubMed] [Google Scholar]
- 7.Brady, R. C., and M. R. Schleiss. 1996. Identification and characterization of the guinea-pig cytomegalovirus glycoprotein H gene. Arch. Virol. 141:2409-2424. [DOI] [PubMed] [Google Scholar]
- 8.Bresnahan, W. A., and T. E. Shenk. 2000. UL82 virion protein activates expression of immediate early viral genes in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. USA 97:14506-14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browne, E. P., and T. Shenk. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. USA 100:11439-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cranmer, L. D., C. L. Clark, C. S. Morello, H. E. Farrell, W. D. Rawlinson, and D. H. Spector. 1996. Identification, analysis, and evolutionary relationships of the putative murine cytomegalovirus homologs of the human cytomegalovirus UL82 (pp71) and UL83 (pp65) matrix phosphoproteins. J. Virol. 70:7929-7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dal Monte, P., C. Bessia, M. P. Landini, and S. Michelson. 1996. Expression of human cytomegalovirus ppUL83 (pp65) in a stable cell line and its association with metaphase chromosomes. J. Gen. Virol. 77:2591-2596. [DOI] [PubMed] [Google Scholar]
- 12.Davison, A. J., A. Dolan, P. Akter, C. Addison, D. J. Dargan, D. J. Alcendor, D. J. McGeoch, and G. S. Hayward. 2003. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 84:17-28. [DOI] [PubMed] [Google Scholar]
- 13.Endresz, V., L. Kari, K. Berencsi, C. Kari, Z. Gyulai, C. Jeney, S. Pincus, U. Rodeck, C. Meric, S. A. Plotkin, and E. Gonczol. 1999. Induction of human cytomegalovirus (HCMV)-glycoprotein B (gB)-specific neutralizing antibody and phosphoprotein 65 (pp65)-specific cytotoxic T lymphocyte responses by naked DNA immunization. Vaccine 17:50-58. [DOI] [PubMed] [Google Scholar]
- 14.Gallina, A., E. Percivalle, L. Simoncini, M. G. Revello, G. Gerna, and G. Milanesi. 1996. Human cytomegalovirus pp65 lower matrix phosphoprotein harbours two transplantable nuclear localization signals. J. Gen. Virol. 77:1151-1157. [DOI] [PubMed] [Google Scholar]
- 15.Gao, M., and H. Isom. 1984. Characterization of the guinea pig cytomegalovirus genome by molecular cloning and physical mapping. J. Virol. 52:436-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert, M. J., S. R. Riddell, B. Plachter, and P. D. Greenberg. 1996. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature 383:720-722. [DOI] [PubMed] [Google Scholar]
- 17.Grefte, J. M., B. T. van-der-Gun, S. Schmolke, M. van-der-Giessen, W. J. van-Son, B. Plachter, G. Jahn, and T. H. The. 1992. The lower matrix protein pp65 is the principal viral antigen present in peripheral blood leukocytes during an active cytomegalovirus infection. J. Gen. Virol. 73:2923-2932. [DOI] [PubMed] [Google Scholar]
- 18.Grefte, J. M. M., M. C. Harmsen, M. van-der-Giessen, S. Knollema, W. J. van-Son, and T. H. The. 1994. Presence of human cytomegalovirus (HCMV) immediate early mRNA but not ppUL83 (lower matrix protein) mRNA in polymorphonuclear and mononuclear leukocytes during active HCMV infection. J. Gen. Virol. 75:1989-1998. [DOI] [PubMed] [Google Scholar]
- 19.Griffith, B. P., and M. J. Aquino-de Jesus. 1991. Guinea pig model of congenital cytomegalovirus infection. Transplant Proc. 23:29-31. [PubMed] [Google Scholar]
- 20.Grundy, J. E., K. M. Lawson, L. P. MacCormac, J. M. Fletcher, and K. L. Yong. 1998. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophil transendothelial migration. J. Infect. Dis. 177:1465-1474. [DOI] [PubMed] [Google Scholar]
