Abstract
The human cytomegalovirus (HCMV) UL76 gene encodes a highly conserved herpesvirus protein, pUL76, which is able to modulate gene expression in either activation or repression. In this study, two specific transcripts were found to contain the reading frame of UL76, one a 4.5-kb and the other a 5.5-kb tricistronic mRNA encoding the UL76, UL77, and UL78 open reading frames. Both transcripts were expressed with true late kinetics, as revealed by data showing inhibition of production in the presence of phosphonoformic acid. Immediately after viral infection, pUL76 was found in the nuclear fraction and was detected in cells in the presence of the protein synthesis inhibitor cycloheximide. Subsequent virus particle purification and Western blot analysis revealed that two forms of pUL76 are associated within mature virions. The high-molecular-mass protein (H-pUL76) was verified as originating from a free form of pUL76 by cross-linking with an unknown protein(s). By performing a biochemical fractionation experiment with purified virions, we provide evidence that pUL76 and H-pUL76 are associated with the detergent-soluble (envelope) and -insoluble (tegument/capsid) fractions, respectively. Both results were consistent with the images exhibited by immunoelectron microscopy, which showed that the distribution of gold particles labeled by the anti-pUL76 antibody juxtaposed the compartments of the envelope and the tegument/capsid of the virion. Evidence indicated that expression of pUL76 at the immediate-early phase of the viral replication cycle leads to the inhibition of HCMV production. The viral constituent pUL76, with a dominant-negative effect on replication, may provide a novel mechanism for HCMV's resumption of latency.
Human cytomegalovirus (HCMV), a ubiquitous pathogen, is a betaherpesvirus. Like other herpesviruses, HCMV may remain as a lifelong asymptomatic infection in healthy persons who have acquired the virus. However, the reactivation of latent HCMV is often linked to a myriad of clinical manifestations, including lung infection, retinitis, and coronary restenosis. Severe clinical complications occur in immunocompromised patients, such as human immunodeficiency virus-infected persons, organ transplant recipients, and infected fetuses (37, 43).
HCMV is characterized by two distinct features, a highly restrictive host range and a lengthy replication period. As the largest virus in the herpesvirus family, the HCMV genome comprises ≈230 kb of double-stranded linear DNA (11, 12, 15, 35, 36, 40, 52). During the lytic replication cycle, the viral genes are expressed in a cascade mode. Sequentially appearing during the replication cycle, the immediate-early gene products are the first to be synthesized, and they govern the production of early and late gene products. After viral maturation, three morphologically distinct particles are released from the infected cells. The infectious particle, the virion, is packaged with the viral genome. The two noninfectious particles, noninfectious enveloped particle and dense body, are devoid of the genome (4, 20, 27, 46).
In the virion particle, the outermost envelope is composed of a lipid bilayer incorporating viral glycoproteins (7). There is an icosahedrally symmetric capsid within the central core; the space between the capsid and the envelope, designated the tegument, can be divided into two parts. The innermost layer of tegument consists of three phosphorylated proteins, the basic phosphoprotein, the upper matrix protein (ppUL82), and ppUL99. These proteins are encoded by UL32, UL82, and UL99, respectively (13). The rest of the tegument space seems to be an amorphously organized area with more than 20 viral proteins (2). Furthermore, recent findings reveal that selective subsets of viral and cellular RNAs are packaged within herpesvirus particles. These disclose the complexity of viral composition (6, 21, 39, 42). Significantly, studies have illustrated that these virion-associated proteins and RNAs may participate in the initial events in the establishment of infection and that they play roles in the alteration of cellular and viral gene expression, initiation of viral replication, disassembly of virion particles, blockade of the cell cycle, and modulation of the immune response (1, 6, 8, 9, 22, 31, 53-55).
The UL76 protein of HCMV is a member of a highly conserved protein family of herpesviruses notable for its high content of basic amino acids (16). In an effort to investigate the function of UL76, we reviewed the published documents related to the family. The UL24 proteins of herpes simplex virus type 1 as well as herpes simplex virus type 2 appear to be present predominantly in the nucleus (25, 38), and the latter was shown to be associated with a purified virion and C-capsid (25). Known to be a nonessential gene in replication (30, 41), the role of UL24 may be critical for acute replication in trigeminal ganglia and reactivation from latency in experimental animals, which indicates that UL24 might be involved in viral replication (29). Moreover, a syncytial plague phenotype (syn) is displayed when certain cell types are infected with viruses mutated in UL24 (30, 47), suggesting that the UL24 protein may be associated with the membrane.
In this report, we provide evidence that transcripts encoding the HCMV UL76 open reading frame (ORF) express true-late kinetics and that the UL76-derived proteins are constituents of viral particles. Specifically, within mature virions, pUL76 is associated with the viral envelope fraction, and a modified form, H-pUL76, is present in the tegument/capsid fraction. Previously, UL76 was cloned for its ability to modulate the expression of the major immediate-early gene promoter (UL122 to UL123) in a dual mode involving both activation and repression (49). In this study, we further demonstrate that exogenous viral pUL76 is delivered to the nuclear fraction, suggesting that pUL76 has the potential to mediate gene regulation immediately after infection. Cells expressing pUL76 at the immediate-early stage display inhibition of HCMV production and repression of viral replication. The dominant inhibitory effect of UL76 on expression might explain the long replication cycle of HCMV and might also provide a novel mechanism for the virus to enter a latent state.
MATERIALS AND METHODS
Construction of plasmids.
For the construction of specific riboprobes used in transcriptional analysis, DNA fragments around the UL76 region were cloned into pBK-CMV (see Fig. 1). The complete coding content of UL76, a 1.2-kb SmaI-SmaI fragment (nucleotides 110228 to 111437; coordinate nucleotides of HCMV AD169 sequence, accession number X17403), was subcloned, and the resulting plasmid was designated pRB-UL76. The construct containing the UL76-specific sequence, a 466-bp SmaI-PstI fragment (nucleotides 110228 to 110694), was designated pRB-A, and pRB-B harbored the 5′ noncoding sequence of UL76, comprising a 1.62-kb HindIII-SpeI fragment (nucleotides 107667 to 109288). The construct containing the UL77-specific sequence, a 500-bp SalI-SacI fragment (nucleotides 112129 to 112629), was designated pRB-C. The construct containing the UL78-specific sequence, a 439-bp SalI-PstI fragment (nucleotides 113266 to 113705), was designated pRB-D. Plasmid pHcGAP, encoding cDNA for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was obtained from the American Type Culture Collection (Rockville, Md.) (48). An internal 0.78-kb PstI-XbaI fragment of the GAPDH gene was subcloned into pGEM-3Z, and the resulting plasmid was designated pGAPDH-G.
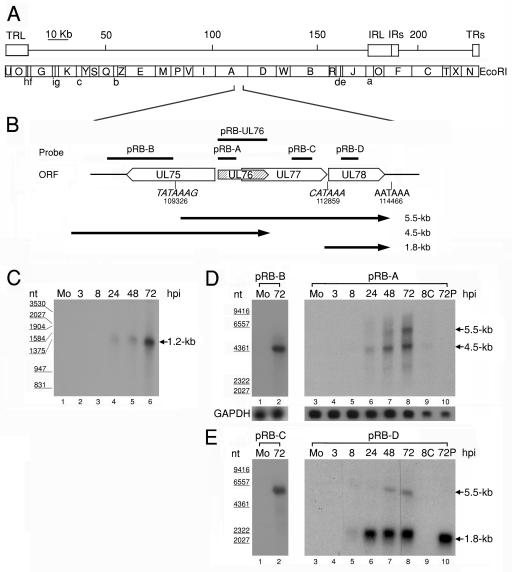
FIG. 1.
(A) Schematic diagram of EcoRI physical maps in the HCMV AD169 genome. The scale indicates the genomic position. The genomic arrangement around UL76 is enlarged in the diagram. (B) Schematic depiction of the genomic region from UL75 to UL78 with the putative transcriptional TATA boxes (nucleotides 109326 and 112859) and the potential poly(A) termination signal (nucleotides 114466). The putative transcripts are indicated by black lines with arrows below the ORFs. Lengths are shown on the right (in kilobases). (C) RNase protection analysis with poly(A)+ RNA purified during the HCMV replication cycle was employed to detect RNA encompassing the UL76 coding sequence. A radiolabeled UL76 antisense transcript complementary to the full-length coding sequence was generated from pRB-UL76 and used for the analysis. Lanes: 1, poly(A)+ RNA from mock-infected cells (Mo); 2 to 6, poly(A)+ RNA harvested at 3, 8, 24, 48, and 72 h postinfection, respectively. The sizes of molecular mass markers are indicated in nucleotides (nt) on the left. Northern blot analyses of UL76 transcriptional expression kinetics during the HCMV replication cycle are shown. Samples of poly(A)+ RNA were electrophoretically separated with a 1% formaldehyde agarose gel. (D) Single-stranded riboprobes specific for UL76 sequence (pRB-A) and the 5′ noncoding sequence of UL76 (pRB-B) were used for hybridization. (E) Northern blots were probed with sequences specific for UL77 (pRB-C) and UL78 (pRB-D). Lanes: 1 and 3, 1 μg of poly(A)+ RNA from mock-infected cells (Mo); 2, 1 μ of poly(A)+ RNA harvested at 72 h postinfection; 3 to 8, 1 μg of poly(A)+ RNA harvested at 3, 8, 24, 48, and 72 h postinfection, respectively; 9, 0.5 μg of poly(A)+ RNA harvested at 8 h postinfection in the presence of cycloheximide (100 μg/ml); 10, 0.5 μg of poly(A)+ RNA harvested at 72 h postinfection in the presence of phosphonoformic acid (150 μg/ml). The transcriptional levels of GAPDH were monitored as an internal control. The sizes of molecular size markers are indicated on the left.
