Abstract
Apoptin, a viral death protein derived from chicken anemia virus, displays a number of tumor-specific behaviors. In particular, apoptin is phosphorylated, translocates to the nucleus, and induces apoptosis specifically in tumor or transformed cells, whereas it is nonphosphorylated and remains primarily inactive in the cytoplasm of nontransformed normal cells. Here, we show that in normal cells apoptin can also be activated by the transient transforming signals conferred by ectopically expressed simian virus 40 (SV40) large T antigen (LT), which rapidly induces apoptin's phosphorylation, nuclear accumulation, and the ability to induce apoptosis. Further analyses with mutants of LT showed that the minimum domain capable of inducing all three of apoptin's tumor-specific properties resided in the N-terminal J domain, a sequence which is largely shared by SV40 small t antigen (st). Interestingly, the J domain in st, which lacks its own nuclear localization signal (NLS), required nuclear localization to activate apoptin. These results reveal the existence of a cellular pathway shared by conditions of transient transformation and the stable cancerous or precancerous state, and they support a model whereby a transient transforming signal confers on apoptin both the upstream activity of phosphorylation and the downstream activity of nuclear accumulation and apoptosis induction. Such a pathway may reflect a general lesion contributing to human cancers.
Apoptin, also known as VP3, is a small protein of 121 amino acids (aa) derived from chicken anemia virus (CAV) (29, 30). The early observation that CAV kills infected chicken thymocytes was given a mechanistic basis when apoptin was shown to cause apoptosis when overexpressed alone (28, 31, 32). Apoptin requires activation of downstream caspases for this activity in tumor cells, indicating that Apoptin induces the classical apoptosis pathway (11). Different assays such as cytochrome c release, caspase-3 activation, and DNA condensation confirmed the tumor-specific apoptin-induced apoptosis (11). Additional studies have shown that apoptin induces apoptosis not only in a great variety of human tumor cells (10, 52, 53) but also in transformed cells, e.g., simian virus 40 (SV40)-transformed human fibroblasts and keratinocytes (10). Intriguingly, apoptin has also been shown to kill UV-irradiated cells from as-yet-healthy individuals with hereditary cancer-prone syndromes (50). Remarkably, apoptin does not induce significant apoptosis in a variety of tested healthy human normal cells, including primary human mesenchymal stem cells, human hepatocytes, and human hematopoietic stem CD34+ cells (12; Y.-H. Zhang, unpublished data), or in a number of primary rodent cells (10, 27, 51). Thus, the sensitivity of a cell to apoptin-induced apoptosis likely depends on alternations related to the tumor or transformed status of the cell.
In tumor or transformed cells, apoptin translocates rapidly to the nucleus prior to morphologically apparent apoptosis, whereas in primary and nontransformed cells under nonstressed conditions, it remains predominately in the cytoplasm in an inactive state (10, 51). However, whereas nuclear localization in tumor cells seems to be important for apoptin-induced apoptosis, forced nuclear translocation and/or expression of apoptin in normal cells is not sufficient for induction of apoptosis (12; Y.-H. Zhang, unpublished), indicating another activation step particular to the tumor or transformed state. More recently, we have shown that apoptin is phosphorylated both in vitro and in vivo on Thr108 specifically in tumor and transformed cells, providing a mechanism for the upstream, tumor-specific activity of apoptin (38).
Tumor formation is thought to progress through multiple steps, with each step giving rise to cells possessing increasingly malignant characteristics. Critical steps in the development of a cancerous cell include the attainment of an unlimited replicative life span and the suppression of growth-inhibiting tendencies. These traits are driven by genetic events such as mutations in tumor suppressor genes or oncogenes or by the acquisition of specific viral transforming sequences (41). DNA tumor viruses have served as a useful model for understanding these processes because they encode dominant acting transforming proteins targeting one or more key regulatory proteins in a cell. One such virus, SV40, possesses potent oncogenic potential, having the ability to induce transformation and tumorigenesis in both rodent and human cells (4, 5, 6, 17). SV40 encodes three tumor antigens: large T antigen (LT), small t antigen (st), and a 17,000-molecular-weight (17K) t antigen (41). While the role of the 17K protein in SV40 infection and transformation is unclear, a number of studies have shown that the full transforming and tumorigenic potential of SV40 resides in both LT and st (19, 39, 41). In particular, LT complexes with and thereby functionally inactivates the cellular tumor suppressor proteins pRb and p53 (36, 40, 41). Moreover, LT interacts with other cellular proteins as a molecule chaperone (13, 21, 41, 46).