- 21.Gyulai, Z., V. Endresz, K. Burian, S. Pincus, J. Toldy, W. I. Cox, C. Meric, S. Plotkin, E. Gonczol, and K. Berencsi. 2000. Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-Exon4, gB, pp150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs. J. Infect. Dis. 181:1537-1546. [DOI] [PubMed] [Google Scholar]
- 22.Hensel, G. M., H. H. Meyer, I. Buchmann, D. Pommerehne, S. Schmolke, B. Plachter, K. Radsak, and H. F. Kern. 1996. Intracellular localization and expression of the human cytomegalovirus matrix phosphoprotein pp71 (ppUL82): evidence for its translocation into the nucleus. J. Gen. Virol. 77:3087-3097. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann, H., H. Sindre, and T. Stamminger. 2002. Functional interaction between the pp71 protein of human cytomegalovirus and the PML-interacting protein human Daxx. J. Virol. 76:5769-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homer, E. G., A. Rinaldi, M. J. Nicholl, and C. M. Preston. 1999. Activation of herpesvirus gene expression by the human cytomegalovirus protein pp71. J. Virol. 73:8512-8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irmiere, A., and W. Gibson. 1983. Isolation and characterization of a non-infectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology 130:118-133. [DOI] [PubMed] [Google Scholar]
- 26.Ishov, A. M., O. V. Vladimirova, and G. G. Maul. 2002. Daxx-mediated accumulation of human cytomegalovirus tegument protein pp71 at ND10 facilitates initiation of viral infection at these nuclear domains. J. Virol. 76:7705-7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson, K. P. 1969. Mouse cytomegalovirus: placental infection. J. Infect. Dis. 120:445-450. [DOI] [PubMed] [Google Scholar]
- 28.Kalejta, R. F., J. T. Bechtel, and T. Shenk. 2003. Human cytomegalovirus pp71 stimulates cell cycle progression by inducing the proteasome-dependent degradation of the retinoblastoma family of tumor suppressors. Mol. Cell. Biol. 23:1885-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kern, F., T. Bunde, N. Faulhaber, F. Kiecker, E. Khatamzas, I. M. Rudawski, A. Pruss, J. W. Gratama, R. Volkmer-Engert, R. Ewert, P. Reinke, H. D. Volk, and L. J. Picker. 2002. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J. Infect. Dis. 185:1709-1716. [DOI] [PubMed] [Google Scholar]
- 30.Marshall, K. R., K. V. Rowley, A. Rinaldi, I. P. Nicholson, A. M. Ishov, G. G. Maul, and C. M. Preston. 2002. Activity and intracellular localization of the human cytomegalovirus protein pp71. J. Gen. Virol. 83:1601-1612. [DOI] [PubMed] [Google Scholar]
- 31.McFarlane, M., J. I. Daksis, and C. M. Preston. 1992. Hexamethylene bisacetamide stimulates herpes simplex virus immediate early gene expression in the absence of trans-induction by Vmw65. J. Gen. Virol. 73:285-292. [DOI] [PubMed] [Google Scholar]
- 32.McGregor, A., A. Roberts, R. W. Davies, J. B. Clements, and A. R. MacLean. 1999. Rapid generation of recombinant herpes simplex virus vectors expressing the bacterial lacZ gene under the control of neuronal promoters. Gene Ther. Mol. Biol. 4:193-202. [Google Scholar]
- 33.McGregor, A., and M. R. Schleiss. 2001. Molecular cloning of the guinea pig cytomegalovirus (GPCMV) genome as an infectious bacterial artificial chromosome (BAC) in Escherichia coli. Mol. Gen. Metab. 72:15-26. [DOI] [PubMed] [Google Scholar]
- 34.McVoy, M. A., D. E. Nixon, and S. P. Adler. 1997. Circularization and cleavage of guinea pig cytomegalovirus genomes. J. Virol. 71:4209-4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lipppincott Williams and Wilkins, Philadelphia, Pa.