For expression in eukaryotic cells, the UL76 ORF was placed under the control of an HCMV major immediate-early gene promoter. For cloning of the construct, the upstream T3 promoter of pRB-UL76 was deleted by removal of the NheI-SpeI fragment, and the resulting plasmid was designated pUL76-CMV. Plasmid pGEM-IE1, encoding HCMV major IE1 cDNA, was kindly provided by John Sinclair (Cambridge University, United Kingdom) and used to assess the accumulation of HCMV DNA.
Cells, transfection, growth curve, viruses, and titration.
Human embryonic lung cells (HEL 299), human glioblastoma U-373 MG cells, and HCMV strain AD169 (VR-538) were purchased from the American Type Culture Collection. Cell cultures were maintained as described previously (49). To establish stably expressing cells, pUL76-CMV or the cloning vector pBK-CMV was transfected into U-373 MG cells with Lipofectamine Plus (Life Biotechnology). After 48 h of transfection, transfected cells were selected by adding G418 at a concentration of 500 μg/ml. After 14 days of selection, cells resistant to G418 were cloned. The selected cells were analyzed by nucleotide sequencing and genomic Southern and Western blotting to confirm the integrity of the UL76 nucleotide sequence, the copy number of the transfected DNA, and the level of protein expression, respectively (49).
To monitor the proliferation of transfected cells, a tetrazolium salt, MTT (3-[4,5-dimethylthiazol-2-yl])-2,5-diphenyl-tetrazolium bromide), was added to cells harvested at the indicated day. In this assay, viable cells converted MTT to formazan, which was then quantified with a spectrophotometer as described previously (10). Each data point is the average of values obtained from two independent experiments.
Routinely, the virus stock was propagated at a low multiplicity of infection (MOI). To titrate infectious viral particles, the harvested viral supernatant was serially diluted and inoculated onto 90% confluent HEL cells grown in six-well culture plates. After 2 h of adsorption, 2 ml of growth medium containing 0.3% agarose was layered on top of the cells. When the incubation was extended for 10 to 21 days, plaques were counted under the microscope. Each data point of titration is a statistical value derived from at least three independent experiments.
RNase protection and Northern and Southern blot analyses.
For the analyses of the transcriptional profile from the UL76 locus, samples of total RNA were harvested from HCMV-infected HEL cells at various time points at an MOI of 2 to 3 PFU/cell, with the acid-phenol-thiocyanate protocol (14). Subsequently, polyadenylated [poly(A)+] mRNA was purified from total RNA with Oligotex resin (Qiagen). Poly(A)+ mRNA (1 μg) from each time interval was separated by electrophoresis on a 1% denaturing agarose-formaldehyde gel at 100 V in electrophoresis buffer (10 mM morpholinepropanesulfonic acid [MOPS, pH 7.0], 4.0 mM sodium acetate, 0.5 mM EDTA). The RNA was capillary transferred onto a Hybond-N+ membrane (Amersham Pharmacia Biotech) in 20× SSC [1× SSC contains 0.15 M NaCl plus 15 mM sodium citrate (pH 7.0)], fixed to the membrane by UV irradiation, and prehybridized for 6 h at 55°C in a hybridization buffer containing 50% formamide, 6× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.4]), 5× Denhardt's solution (0.1% Ficoll, 0.1% polyvinylpyrrolidone, and 0.1% bovine serum albumin), 0.5% sodium dodecyl sulfate (SDS), and 100 μg of fragmented salmon sperm DNA per ml.
Radioactively labeled riboprobes antisense to the UL76 ORF could be transcribed from linearized plasmids when plasmids pRB-A, pRB-B, pRB-C, and pRB-D were linearized by digestion with SmaI, HindIII, SalI, and SalI, respectively (45). Hybridization was performed for 16 h at 55°C in hybridization buffer with a [32P]cytidine-labeled riboprobe at 106 cpm per ml. The membrane, monitored with a Phosphorimager, was repeatedly washed with buffer containing 0.1% SDS and decreasing concentrations of SSC. Labeled bands were recorded until the radioactive background was invisible. For reprobing the membrane, the radiolabeled probe was stripped with a large volume of boiling 0.5% SDS. To determine whether UL76 expression was required before viral replication or protein synthesis, RNA samples were purified from cells cultured with the viral replication inhibitor phosphonoformic acid (150 μg/ml) or the protein synthesis inhibitor cycloheximide (100 μg/ml).
For the RNase protection assay, a radiolabeled riboprobe antisense to the complete UL76 ORF was transcribed from pRB-UL76. In each protection reaction, 1 μ of poly(A)+ RNA was mixed with a labeled riboprobe (105 cpm). The RNA mixture was pelleted and resuspended in buffer containing 40 mM PIPES [piperazine N,N′-bis(2-ethanesulfonic acid)]-sodium salt (pH 6.8), 1 mM EDTA (pH 8.0), 0.4 M NaCl, and 80% deionized formamide. To denature the RNA mixture, samples were heated at 85°C for 10 min. Next, cRNA was reannealed at 55°C for 12 h. RNase I was subsequently added to digest the remaining unprotected single-stranded RNA, and the enzymatic digestion was terminated by the addition of 0.5% SDS and 300 μg of proteinase K per ml. Following phenol-chloroform extraction, protected RNA was pelleted and resuspended in gel loading buffer containing 95% deionized formamide, 5 mM EDTA, 0.025% SDS, and trace amounts of xylene cyanol FF. Protected RNA samples were resolved on a denaturing 3.5% polyacrylamide gel (acrylamide- bisacrylamide, 29:1) containing 8 M urea. After 4 h of electrophoresis at 100 V, the gel was fixed in 10% trichloroacetic acid and dried under a heated vacuum for 2 h. The radiographic image was recorded by exposure to BioMax MS film (Eastman Kodak).
Genomic DNA samples of U-373 MG cells or cells stably transfected with pUL76-CMV or the cloning vector pBK-CMV were prepared as described previously (5). Five micrograms of genomic DNA was subjected to XmaI digestion, separated by electrophoresis on a 0.7% agarose gel at 35 V in 1× TBE (Tris-borate-EDTA) buffer for 3 h, and then capillary-transferred onto a NytranN+ membrane. As described in the previous section, probes for detecting the UL76 and GAPDH genes were generated from pRB-UL76 and pGAPDH-G, respectively.
A genomic slot blotting assay was carried out to monitor the accumulation of HCMV DNA during the infection. HCMV-infected cells and released viral particles were harvested at the indicated time points and subjected to genomic DNA purification (5). Five micrograms of each DNA sample was immobilized onto a Hybond N+ membrane with a slot blot apparatus (Bio-Dot SF; Bio-Rad). The membrane bound with DNA was probed with HCMV IE1 cDNA generated from pGEM-IE1. Subsequently, the same blot was stripped and reprobed with the GAPDH gene.
Virus-associated proteins.
HCMV-infected cells were treated with the protein synthesis inhibitor cycloheximide (100 μg/ml) 1 h before and during the period of infection in order to investigate the presence of viral constituents before the onset of viral protein synthesis. At the indicated times, cells infected with HCMV AD169 at an MOI of 2 to 3 PFU/cell were harvested, washed with phosphate-buffered saline and lysed in a buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.5% SDS) containing complete protease inhibitor cocktail (Roche). Protein extracts were recovered from the supernatant of cell lysates by centrifugation at 12,000 × g for 10 min at 4°C.
Cytoplasmic and nuclear fractions.
Mock- and HCMV-infected HEL cells were harvested. Each sample preparation contained pooled cells from 10 15-cm-diameter dishes. Nuclear and cytoplasmic/membrane fractions were prepared by following a hypotonic separation protocol essentially as described previously (17). HEL cells were infected with HCMV at an MOI of 10 PFU/cell. After washing the cells in phosphate-buffered saline, the nuclei were separated from the cytoplasm and membrane by swelling the cells for 10 min on ice in hypotonic buffer (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol) supplemented with a complete protease inhibitor cocktail. To assist the release of nuclei, swollen cells were subjected to Dounce homogenization. Nuclei were pelleted by spinning at 3,300 × g for 10 min at 4°C and washed twice in phosphate-buffered saline. The cytoplasmic/membrane fraction was cleared of contaminants by centrifugation at 25,000 × g for 1 h at 4°C. For verifying the purity of the nuclear and cytoplasmic/membrane fractions, Western blotting was carried out, and the blots were incubated with antibodies for PML protein and actin, respectively.