Our studies thus far have suggested that at least a (pre)cancerous state is essential for apoptin to function as a death effector (10, 50). Because expression of SV40 LT is sufficient to induce transformation in multiple cell types as described above and because apoptin is activated in cells stably transformed by SV40 (10), we sought to understand whether and how a transient transforming situation might activate apoptin. In this study, we show that, when transiently coexpressed in normal cells, the viral oncoprotein SV40 LT renders apoptin functional in a tumor-specific manner; namely, apoptin's phosphorylation, nuclear translocation, and cell-killing ability are induced. We mapped the apoptin-activating determinant to an N-terminal region of LT that includes the J domain common to both SV40 LT and st. These results show that apoptin's upstream activation is mediated by a pathway initiated by N-terminal sequences in LT, a pathway that by inference may be shared by all transformed and tumorigenic cells.
MATERIALS AND METHODS
Cell strains and culture.
Two human diploid foreskin fibroblast strains derived from healthy individuals, VH10 and VH25 (1); the SV40 stably transformed human foreskin fibroblast line, VH/SV (kindly provided by B. Klein, Leiden University Medical Center, Leiden, The Netherlands); primary mouse embryonic fibroblasts (MEFs) (kindly donated by A. G. Jochemsen, Leiden University Medical Center, Leiden, The Netherlands); and the human osteosarcoma cell line Saos-2 were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin (Life Technologies)/ml. VH10 and VH25 cells were used below passage 13, and MEF cells were used at passages 4 to 6. CD31-negative human foreskin-derived primary fibroblasts (kindly provided by D. Mumberg, Schering AG, Berlin, Germany), regularly and freshly prepared from foreskin circumcisions by negative selection with an CD31-antibody to remove endothelial cells, were cultured in MEM-Earle medium (BIOCHROM AG, Berlin, Germany) containing 2.2 g of NaHCO3/liter and 0.518 g of N-acetyl-l-alanyl-l-glutamine/liter and supplemented with 10% fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml and used at passage 5. For retroviral infection, MEFs, generated at day 15 of conception from FVB mice (Charles River, Lyon, France), and EcoPack2-293 packaging cells (Clontech) for the production of retroviral vector capable of infecting rodent cells (ecotropic retrovirus), were cultured in DMEM supplemented with 10% fetal calf serum, penicillin, and streptomycin as described above. Human primary epidermal keratinocytes (FSK-1) were initiated in a modified complete medium, cultured as previously described (10), and were used at passage 3. SVK14, a SV40-stably transformed epidermal keratinocyte strain, was cultured in DMEM-F12 medium (3:1) supplemented with 5% fetal calf serum, 0.4 μg of hydrocortisone/ml, 0.25 μg of soproleronol/ml, 100 U of penicillin/ml, and 100 μg of streptomycin/ml.
DNA plasmids.
pCMV-VP3, encoding apoptin, and pCMV-neo, the empty control plasmid, have been described previously (31, 53). In pRSV-SV40(LT/st) (formerly named pR-SV40), the coding region for SV40 LT and st was placed downstream of the strong heterologous enhancer-promoter of the Rous sarcoma virus (RSV) long terminal repeat and constructed by replacing the neomycin resistance coding sequence of pRSV-neo (18) between the unique HindIII and BamHI sites with the LT- and st-coding region of SV40 (15). In pRSV-LT (formerly named pR-S884), a complete SV40 LT together with 62-aa C-terminal-truncated st was expressed under the RSV promoter, created by substituting the 1,169-bp HindIII fragment of pRSV-SV40 encompassing the st-coding region with the corresponding 922-bp HindIII fragment of pR-SV40-dl884 with a deletion in the C terminus of st (15). The plasmid pRSV-st (previously named pR-SVt-cDNA) contains cDNA sequences coding for the SV40 st gene fused to the RSV long terminal repeat and was constructed from pW2t/cDNA (35, 42). The above-described SV40 LT- and st-expressing constructs were kindly provided by A. G. Jochemsen.