- 36.Morello, C. S., L. D. Cramer, and D. H. Spector. 1999. In vivo replication, latency, and immunogenicity of murine cytomegalovirus mutants with deletions in the M83 and M84 genes, the putative homologs of human cytomegalovirus pp65 (UL83). J. Virol. 73:7678-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morello, C. S., L. D. Cranmer, and D. H. Spector. 2000. Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84 (a homolog of human cytomegalovirus pp65). J. Virol. 74:3696-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nefedov, M., R. Williamson, and P. A. Ioannou. 2000. Insertion of disease-causing mutations in BACs by homologous recombination in Escherichia coli. Nucleic Acids Res. 28:E79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pepperl, S., J. Munster, M. Mach, J. R. Harris, and B. Plachter. 2000. Dense bodies of human cytomegalovirus induce both humoral and cellular immune responses in the absence of viral gene expression. J. Virol. 74:6132-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 75:8909-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rawlinson, W. D., H. E. Farrell, and B. G. Barrell. 1996. Analysis of the complete DNA sequence of murine cytomegalovirus. J. Virol. 70:8833-8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redhasse, M. J. 2000. The immunogenicity of human and murine cytomegaloviruses. Curr. Opin. Immunol. 12:390-396. [DOI] [PubMed] [Google Scholar]
- 43.Riddell, S. R., B. A. Walter, M. J. Gilbert, and P. D. Greenberg. 1994. Selective reconstitution of CD8+ cytotoxic T lymphocyte responses in immunodeficient bone marrow transplant recipients by the adoptive transfer of T cell clones. Bone Marrow Transplant 14(Suppl. 4):S78-S84. [PubMed] [Google Scholar]
- 44.Rixon, F. J., C. Addison, A. McGregor, S. J. Macnab, P. Nicholson, V. G. Preston, and J. D. Tatman. 1996. Multiple interactions control the intracellular localization of the herpes simplex virus type 1 capsid proteins. J. Gen. Virol. 77:2251-2260. [DOI] [PubMed] [Google Scholar]
- 45.Roizmann, B., and A. E. Sears. 1996. Herpes simplex viruses and their replication, p. 2231-2295. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 46.Ruger, B., S. Klages, B. Walla, J. Albrecht, B. Fleckenstein, P. Tomlinson, and B. Barrell. 1987. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J. Virol. 61:446-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez, V., P. C. Angeletti, J. A. Engler, and W. J. Britt. 1998. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J. Virol. 72:3321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schleiss, M. R. 1994. Cloning and characterization of the guinea pig cytomegalovirus glycoprotein B gene. Virology 202:173-185. [DOI] [PubMed] [Google Scholar]
- 49.Schleiss, M. R., A. McGregor, N. J. Jensen, G. Erdem, and L. Aktan. 1999. Molecular characterization of the guinea pig cytomegalovirus UL83 (pp65) protein homolog. Virus Genes 19:205-221. [DOI] [PubMed] [Google Scholar]
- 50.Schleiss, M. R., N. Bourne, N. J. Jensen, F. Bravo, and D. I. Bernstein. 2000. Immunogenicity evaluation of DNA vaccines that target guinea pig cytomegalovirus proteins glycoprotein B and UL83. Viral Immunol. 13:155-167. [DOI] [PubMed] [Google Scholar]
- 51.Schleiss, M. R., N. Bourne, F. Bravo, N. J. Jensen, and D. I. Bernstein. 2003. Quantitative competitive PCR (qcPCR) analysis of viral load following experiment guinea pig cytomegalovirus (GPCMV) infection. J. Virol. Methods 108:103-110. [DOI] [PubMed] [Google Scholar]
- 52.Schmolke, S., P. Drescher, G. Jahn, and B. Plachter. 1995. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J. Virol. 69:1071-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmolke, S., H. F. Kern, P. Drescher, G. Jahn, and B. Plachter. 1995. The dominant phosphoprotein pp65 (UL83) of human cytomegalovirus is dispensable for growth in cell culture. J. Virol. 69:5959-5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Staczek, J. 1990. Animal cytomegaloviruses. Microbiol. Rev. 54:247-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vieira, J., T. J. Schall, L. Corey, and A. P. Geballe. 1998. Functional analysis of the human cytomegalovirus US28 gene by insertion mutagenesis with the green fluorescent protein gene. J. Virol. 72:8158-8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vink, C., E. Beuken, and C. A. Bruggeman. 2000. Complete DNA sequence of the rat cytomegalovirus genome. J. Virol. 74:7656-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinheimer, S. P., B. A. Boyd, S. K. Durham, J. L. Resnick, and D. R. O'Boyle. 1992. Deletion of the VP16 open reading frame of herpes simplex virus type 1. J. Virol. 66:258-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhan, X., M. Lee, J. Xiao, and F. Liu. 2000. Construction and characterization of murine cytomegaloviruses that contain transposon insertions at open reading frames m09 and M83. J. Virol. 74:7411-7421. [DOI] [PMC free article] [PubMed] [Google Scholar]