Viral particle purification.
The released virus particles were purified by rate velocity glycerol-tartrate gradient centrifugation as described previously (27, 46). HEL cells were grown to confluence in a 15-cm-diameter dish and inoculated with HCMV at an MOI of 1 to 2 PFU/cell in Eagle's minimal essential medium supplemented with 5% fetal bovine serum. At 4 days postinfection, the culture medium was replaced with fresh Eagle's minimal essential medium supplemented with 2% fetal bovine serum. HCMV particles were harvested from the supernatant of infected HEL cells at 7 to 8 days postinfection. Supernatant fractions were collected and cleared of cell debris by centrifugation at 1,500 × g for 15 min. Viral particles were further concentrated in a stirred-cell unit (model 8200; Millipore) by filtering them through a PM-100 membrane with a cutoff of 100 kDa. The concentrated supernatant was layered onto 9-ml sodium tartrate-glycerol gradients prepared in TN buffer (50 mM Tris-HCl, 100 mM NaCl [pH 7.4]) and centrifuged at 284,000 × g for 20 min in Hitachi swing-out rotor RPS40T. Bands corresponding to noninfectious enveloped particles, virions, and dense bodies were removed by puncturing the sidewall of the tube with a needle. To ensure purity, the virion particles were subjected to additional ultracentrifugation with the same gradient. Viral fractions recovered from the gradients were diluted in 3 volumes of TN buffer, pelleted by centrifugation at 25,000 × g for 1 h at 4°C, and stored at −80°C.
Trypsin treatment of virions.
Virion protein (20 μg) was digested for 1 h at 37°C in 10 μl of trypsin reaction mixture (TN buffer supplemented with 90 μg of trypsin per ml and 1 mM CaCl2) in either the presence or absence of 1% Triton X-100. The digestion reaction was terminated by the addition of 0.5 mM phenylmethylsulfonyl fluoride and 0.5 mg of soybean trypsin inhibitor (Roche) per ml (51).
Fractionation of virion protein by detergent treatment.
Purified virion particles were incubated with 0.5% deoxycholate and 2% Nonidet P-40 (NP-40) on ice for 1 h. The protein mixture was fractionated into a detergent-soluble supernatant and an insoluble pellet by centrifugation at 25,000 × g for 1 h at 4°C (33, 51). Prior to SDS-polyacrylamide gel electrophoresis (PAGE) analysis, the viral proteins were prepared by adding equal volumes of 2× viral sample buffer (4% SDS, 10% β-mercaptoethanol, 100 mM Tris [pH 7.0], 10% glycerol, 0.005% bromophenol blue). In some experiments, 10% β-mercaptoethanol was omitted and replaced with 6 M urea, 200 mM dithiothreitol, or 135 mM iodoacetamide.
Silver staining.
Denaturing SDS-10% PAGE was employed to resolve the viral protein bands, which were visualized with the PlusOne silver staining kit (Pharmacia Biotech). After electrophoresis, the gel was fixed sequentially in a solution containing 40% ethanol and 10% acetic acid in water for 30 min and in a solution containing 10% ethanol and 5% acetic acid in water for 15 min. After immersion in a sensitized solution (10% ethanol, 0.125% glutaraldehyde, 0.2% sodium thiosulfate, 0.8 M sodium acetate) for 30 min, it was incubated in a solution containing 0.25% silver nitrate and 0.015% formaldehyde in water for 20 min, rinsed twice with double-distilled water for 1 min, and developed in 0.02% formaldehyde (37% in water) in 2.5% sodium carbonate for 2 to 5 min with shaking. At the desired intensity, the developing reaction was terminated immediately by placing the gel in a stopping solution (4 mM EDTA).
Antibodies.
The polyclonal antibody against HCMV pUL76 was described previously (49). The characterized anti-IE2 polyclonal antibody was a generous gift from Young-Sun Lin (Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan) (26). The antibodies recognizing promyelocytic leukemia (PML) protein and HCMV IE1 and IE2 were obtained from Chemicon (Temecula, Calif.). The mouse monoclonal antibodies against lower matrix protein ppUL83 (encoded by UL83), envelope glycoprotein gpUL55 (gB, encoded by UL55), DNA polymerase processivity factor ppUL44 (encoded by UL44), single-stranded-DNA-binding protein ppUL57 (encoded by UL57), and tegument protein ppUL99 (encoded by UL99) were purchased from the Goodwin Institute, Plantation, Fla. (RGI catalog nos. 1205S, 1201, 1202S, 1209, and 1207, respectively). The antibody recognizing actin was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Western blot analysis.
Equal amounts of prepared proteins were resolved on an SDS-polyacrylamide gel, and proteins were immobilized onto polyvinylidene difluoride membranes. CABS (4-[cyclohexylamino]-1-butanesulfonic acid) electrophoretic transfer buffer (25 mM [pH 11.4]) was used for the immobilization of pUL76 to polyvinylidene difluoride. For Western blotting of other proteins, transfer to polyvinylidene difluoride was performed in a buffer containing 48 mM Tris and 39 mM glycine, pH 9.2. Polyvinylidene difluoride blots were incubated with the antibodies specified in the text. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin G (Amersham Pharmacia Biotech) was diluted (1:30,000) for use as a secondary antibody. Antigen detection was accomplished by incubation with the Lumi-Light Plus Western blotting substrate (Roche), and chemiluminescent signals were recorded with BioMax light film (Eastman Kodak).
Immunoelectron microscopy.
HCMV-infected cells were seeded as described above. After being washed in phosphate-buffered saline, HEL cells harvested from 96 h postinfection were fixed in 2.25% paraformaldehyde-1.25% glutaraldehyde in 100 mM sodium cacodylate for 30 min at room temperature. Cells were contrast stained in 1% OsO4, soaked in 0.3% uranyl acetate in 50 mM maleic acid for 60 min, and subsequently embedded in Epon. Sections of 70 nm were cut, washed several times with double-distilled water, and blocked in 1% bovine serum albumin and 1% goat serum in phosphate-buffered saline. Samples were incubated with anti-pUL76 antibody (1:1,000 dilution) for 1 h before extensive washing with phosphate-buffered saline. Next, the samples were incubated overnight with a secondary antibody, 10-nm gold colloid-conjugated goat anti-rabbit immunoglobulin G antiserum (Sigma-Aldrich) (diluted 1:100 in phosphate-buffered saline containing 1% bovine serum albumin and 1% goat serum). Following several washes in phosphate-buffered saline, samples were contrast stained with lead citrate prior to examination (23).
Indirect immunofluorescent staining.
To visualize subcellular protein expression, stably transfected U-373 MG cells were seeded onto a coverslip (20 × 104 cells per well) in six-well culture plates 1 day before viral inoculation. HCMV-infected cells grown on coverslips at an MOI of 2 to 3 PFU/cell were removed at various postinfection time points, washed twice with phosphate-buffered saline, fixed with 1% paraformaldehyde in phosphate-buffered saline for 10 min, permeabilized with 0.1% NP-40 in phosphate-buffered saline on ice for 30 min, and incubated with antibodies for IE1/IE2, ppUL44, ppUL57, or ppUL99 at 37°C for 30 min in a humid chamber. After extensive washing in phosphate-buffered saline, the cells were immersed in a solution containing the secondary antibody fluorescein isothiocyanate-conjugated goat anti-mouse and -rabbit immunoglobulin G, at 37°C for 30 min. Confocal images were acquired with a laser scanning confocal microscope (Leica TCS SP2). Images were processed with Adobe Photoshop (version 4.0) software.
RESULTS
Analysis of the transcripts from the UL76 region.
The UL76 gene of HCMV encodes a regulator that modulates gene expression in a transient-expression cell culture system (49). RNase protection and Northern blot analyses were employed to examine the sizes of the transcripts found around the UL76 locus and its transcriptional expression kinetics during the HCMV infection cycle (Fig. 1A and B). In the initial experiment, the 1.2-kb riboprobe antisense to the reading frame of UL76 was generated from pRB-UL76 with an insert corresponding to the entire UL76 ORF (Fig. 1B). Following annealing of the complementary regions between the riboprobe and transcripts, those unprotected single-stranded transcripts were degraded with RNase I. The sizes of the protected transcripts were resolved on a denaturing 3.5% polyacrylamide gel containing 8 M urea. As shown in Fig. 1C, lanes 1 to 3, no signal could be detected in mock- or virus-infected cells in the early stages. The protected signal, a 1.2-kb transcript, was first observed 24 h after infection, and it increased in intensity during the late stage of the replication cycle (Fig. 1C, lanes 4 to 6). This result indicates that the transcript(s) encoded by UL76 contained the complete UL76 ORF; in other words, no apparent transcriptional initiation and termination took place within the UL76 ORF.