The following four plasmids expressing mutated SV40 LT were kind gifts from J. M. Pipas (Department of Biological Sciences, University of Pittsburgh, Pittsburgh, Pa.). pRSV-3213, containing two amino acid substitutions (E107K and E108K) within the cr2-like sequence is defective for LT association with pRb (37, 43). pRSV-5031, with three amino acid substitutions (D402N, V404M, and V413M) within the ATPase/p53 binding domain, loses the p53 binding ability of LT (7, 33, 40). The J domain-deleted mutant, pRSV-dl1135(Δ17-27), synthesizes an LT lacking the cr1-like motif (36) and fails to transform established cell lines and to immortalize primary cells (9, 46, 48). Truncated mutant pRSV-TN136 (43) directs the synthesis of the first 136 N-terminal aa of SV40 LT, including the amino-terminal-transforming domain and NLS.
We generated a plasmid pRSV-nls-st, encoding SV40 st N-terminally fused to the NLS of SV40 LT (PPKKKRKV), by first ligating a BglII/KpnI/BglII linker containing an ATG start codon and the NLS to the N terminus of a BglII/BamHI fragment of SV40 st contained in pWZT-Bg-st (kindly offered by K. Rundell, Northwestern University, Evanston, Ill.) to form a KpnI/BamHI fragment of nls-st and then religating the KpnI/BamHI fragment of nls-st to the KpnI/BamHI pRSV vector which is identical to those of the SV40 LT mutant constructs described above.
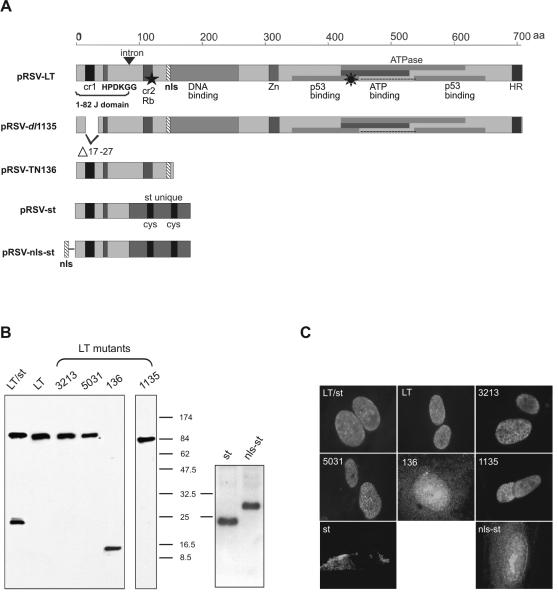
A schematic representation of SV40 proteins encoded by all constructs is shown in Fig. 1A. The comparable expression level, size, and subcellular localization of these SV40 proteins after transfection into Saos-2 cells were confirmed by Western blot analysis (Fig. 1B) and indirect immunofluorescence (Fig. 1C), respectively. SV40 LT (both wild type and mutated) was localized in the nucleus, SV40 st was localized mainly in the cytoplasm of cells, and NLS-tagged SV40 st (nls-st) translocated to the nucleus.
FIG. 1.