Northern blot experiments were subsequently carried out. Samples of poly(A)+ mRNA were prepared during the viral lytic reproduction period, resolved on 1% formaldehyde-agarose gels, and capillary-transferred to Hybond N+ membranes. The expression of GAPDH was used as an internal loading control (Fig. 1D, lower panel). For the generation of labeled riboprobes, plasmids harboring various fragments around the UL76 locus were constructed in the pBK-CMV vector (Stratagene) (Fig. 1B). The Northern blot was first hybridized with a UL76-specific antisense riboprobe derived from pRB-A. In this experiment, no transcript could be detected when RNA samples were harvested from mock-infected cells or cells infected with HCMV at 3 and 8 h postinfection (Fig. 1D, lanes 3 to 5). A 4.5-kb transcript was initially detected in cells infected with HCMV for 24 h, and a 5.5-kb transcript appeared at 48 h postinfection (Fig. 1D, lanes 6 and 7). The intensities of both the 4.5- and 5.5-kb transcripts increased in the late stage of the lytic cycle (Fig. 1D, lane 8). To further analyze whether these two transcripts extended from UL76 to UL77 or even to UL78, we set out the hybridization assays with UL77- and UL78-specific riboprobes derived from pRB-C and pRB-D, respectively. As shown in Fig. 1E (lanes 1, 2, 7, and 8), the signals corresponding to the 5.5-kb transcript were detected within UL77 and UL78. These results suggest that the 5.5-kb transcript encodes a tricistron encompassing the UL76, UL77, and UL78 ORFs.
To gain insight into the nucleotide sequence around UL76, we analyzed the region for potential transcriptional promoter and termination signals with the gene feature launcher programs from the Baylor College of Medicine. The TSSW program predicted a putative promoter located upstream of the UL76 ORF. The HCMV genomic sequence (nucleotides 109054 to 109347) comprises an array of transcriptional factor-binding sites, a TATA box (TATAAA), a transcription initiation site (nucleotide 109326), and a consensus polyadenylation AATAAA motif downstream of UL78 (nucleotide 114466) (Fig. 1B). The prediction indicates the synthesis of a nonpolyadenylated 5.2-kb tricistronic transcript containing the UL76, UL77, and UL78 ORFs, which is consistent with the features of the 5.5-kb mature mRNA including a polyadenylated tail. In view of our findings that the signal for the 4.5-kb transcript could only be detected by probing with pRB-A and pRB-B (Fig. 1D, lanes 2 and 6 to 8) and that the 5.5-kb transcript was absent by probing with pRB-B, we suggest that the two transcripts may utilize different transcription initiation sites (Fig. 1B).
When infected cells were treated with cycloheximide and RNA was harvested 8 h postinfection, the signals for the 4.5- and 5.5-kb transcripts could not be detected (Fig. 1D and E, lane 9). Additionally, signals for both transcripts disappeared when infected cells were grown in the presence of phosphonoformic acid (Fig. 1D and E, lane 10). Taken together, these results indicate that both transcripts are accordingly classified as true late transcripts. Moreover, a UL78-specific 1.8-kb transcript was synthesized, and it might have originated from a putative promoter upstream of the UL78 ORF (transcription initiates at nucleotide 112859) and shared a termination signal with the tricistronic transcript (Fig. 1B). Evidently, the 1.8-kb transcript was not detected under immediate-early conditions and was not inhibited by phosphonoformic acid, suggesting that it is expressed with early kinetics.
pUL76 is present in cells prior to de novo viral protein synthesis.
In our previous studies, pUL76 could be detected in cell lysates harvested at 2 h postinfection (49). This result appears contradictory to the expression of a true late UL76 gene. Recently, the groups of Shenk and Roizman presented evidence that subsets of selective transcripts are packaged within herpesvirus virion particles (6, 42). We propose a hypothesis to account for the originality of the protein: pUL76 is a virus-associated protein, which might be delivered from the viral inoculum very early after infection. To verify this speculation, we cultured HCMV-infected HEL cells in the presence of cycloheximide to inhibit protein synthesis. Cell lysates harvested at specific time points after HCMV infection were resolved by SDS-PAGE. Subsequently, Western blot analysis was performed with an anti-pUL76 antibody. As a positive control, the tegument protein ppUL83 was detected with its specific antibody. In a parallel control experiment, virus-infected cells were cultured without cycloheximide in the medium (Fig. 2A).
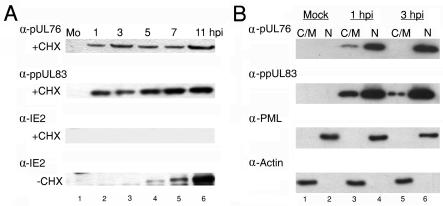
FIG. 2.
(A) Detection of pUL76 in HCMV-infected cells when protein synthesis was inhibited. For 1 h before infection with HCMV and continuing during the period of infection, cells were incubated in the presence of the protein synthesis inhibitor cycloheximide (+CHX; 100 μg/ml). Cell lysates harvested at the indicated times (hours postinfection) were subjected to Western blot analyses by incubating them with anti-pUL76, anti-ppUL83, and anti-IE2 antibodies (panels from the top down, respectively). As controls, infected cultures were grown in the absence of cycloheximide (−CHX). Cell lysates prepared at the indicated times (hours postinfection) were analyzed by Western blotting with an anti-IE2 antibody (last panel). Lanes 1 to 6, Western blotting of treated cell lysates harvested from mock-infected (Mo) cells and 1, 3, 5, 7, and 11 h postinfection with HCMV, respectively. (B) Western blot analyses of pUL76 and ppUL83 prepared from HCMV-infected cell subcellular fractions. The cytoplasmic/membrane (C/M) fractions of mock-infected (lane 1) and HCMV-infected HEL cells at 1 and 3 h postinfection (lanes 3 and 5, respectively) as well as nuclear fractions (N) of mock-infected (lane 2) and HCMV-infected HEL cells at 1 and 3 h postinfection (lanes 4 and 6, respectively) were resolved by SDS-10% PAGE. Proteins on Western blots were detected with anti-pUL76 and anti-ppUL83 antibodies. As controls, the membranes were subsequently stripped and analyzed with anti-PML protein and antiactin antibodies.
Our data demonstrate that in the absence of cycloheximide in virus-infected cells, increasing levels of the IE2 protein appeared from 3 to 11 h postinfection, whereas IE2 synthesis was abolished in the presence of cycloheximide (Fig. 2A, last two panels). This indicates that viral protein translation was inhibited by cycloheximide. We consistently observed that both pUL76 and the identified virus-associated lower matrix protein ppUL83 were detectable under conditions in which protein synthesis was inhibited (Fig. 2A, top two panels). Evidently, the pUL76 appearing at 3 h postinfection is unlikely to be derived from translation after viral infection. As a result, it is suggested that pUL76, like ppUL83, is acquired via entry of the viral inoculum.
To further investigate the targeting site of pUL76, which was transported into the cell by the infecting virus, cytoplasmic/membrane and nuclear fractions were prepared from cells after 1 and 3 h of infection (Fig. 2B, lanes 3 to 6). In a parallel negative control experiment, cells were mock infected with HCMV (Fig. 2B, lanes 1 and 2). SDS-PAGE was employed to resolve protein bands from the respective fractions. To ensure the purity of proteins, proteins of the cytoplasmic/membrane (Fig. 2B, lanes 1, 3, and 5) and nuclear (Fig. 2B, lanes 2, 4, and 6) fractions were analyzed by Western blotting with antibodies that recognize nucleus-specific PML protein and cytoplasm-specific actin, respectively. The results showed that both fractions were recognized by their respective antibodies, indicating no measurable cross-contamination (Fig. 2B, last two panels). In a subsequent Western blot experiment, the pUL76 band could be identified in both the nuclear and cytoplasmic/membrane fractions after 1 h of infection. When the infection wascontinued for 3 h, pUL76 was consistently present in the nuclear fraction but was undetectable in the cytoplasmic/membrane fraction (Fig. 2B, top panel, lanes 3 to 6). The major viral matrix protein ppUL83, used as a positive control, was present in both the cytoplasmic/membrane and nuclear fractions as early as 1 h postinfection. At 3 h postinfection, ppUL83 was predominantly associated with the nuclear fraction (Fig. 2B, second panel, lanes 3 to 6). These results show that ppUL83 as well as pUL76 derived from exogenous viral particles appear to target mainly the nuclear fraction at the onset of infection (23, 24). To confirm the identity of the protein that immunoreacted with the anti-pUL76 antibody, the nucleus fraction at 48 h postinfection was subjected to immunoprecipitation, in-gel digestion, and mass spectrometry analysis, and the resulting data verified this (data not shown).
pUL76 is associated with mature viral particles.
To directly gain insight into whether pUL76 is a protein constituent of mature viral particles, we set out to purify HCMV particles via negative-viscosity-positive-gradient ultracentrifugation (2, 27, 28). Viral particle bands corresponding to noninfectious enveloped particles and virions were collected from the gradients. Subsequently, protein constituents of individual particles were resolved in equal amounts by SDS-10% PAGE. After silver staining of the gel, the viral protein patterns became visible, and those profiles were consistent with the characteristics of each viral particle as documented previously (Fig. 3A, lanes 1 to 3) (2).
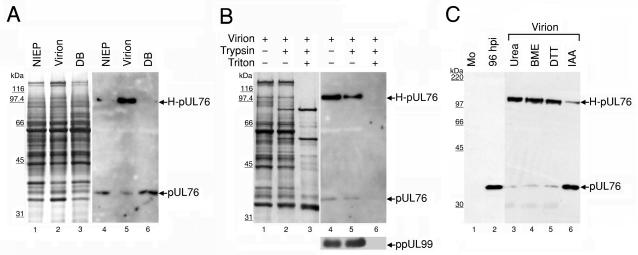
FIG. 3.
Association of pUL76 with gradient-purified HCMV particles. (A) Protein composition of three distinct viral particles. The released viral particles were purified, and equal amounts of the viral proteins were resolved on an SDS-10% polyacrylamide gel and visualized by silver staining (lanes 1 to 3). The presence of pUL76 within viral particles was analyzed by Western blotting with an anti-pUL76 antibody (lanes 4 to 6). Lanes 1 and 4, noninfectious enveloped particles (NIEP); lanes 2 and 5, virions; lanes 3 and 6, dense bodies (DB). (B) Demonstration that pUL76 is associated with HCMV virions and is insensitive to trypsin digestion. Purified virions were treated with trypsin in the presence (+) or absence (−) of Triton X-100. The proteins were resolved after digestion on an SDS-10% polyacrylamide gel. The presence of proteins was analyzed by Western blotting with an anti-pUL76 antibody and, as a control, with an anti-ppUL99 antibody. (C) Evidence that H-pUL76 within the virions is derived from pUL76. Lanes: 1, Western blot of lysate prepared from mock-infected (Mo) HEL cells; 2, cell lysate at 96 h postinfection; 3 to 6, gradient-purified virions treated with 6 M urea (lane 3), 10% β-mercaptoethanol (BME; lane 4), 200 mM dithiothreitol (DTT, lane 5), or 135 mM iodoacetamide (IAA, lane 6). The sizes of molecular mass markers are indicated on the left.
Next, the presence of pUL76 in the viral proteins was examined by Western blotting with the anti-pUL76 antibody. The results are shown in Fig. 3A, lanes 4 to 6. The pUL76-specific antibody recognized two signals on the Western blot. One signal migrated to the position of monomeric pUL76, and the other signal had an apparent molecular mass of 110 kDa and was tentatively designated H-pUL76 (Fig. 3A, lane 5). Given that equal amounts of the viral proteins were analyzed, it is noted that H-pUL76 accumulated distinctly in the virion. In the other two particles, noninfectious enveloped particles and dense bodies, the signal for H-pUL76 was barely detectable (Fig. 3A, lanes 4 and 6). Because H-pUL76 was first observed in the highly purified virion, we proposed two possibilities to account for the appearance of H-pUL76: either H-pUL76 was a contaminant stuck outside the virion particles and enriched after ultrafiltration and gradient separation, or it was a new form of pUL76 modified at the final stage of virion maturation.
We assumed that contaminants retained outside the virions would be removed by proteolytic digestion, whereas proteins embedded within virions would resist digestion. Once the embedded viral proteins were exposed by treatment with the detergent Triton X-100, proteolytic degradation occurred. Consequently, we performed this experiment with purified virion particles. The outcomes demonstrated that both pUL76 and H-pUL76 were associated within the virion. The protein compositions of intact virions and virions treated with trypsin in the presence and absence of Triton X-100 were resolved by denaturing PAGE (Fig. 3B). The migration positions of pUL76 and H-pUL76 showed no apparent difference regardless of whether the virions were treated with trypsin (Fig. 3B, lanes 4 and 5). This eliminates the possibility that the signals appeared as the result of contaminants derived from the virion purification process. This result confirms that both proteins are embedded within virions and become sensitive to trypsin digestion when Triton X-100 is used to solubilize virions, which made the virions accessible to trypsin digestion (Fig. 3B, lanes 4 to 6). Obviously, H-pUL76 is a virion-associated protein that is recognized by the anti-pUL76 antibody.
We observed that H-pUL76 was not detectable in the cell lysate 96 h after infection with HCMV (Fig. 3C, lanes 1 and 2) and remained undetectable until 7 days postinfection, when the H-pUL76 appeared in purified virions. We next investigated the possibility that H-pUL76 is a modified form of pUL76 which emerges at a late stage of virion maturation. Virions were denatured in lysis buffer supplemented with 6 M urea, 10% β-mercaptoethanol, or 200 mM dithiothreitol (Fig. 3C, lanes 3 to 5). However, following these treatments, the relative amounts of these two proteins were unaffected. When purified viral proteins were incubated with 135 mM iodoacetamide in lysis buffer, the amount of H-pUL76 decreased in conjunction with an increase in pUL76 (Fig. 3C, lane 6). Iodoacetamide is known to alkylate sulfhydryls on cysteines and irreversibly prevent the formation of disulfide bonds. Our results suggest that H-pUL76 is composed of pUL76 in disulfide cross-linkage with another protein(s).
Localization of UL76 protein within virions.
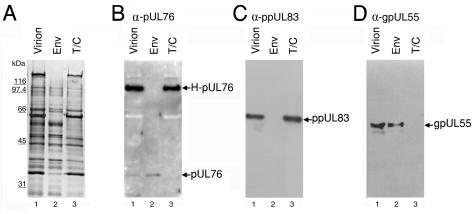
Next, we investigated the localization of these two UL76-derived proteins within the virion particles. Basically, by treatment with detergents, virion proteins can be fractionated into two portions. Many envelope proteins were solubilized by the detergents and retained in the supernatant, whereas tegument/capsid proteins were mainly retained in the insoluble pellet. Accordingly, virion proteins were fractionated by incubation with NP-40 and deoxycholate. The virions and the detergent-soluble and -insoluble proteins were resolved by SDS-PAGE. On inspection of the silver-stained gel, tegument proteins ppUL83 and ppUL82 could be easily recognized in the tegument/capsid fraction (Fig. 4A). Subsequently, the same amounts of proteins were subjected to Western blot analysis with anti-pUL76 antibody (Fig. 4B). As controls, the Western blots were analyzed with antibodies to tegument protein ppUL83 and envelope protein gpUL55 to examine the purity of the fractionated proteins in this experiment (Fig. 4C and D). As shown in lane 2 of Fig. 4B and D, pUL76 and gpUL55 were both found in the soluble envelope fraction, whereas H-pUL76 and ppUL83 were detected exclusively in the insoluble tegument/capsid fraction (Fig. 4B and C, lane 3).
FIG. 4.
pUL76 and H-pUL76 are associated with the virion envelope and tegument/capsid fractions, respectively. After treatment with detergents, virion proteins were fractionated into soluble supernatant and insoluble pellet, which were then resolved on an SDS-10% polyacrylamide gel. Lanes: 1, virion; 2, virion envelope proteins (Env) soluble in detergents; 3, virion tegument/capsid (T/C) proteins insoluble in detergents. (A) The protein compositions of the virions and fractions were visualized by silver staining. In parallel experiments, the proteins were subjected to Western blotting analyses with anti-pUL76 (B), anti-ppUL83 (C), and anti-gpUL55 (D) antibodies. As controls, gpUL55 and ppUL83, present in the envelope and tegument/capsid, respectively, were used to assess the purity of the fractions.
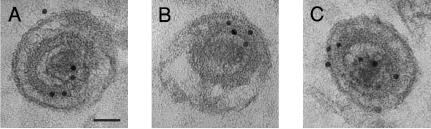
To further inspect the ultrastructural localization of pUL76 within released virion particles, immunoelectron microscopy was performed. Microsections of infected cells were stained with the anti-pUL76 antibody, which was labeled with a gold-conjugated secondary antibody. A detailed dissection of the virions revealed that the gold particles were localized in the inner tegument layer adjacent to the capsid (Fig. 5). We also observed in a few images that the gold particles were juxtaposed with the envelope (Fig. 5C). These ultrastructural images were consistent with the results obtained from the virion fractionation experiment, indicating that UL76-derived proteins are associated with the virion tegument/capsid and envelope compartments.
FIG. 5.
Immunoelectron microscopic illustration of the ultrastructural localization of UL76 protein within HCMV virions. HCMV-infected HEL cells were fixed at 96 h postinfection and immunogold labeled with the anti-pUL76 antibody. Gold particles were found juxtaposed to the tegument/capsid of extracellular virions (A, B, and C) and the envelope (C). Bar, 50 nm.
Stably transfected cells expressing UL76.