Schematic representation of the domain structure, expression, and subcellular localization of SV40 proteins encoded by related plasmids. (A) Schematic representation of the domain structure of SV40 proteins. pRSV-LT encodes the 708-aa-long SV40 LT, in which the depiction of the domain structure of LT has been adapted from Srinivasan et al. (43). The first 1 to 82 aa (1-82 J domain) in the N terminus represent the J domain containing cr1 and HPDKGG motifs. cr2 is a conserved motif allowing LT to bind pRb. The positions of the DNA binding domains, zinc finger motif (Zn), and sequences required for ATPase activity and p53 association are shown. HR, host range domain required for viron assembly. pRSV-dl1135 synthesizes a LT missing the cr1 motif due to a deletion (Δ17-27). PRSV-TN136 expresses the 136 N-terminal aa of LT containing the J domain, pRb binding domain, and NLS. pRSV-st directs the synthesis of wild-type SV40 st, which contains the first 1 to 82 aa of the J domain identical to that of LT and its unique C terminus. pRSV-nls-st expresses an st N terminally fused to an engineered NLS. The eight-pointed star indicates LT mutant pRSV-5031 lacking p53 binding function because of multiple mutations (D402N, V404M, and V423M) in the ATPase/p53 binding domain. ★, the position of two amino acid substitutions (E107K and E108K) within cr2 leading to the mutant pRSV-3213, which is defective in pRb binding ability; ▾, 448-bp intron (bp 4918 to 4571) in the SV40 LT early region following a 246-bp sequence (5163 to 4918) that is shared in both LT and st and encodes the identical 1 to 82 aa of the J domain (3). Plasmid pRSV-SV40(LT/st) encodes two early region products, both wild-type LT and st. (B) Expression of SV40 proteins. Two days after DOTAP transfection, Saos-2 cells were lysed and subjected to Western blot analysis. (C) Subcellular localization of SV40 proteins. Five days after DOTAP transfection, Saos-2 cells were fixed for immunofluorescence microscopy. For both Western blot analysis and immunofluorescence assay, LT-mutated 1135 protein was detected by using antibody KT3 raised specifically against the C-terminal 11 aa of LT, whereas the other SV40 proteins were detected by using antibody 419, which is reactive with the first 82 N-terminal aa shared by both LT and st.
In addition, we also constructed a retroviral vector expressing wild-type SV40 LT, pBabe-puro-LT, by digestion of SV40 LT (cDNA, without intron) from pSG5-SV40-LTcDNA (a kind gift from J. De Caprio, Division of Hematology and Oncology, Massachusetts General Hospital, Boston, Mass.) with BamHI and inserting it into a BamHI-digested pBabe-puro retroviral vector (26). pBabe-puro-eGFP expressing green fluorescent protein (GFP) was used as a control plasmid (a kind gift from S. Olijslagers from our laboratory).
DNA delivery methods.
We used the transfection agent N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (DOTAP), essentially as described by Fischer et al. (16), to introduce DNA into VH10, FSK-1, and MEF cells at a 40% confluence. In addition, FuGENE 6 transfection reagent (Roche Molecular Biochemicals) was used to transfect VH/SV, SVK-14, Saos-2 cells, and (in some cases) VH10 cells at a ratio of 1 μg of DNA to 3 μl of FuGENE 6, according to the manufacturer's protocol. For cotransfections, the ratio of each DNA was 1:1. To deliver DNA by microinjection, VH10 cells were seeded at a 40% confluence on glass-bottomed microinjection dishes (MatTek Corporation) 1 day before, and DNA (50 ng/μl) was microinjected into the nucleus with an Eppendorf microinjector under the injection-pressure condition of 50 hPa. In each sample, at least 100 cells were injected. When CD31-negative fibroblasts were transfected, Nucleofector technology was used in conjunction with the normal human dermal fibroblast cell type-specific solution (AMAXA Biosystems, Cologne, Germany). Each nucleofection sample contained 5 μg of DNA, 106 cells, and 100 μl of normal human dermal fibroblast Nucleofector solution; the transfection was carried out under the program U23 of the Nucleofector device, as recommended by the manufacturer. In the case of CD31-negative fibroblast transfection, in addition to the analysis on a population level by Western blotting, the cells were seeded on several glass coverslips to allow parallel analysis at the single-cell level by immunofluorescence assay. The transfected cells were then suspended in an appropriate volume of medium and seeded for further culture. For retroviral infection of MEFs with pBabe-LT and pBabe-eGFP, virus was produced by transfecting approximately 30% confluent cultures of EcoPack2-293 cells with DNA to FuGENE 6 in a ratio of 1 μg of DNA to 3 μl of FuGENE 6. After overnight incubation, the FuGENE 6-containing medium was replaced by a minimum amount of fresh medium to obtain concentrated virus supernatant. After a 32-h incubation, the virus-containing medium was collected and filtered through a low protein binding filter (Millex; Millipore). Virus supernatant was aliquoted and stored at −80°C prior to viral infection. Then, MEFs at passage 4 were seeded in medium containing 10 μg of polybrene (Sigma)/ml for 4 h and then cultured in virus supernatant diluted with medium at a 1:2 ratio. After an overnight incubation, the virus-polybrene medium was replaced with ordinary DMEM medium.