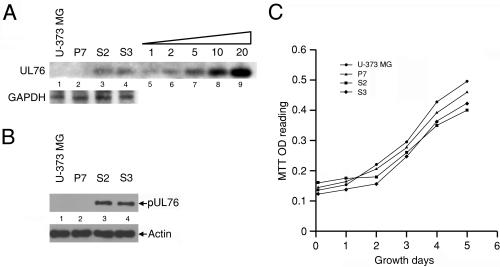
We were interested to investigate the impact of pUL76 at the immediate-early phase of infection, the moment when pUL76 is delivered from the viral inoculum and apparently targeted to the nucleus. In order to fulfill this goal, the UL76 gene was placed under the control of an HCMV major immediate-early promoter (pCMV-UL76) and then transfected into an HCMV-permissive cell line, human glioblastoma U-373 MG cells. Cells resistant to G418 selection, S2 and S3, were cloned. As a negative control, a P7 cell transfected with cloning plasmid pBK-CMV was also selected. The expression of UL76 in the cells was investigated in three experiments. Genomic DNA were prepared from P7, S2, and S3 cells and used for PCR to check for the presence of the UL76 gene in S2 and S3 cells. The PCR products were subjected to nucleotide sequencing, and the results confirmed UL76 sequence integrity in the genome of S2 and S3 cells (data not shown).
With the aim of relatively quantifying the transfected UL76 gene, genomic Southern blotting was performed. In the genomes of S2 and S3 cells, the 1.2-kb signals were hybridized to the UL76 probe (Fig. 6A, lanes 3 and 4), and both signals displayed almost equal intensity. The intensity of the hybridized signal is relative to 2 pg of purified fragment; therefore, it is equivalent to the level of a 1.2-kb single-copy gene in 5 μg of genomic DNA (lanes 5 to 9). As negative controls, the hybridized signals were not detected in either U-373 MG or P7 cells (lanes 1 and 2). In this experiment, the levels of GAPDH were assessed as internal controls. Taking these results together, we showed that S2 and S3 cells are stably transfected with a single copy of an intact UL76 gene.
FIG. 6.
Analyses of U-373 MG cells and cells stably transfected with the cloning vector (P7 cells) or the UL76 gene (S2 and S3 cells). (A) Genomic Southern blotting for quantification of the UL76 gene. Lanes: 1, U-373 MG cells; 2, P7 cells transfected with the cloning vector pBK-CMV; 3 and 4, S2 and S3 cells, respectively, transfected with pUL76-CMV digested with SmaI. In lanes 5 to 9, 1, 2, 5, 10, and 20 pg, respectively, of purified UL76 DNA (SmaI-SmaI fragment, nucleotides 110228 to 111437) was loaded on the gel to be used as standards for relative quantification of the UL76 signal. The transfected UL76 gene was detected by probing with a riboprobe generated from pRB-UL76. As an internal control, the membrane was stripped and probed with the GAPDH gene. (B) Cell lysates prepared from the cells were immunoblotted with an anti-pUL76 antibody. As a control, the membrane was stripped and analyzed with an antiactin antibody. (C) Growth curve for various cells. A thousand cells were seeded per well in 96-well dishes and incubated until harvested at the indicated times. Cell growth was monitored by an MTT reduction assay as described in Materials and Methods. OD, optical density.
As shown in Fig. 6B, only the cell lysates prepared from S2 and S3 cells produced signals that were recognized by an anti-pUL76 antibody (lanes 3 and 4). This result also confirms that the S2 and S3 cells produced equivalent levels of pUL76. Moreover, the specific signals were not detected in lysates obtained from U-373 MG or control P7 cells (lanes 1 and 2). Similar levels of actin, which served as an internal loading control, were detected in all cells tested. Next, the influence of pUL76 on cell growth was assessed, and our data showed no obvious adverse effect on the growth rate of P7 cells in comparison with that of U-373 MG, although a moderate reduction in cell growth for both S2 and S3 cells was exhibited (Fig. 6C).
Inhibition of HCMV production and replication in cells stably expressing pUL76.
Previously, UL76 was demonstrated to be a gene regulator exerting activation or repression, depending on the concentration of the transfected DNA. Within S2 and S3 cells, the conditions resemble those in priming cells with existing pUL76 prior to initiation of transcription from the infected viral genome. Thus, the impact of viral infection was examined. Several experiments were conducted, and the results demonstrated that viral production is inhibited in pUL76-expressing cells. First, though cytopathic effect was extensive in control P7 cells at 7 and 14 days postinfection (Fig. 7A, panels 1 to 3), it did not progress obviously in S2 and S3 (Fig. 7A, panels 4 to 9). Consistent with this observation, the titer of infectious HCMV was reduced by more than two logs when the virus was cultured in either S2 or S3 cells. The reduction in viral production was sustained continuously after 21 days of infection (Fig. 7B), indicating that pUL76 is capable of maintaining long-term inhibition of virus production.
FIG. 7.
Production of HCMV is inhibited in S2 and S3 cells infected with HCMV. Cells were mock or HCMV infected at an MOI of 1 PFU/cell. (A) Inhibition of the development of cytopathic effect in S2 and S3 cells. Panels: 1 to 3, control P7 cells; 4 to 6, UL76-expressing S2 cells; 7 to 9, S3 cells. The progress of cytopathic effect was recorded at 7 and 14 days postinfection with HCMV. (B) Titration of HCMV production on P7 (open bars), S2 (hatched bars), and S3 (shaded bars) cells at 3, 7, 14, and 21 days postinfection. (C) The accumulation of HCMV DNA is delayed and reduced in virus-infected S2 and S3 cells. Total DNA was harvested from infected cells at 1, 3, 5, and 7 days postinfection (dpi). The viral DNA was analyzed by slot blotting and probed with the IE1 cDNA. As a control, the blot was stripped and reprobed with the GAPDH gene.
In order to investigate whether the replication of the HCMV genome was affected, P7, S2, and S3 cells were infected with HCMV, and the accumulation of viral DNA was analyzed by a slot blot experiment (Fig. 7C). Total DNA of the infected cells was assessed for the presence of HCMV DNA with a labeled IE1 cDNA riboprobe. In this analysis, cellular GAPDH were used as an internal loading control. As shown in Fig. 7C, lanes 2 and 3, in virus-infected S2 and S3 cells, the accumulation of viral DNA was considerably delayed and reduced during the infection period. In a parallel experiment, the amount of viral DNA in infected P7 cells was used for comparison.
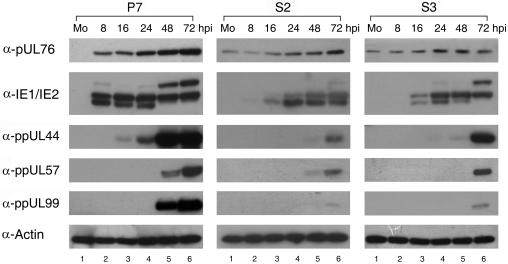
For monitoring the expression of viral replicative proteins during the HCMV infectious cycle, Western blotting and immunofluorescent cell staining experiments were performed. UL76-positive cells S2 and S3 as well as negative P7 cells were inoculated with HCMV. The protein expression kinetics in HCMV-infected P7 cells served as the control (Fig. 8, column 1). We observed that the expression of immediate-early proteins IE1 and IE2 was repressed in S2 and S3 cells (Fig. 8, columns 2 and 3). Consequently, a reduction and delay in expression were exhibited for two early proteins, ppUL44 (DNA polymerase processivity factor) and ppUL57 (single-stranded-DNA-binding protein), and two late proteins, pUL76 and ppUL99 (tegument protein).
FIG. 8.
Reduction in HCMV protein production in S2 and S3 cells stably expressing pUL76. Cell lysates were prepared from mock-infected (Mo) and HCMV-infected cells at the indicated times (hours postinfection) and analyzed by Western blotting with antibodies that recognize pUL76, IE1/IE2, ppUL44, ppUL57, and ppUL99. Columns 1 to 3, cell lysates prepared from P7, S2, and S3 cells, respectively. Lanes 1 to 6, Western blotting of cell lysates harvested from mock-infected cells (Mo) and cells obtained 8, 16, 24, 48, and 72 h postinfection with HCMV at an MOI of 1 PFU/cell, respectively.
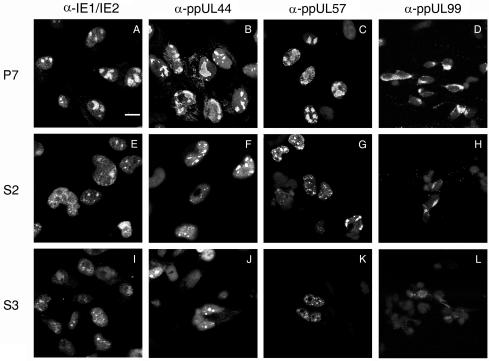
To visualize the distribution of viral proteins, we next performed immunofluorescent cell staining. After 48 h of infection, the cells were stained with antibodies to IE1/IE2, ppUL44, or ppUL57. Without the existing UL76 in P7 cells, IE1/IE2, ppUL44, and ppUL57 were found within the large globular replication compartment (Fig. 9A, B, and C). In contrast, in S2 and S3 cells, the four proteins were diffusely distributed in the nuclei, and there were sporadic punctuate foci in some nuclei (Fig. 9E, F, G, I, J and K). During the late stage of viral production, ppUL99 developed a perinuclear capping structure in P7 cells which was barely seen or poorly developed in S2 and S3 cells (Fig. 9D, H, and L). Therefore, the cells transfected with UL76 exhibited a blockade of replication complex formation and inhibition of viral production. These results are consistent with a novel virion protein with a functional role in gene repression.
FIG. 9.
Inhibition of HCMV replicative proteins upon infection of stably transfected U-373 MG cells expressing pUL76. P7, S2, and S3 cells were infected with HCMV at an MOI of 1 PFU/cell and processed with immunofluorescent cell staining. P7 (A to D), S2 (E to H), and S3 (I to L) cells were stained with antibodies against replication-related proteins IE1/IE2 (A, E, and I), ppUL44 (B, F, and J), and ppUL57 (C, G, and K) at 48 h postinfection as well as late tegument protein (ppUL99) (D, H, and L) at 96 h postinfection. (A) Bar, 20 μm.
DISCUSSION
This report provides considerable evidence that HCMV UL76 encodes a virion-associated protein with the potential to inhibit viral replication. We demonstrate that the UL76 ORF is included in two transcripts expressed with true-late kinetics (Fig. 1). The evidence led us to hypothesize that pUL76 is associated with viral particles. First of all, pUL76 was noted to be present at the very immediate-early time of infection. At this time point, it is unlikely that UL76, expressed as a late gene, is transcribed and then translated from the viral genome after infection. Then, when translation was inhibited by cycloheximide, pUL76 appeared immediately following virus entry, suggesting that pUL76 might have been transported along with the viral particles.
In the initial effort to prove this hypothesis, three types of viral particles were highly purified with a negative viscosity-positive gradient, and Western blotting was employed to demonstrate that pUL76 was present in all three types of viral particles. In addition to pUL76, as expected, the anti-pUL76 antibody also recognized a new form, H-pUL76, specifically associated with the virions (Fig. 3C). The result was confirmed by an experiment showing that both proteins were resistant to proteolytic digestion when the virions were incubated with trypsin, suggesting that they were embedded within the virions (Fig. 3B). We also disrupted virion proteins with various chemicals. With iodoacetamide (an alkylating agent for cysteine residues), a relative decrease in H-pUL76 proportionate to an increase in monomeric pUL76 (Fig. 3C) was obtained. In conclusion, we demonstrated that H-pUL76 is a distinct constituent of the virion that originates from monomeric pUL76. The cross-linking modification that generates H-pUL76 apparently occurs at the late stage of virion maturation.
The localization of pUL76 and H-pUL76 within the virion was revealed by biochemical fractionation of purified virions. Western blot analysis of the protein fractions showed that pUL76 was retained in the detergent-soluble fraction, consisting of many envelope proteins, whereas H-pUL76 was retained in the detergent-insoluble fraction, characteristic of tegument/capsid proteins. Immunoelectron microscopic images provided direct visualization of the ultrastructural localization of the UL76-derived proteins. Labeled gold particles recognized by the anti-UL76 antibody were found in two compartments of the virion, the envelope and the inner layer of the tegument/capsid space, presumably the localization sites of pUL76 and H-pUL76, respectively.
The herpes simplex virus type 2 UL24 protein, a counterpart of HCMV pUL76, is found associated specifically with purified virions and the C-capsid (25), which is consistent with the characteristics of H-pUL76. However, there has been no report of any cross-linking modification for the herpes simplex virus type 2 UL24 protein. H-pUL76 may be derived from such a strong protein-protein interaction of a homo- or hetero-oligomer of pUL76 in HCMV virion. However, at the initial time of infection and during the following infectious cycle, we were not able to detect H-pUL76 in the cytoplasmic/membrane and nuclear fractions, suggesting that the interaction may be dissociated following virus entry (Fig. 2 and 3). In view of the high content of positively charged residues and the ultrastructural localization within virions, we propose that H-pUL76 may be implicated in the formation of virion structures. It may confer stability to the structural conformation by neutralizing the negatively charged phosphoproteins basic phosphoprotein, ppUL83, and ppUL99 that reside in the inner layer tegument (3, 13, 32).
Many types of specific cells show syncytial phenotypes when infected with herpes simplex virus type 1 mutated in UL24, which was hypothesized to encode a membrane-associated protein. Likewise, HCMV pUL76 could be detected in the cytoplasmic/membrane fraction immediately following infection (Fig. 2). In the virion particles, pUL76 was found to associate with the envelope fraction (Fig. 3), which is presumably derived from the membrane during viral egress (34).
In contrast to previous observations in cells transfected with enhanced green fluorescent protein-tagged pUL76, we determined from the images that the nucleus is the target site for pUL76. Therefore, a variety of computational methods (PSORT II, PrositeScan, Predotar, MitoPro, SOSUI, and TMpred) were employed to analyze the putative target sites for pUL76. In summary, pUL76 is predicted to be a soluble protein containing six nuclear localization signals, and no transmembrane domain or mitochondrial targeting sequence was predicted. During the preparation of the manuscript, Rigoutsos et al. used the Bio-Dictionary-based protein annotation and MUSCA (pattern-based multiple sequence alignment) algorithms to predict that the UL76 ORF contains the features of transmembrane domains (40). Therefore, we believe that pUL76 may be associated with the membrane or may be a tegument protein that cofractionated with detergent-soluble virion proteins. The different distributions of pUL76 and H-pUL76 suggest that, through protein interaction, they are involved in different processes of virus maturation.
UL76 was cloned with a promoter-targeting gene screening strategy and identified by its ability to modulate expression of the major immediate-early gene (UL122 to UL123) promoter of HCMV (49). During the screening process, randomly selected plasmids were expressed collectively in virus-permissive cells. This may have led to recovery of cryptic regulators that function in activation and particularly in repression (44). As shown previously, pUL76 in transiently transfected cell cultures may function as a negative gene regulator. At the onset of infection, when virion protein pUL76 is deposited in the nucleus, it likely exerts its regulatory function. Thus, when UL76 is driven to high-level expression in transfected cells, the repressive function may become dominant.
As expected, the negative impact of pUL76 are revealed by the development of cytopathic effect, virus production, and impeded replication of viral DNA after infection of HCMV in cells expressing pUL76. Consequently, the inhibitory effect was elicited by the reduction of expression in replication-essential proteins and the late-expressed tegument protein. These behaviors are in contrast to the negative effects exerted by the dominant repressor UL84 of HCMV. UL84 is one of the essential genes required for transient replication in the cell transfection system. When HCMV-infected cells stably expressed UL84, the synthesis of immediate-early proteins was not affected, whereas the production of early and late proteins was completely abolished (19, 50). Recently, two groups of researchers globally assessed the growth ability of HCMV ORFs by introducing specific deletions or transposon insertions (18, 52). In contrast to the dominant-negative effects during stable expression, in both approaches UL76 and UL84 were defined to be required for efficient growth of HCMV. The mechanisms underlying the regulation of HCMV replication and the roles played by UL76 and UL84 remain to be investigated.
In this study, we revealed a potential gene repressor associated with HCMV particles. To our understanding, this is the first identified viral constituent of HCMV with the ability to repress gene expression and inhibit viral replication, which may explain the long production cycle of HCMV or indicate a novel mechanism employed by HCMV to resume latency.
Acknowledgments
This work was supported by research grants NSC90-2320-B-037-027, NSC91-2320-B-037-018 and NSC92-2320-B-037-049 from the National Science Council, Taiwan, awarded to S.-K. Wang.
Thanks to Young-Sun Lin for providing the IE2 antibody, members of the confocal microscopy room, MEMS Research Center, and proteomics research core laboratory, National Cheng Kung University, for technical assistance.
REFERENCES
- 1.Arrode, G., C. Boccaccio, J. Lule, S. Allart, N. Moinard, J. P. Abastado, A. Alam, and C. Davrinche. 2000. Incoming human cytomegalovirus pp65 (UL83) contained in apoptotic infected fibroblasts is cross-presented to CD8+ T cells by dendritic cells. J. Virol. 74:10018-10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldick, C. J., and T. Shenk. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 70:6097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter, M. K., and W. Gibson. 2001. Cytomegalovirus basic phosphoprotein (pUL32) binds to capsids in vitro through its amino one-third. J. Virol. 75:6865-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhella, D., F. J. Rixon, and D. J. Dargan. 2000. Cryomicroscopy of human cytomegalovirus virions reveals more densely packed genomic DNA than in herpes simplex virus type 1. J. Mol. Biol. 295:155-161. [DOI] [PubMed] [Google Scholar]
- 5.Blin, N., and D. W. Stafford. 1976. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 3:2303-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bresnahan, W. A., and T. Shenk. 2000. A subset of viral transcripts packaged within human cytomegalovirus particles. Science 288:2373-2376. [DOI] [PubMed] [Google Scholar]
- 7.Britt, W. J., and M. Mach. 1996. Human cytomegalovirus glycoproteins. Intervirology 39:401-412. [DOI] [PubMed] [Google Scholar]
- 8.Browne, E. P., and T. Shenk. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. USA 100:11439-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browne, E. P., B. Wing, D. Coleman, and T. Shenk. 2001. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J. Virol. 75:12319-12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campling, B. G., J. Pym, P. R. Galbraith, and S. P. C. Cole. 1988. Use of the MTT assay for rapid determination of chemosensitivity of human leukemic blast cells. Leukoc. Res. 12:823-831. [DOI] [PubMed] [Google Scholar]
- 11.Cha, T. A., E. Tom, G. W. Kemble, G. M. Duke, and E. S. Mocarski. 1996. Hum. cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, I. Hutchison, C. A., T. Kouzarides, J. A. Martignetti, E. Preddie, S. C. Satchwell, P. Tomlinson, K. M. Weston, and B. G. Barrell. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-169. [DOI] [PubMed] [Google Scholar]
- 13.Chen, D. H., H. Jiang, M. Lee, F. Liu, and Z. H. Zhou. 1999. Three-dimensional visualization of tegument/capsid interactions in the intact human cytomegalovirus. Virology 260:10-16. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski, P., and N. Sacchi. 1987. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 15.Davison, A. J., A. Dolan, P. Akter, C. Addison, D. J. Dargan, D. J. Alcendor, D. J. McGeoch, and G. S. Hayward. 2003. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 84:17-28. [DOI] [PubMed] [Google Scholar]
- 16.Dezélée, S., F. Bras, P. Vende, B. Simonet, X. Nguyen, A. Flamand, and M. J. Masse. 1996. The BamHI fragment 9 of pseudorabies virus contains gene homologous to the UL24, UL25, UL26, and UL26.5 gene of herpes simplex virus type 1. Virus Res. 42:27-39. [DOI] [PubMed] [Google Scholar]
- 17.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebert, S., S. Schmolke, G. Sorg, S. Floss, B. Plachter, and T. Stamminger. 1997. The UL84 protein of human cytomegalovirus acts as a transdominant inhibitor of immediate-early-mediated transactivation that is able to prevent viral replication. J. Virol. 71:7048-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson, W. 1996. Structure and assembly of the virion. Intervirology 39:389-400. [DOI] [PubMed] [Google Scholar]
- 21.Greijer, A. E., C. A. Dekkers, and J. M. Middeldorp. 2000. Human cytomegalovirus virions differentially incorporate viral and host cell RNA during the assembly process. J. Virol. 74:9078-9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi, M. L., C. Blankenship, and T. Shenk. 2000. Hum. cytomegalovirus UL69 protein is required for efficient accumulation of infected cells in the G1 phase of the cell cycle. Proc. Natl. Acad. Sci. USA 97:2692-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hensel, G., H. Meyer, S. Gartner, G. Brand, and H. F. Kern. 1995. Nuclear localization of the human cytomegalovirus tegument protein pp150 (ppUL32). J. Gen. Virol. 76:1591-1601. [DOI] [PubMed] [Google Scholar]
- 24.Hensel, G. M., H. H. Meyer, I. Buchmann, D. Pommerehne, S. Schmolke, B. Plachter, K. Radsak, and H. F. Kern. 1996. Intracellular localization and expression of the human cytomegalovirus matrix phosphoprotein pp71 (ppUL82): evidence for its translocation into the nucleus. J. Gen. Virol. 77:3087-3097. [DOI] [PubMed] [Google Scholar]
- 25.Hong-Yan, Z., T. Murata, F. Goshima, H. Takakuwa, T. Koshizuka, Y. Yamauchi, and Y. Nishiyama. 2001. Identification and characterization of the UL24 gene product of herpes simplex virus type 2. Virus Genes 22:321-327. [DOI] [PubMed] [Google Scholar]
- 26.Huang, C. F., Y. C. Wang, D. A. Tsao, S. F. Tung, Y. S. Lin, and C. W. Wu. 2000. Antagonism between members of the CNC-bZIP family and the immediate-early protein IE2 of human cytomegalovirus. J. Biol. Chem. 275:12313-12320. [DOI] [PubMed] [Google Scholar]
- 27.Irmiere, A., and W. Gibson. 1983. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology 130:118-133. [DOI] [PubMed] [Google Scholar]
- 28.Irmiere, A., and W. Gibson. 1985. Isolation of human cytomegalovirus intranuclear capsids, characterization of their protein constituents, and demonstration that the B-capsid assembly protein is also abundant in noninfectious enveloped particles. J. Virol. 56:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson, J. G., S.-H. Chen, W. J. Cook, M. F. Kramer, and D. M. Coen. 1998. Importance of the herpes simplex virus UL24 gene for production ganglionic infection in mice. Virology 242:161-169. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson, J. G., S. L. Martin, and D. M. Coen. 1989. A conserved open reading frame that overlaps the herpes simplex virus thymidine kinase gene is important for viral growth in cell culture. J. Virol. 63:1839-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalejta, R. F., and T. Shenk. 2003. The human cytomegalovirus UL82 gene product (pp71) accelerates progression through the G1 phase of the cell cycle. J. Virol. 77:3451-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landini, M. P., B. Severi, G. Furlini, and L. Badiali De Giorgi. 1987. Human cytomegalovirus structural components: intracellular and intraviral localization of p28 and p65-69 by immunoelectron microscopy. Virus Res. 8:15-23. [DOI] [PubMed] [Google Scholar]
- 33.Lehner, R., H. Meyer, and M. Mach. 1989. Identification and characterization of a human cytomegalovirus gene coding for a membrane protein that is conserved among human herpesviruses. J. Virol. 63:3792-3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy, E., I. Rigoutsos, T. Shibuya, and T. E. Shenk. 2003. Reevaluation of human cytomegalovirus coding potential. Proc. Natl. Acad. Sci. USA 100:13585-13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 100:14976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 38.Pearson, A., and D. M. Coen. 2002. Identification, localization, and regulation of expression of the UL24 protein of herpes simplex virus type 1. J. Virol. 76:10821-10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prichard, M. N., S. Jairath, M. E. T. Penfold, S. St. Jeor, M. C. Bohlman, and G. S. Pari. 1998. Identification of persistent RNA-DNA hybrid structures within the origin of replication of human cytomegalovirus. J. Virol. 72:6997-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rigoutsos, I., J. Novotny, T. Huynh, S. T. Chin-Bow, L. Parida, D. Platt, D. Coleman, and T. Shenk. 2003. In silico pattern-based analysis of the human cytomegalovirus genome. J. Virol. 77:4326-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 42.Sciortino, M. T., M. Suzuki, B. Taddeo, and B. Roizman. 2001. RNAs extracted from herpes simplex virus 1 virions: apparent selectivity of viral but not cellular RNAs packaged in virions. J. Virol. 75:8105-8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soderberg-Naucler, C., and J. Y. Nelson. 1999. Hum. cytomegalovirus latency and reactivation-a delicate balance between the virus and its host's immune system. Intervirology 42:314-321. [DOI] [PubMed] [Google Scholar]
- 44.Stamminger, T., M. Gstaiger, K. Weinzierl, K. Lorz, M. Winkler, and W. Schaffner. 2002. Open reading frame UL26 of human cytomegalovirus encodes a novel tegument protein that contains a strong transcriptional activation domain. J. Virol. 76:4836-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sturzl, M., and W. K. Roth. 1989. “Run-off” synthesis and application of defined single-stranded DNA hybridization probes. Anal. Biochem. 185:164-169. [DOI] [PubMed] [Google Scholar]
- 46.Talbot, P., and J. D. Almeida. 1977. Human cytomegalovrius: purification of enveloped virions and dense bodies. J. Gen. Virol. 26:345-349. [DOI] [PubMed] [Google Scholar]
- 47.Tognon, M., R. Guandalini, M. Romanelli, G. R. Manservigi, and B. Trevisani. 1990. Phenotypic and genotypic characterization of locus Syn5 in herpes simplex virus 1. Virus Res. 18:135-150. [DOI] [PubMed] [Google Scholar]
- 48.Tso, J. Y., X. H. Sun, T. H. Kao, K. S. Reece, and R. Wu. 1985. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 13:2485-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, S.-K., C.-Y. Duh, and T.-T. Chang. 2000. Cloning and identification of regulatory gene UL76 of human cytomegalovirus. J. Gen. Virol. 81:2407-2416. [DOI] [PubMed] [Google Scholar]
- 50.Xu, Y., K. S. Colletti, and G. Pari. 2002. Human cytomegalovirus UL84 localizes to the cell nucleus via a nuclear localization signal and is a component of viral replication compartments. J. Virol. 76:8931-8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao, F., and R. J. Courtney. 1992. Association of ICP0 but not ICP27 with purified virions of herpes simplex virus type 1. J. Virol. 66:2709-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. USA 100:12396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yurochko, A. D., and E. S. Huang. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162:4806-4816. [PubMed] [Google Scholar]
- 54.Yurochko, A. D., E. S. Hwang, L. Rasmussen, S. Keay, L. Pereira, and E. S. Huang. 1997. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J. Virol. 71:5051-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu, Y., L. Huang, and D. G. Anders. 1998. Human cytomegalovirus oriLyt sequence requirements. J. Virol. 72:4989-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]