Immunofluorescence microscopy.
Cells were washed once with phosphate-buffered saline, fixed with 80% acetone (in H2O) for 10 min, and stored at −20°C until further analysis. Immunocytochemical staining was performed essentially as described previously (10). Antibodies used to detect the presence of apoptin were α-VP3-C, a polyclonal rabbit serum against the C terminus of apoptin (11); 111.3, a mouse monoclonal antibody against the N terminus of apoptin (10); and α-108-P, a purified phosphospecific rabbit polyclonal antibody against phosphorylated Thr108 of apoptin (38). Antibodies used to detect the expression of SV40 proteins were 419, a mouse monoclonal antibody reactive with both SV40 LT and st by recognizing a shared epitope within the first 82 N-terminal aa (Research Diagnostic, Inc.); and KT3, a mouse monoclonal antibody raised against an 11-aa peptide (KPPTPPPEPET) derived from the carboxy-terminal sequence of SV40 LT and nonreactive with SV40 st (Abcom, Ltd., Cambridge, United Kingdom). Secondary antibodies were either conjugated to fluorescein isothiocyanate or Rhodamine (Jackson ImmunoResearch Laboratories). 2,4-Diamidino-2-phenylindole (DAPI) (1 μg/ml) was used to stain cellular DNA to determine the morphological appearance of apoptosis essentially as described previously (10). Images of representative cells were taken with digital image analysis equipment (Sony DXC-950P 3CCD color video camera) and processed with Analysis software (version 3.00; Soft Imaging System). To determine whether apoptin colocalized with SV40 LT, cells were analyzed with the Bio-Rad MRC 600 confocal laser scan microscope equipped with an argon-crypton laser.
Immunoprecipitation assay and Western blot analysis.
Transfected cells were lysed with lysis buffer (50 mM Tris [pH 7.5], 250 mM NaCl, 5 mM EDTA, 0.1% Triton X-100, 20 mM β-glycerophosphate, 5 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin/ml, 200 μg of trypsin inhibitor/ml, and 1 μg of pepstatin/ml) on ice for 30 min. After being centrifuged at 13,000 × g and 4°C for 10 min, the supernatant of lysates was harvested for further analysis. To determine whether apoptin is associated with SV40 LT, we used Western blot analysis along with immunoprecipitation (IP). Briefly, 9/10 of the supernatant of lysates was first incubated at 4°C for 2 to 3 h in a final volume of 400 μl of lysis buffer containing 40 μl of 10 protein A-Sepharose beads (Sigma-Aldrich, Zwijndrecht, The Netherlands), which were first coupled to anti-apoptin rabbit polyclonal antibody α-VP3-C or mouse monoclonal antibody 416 specific for antigenic determinants unique to SV40 LT (Research Diagnostic, Inc.). One-tenth of the lysate supernatant was left for direct Western blot analysis without IP. The IP and non-IP samples were then added to 4× denaturing Laemmli sample buffer to a maximal volume of 50 μl for fractionation on sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene difluoride (Immobilon) membranes. Blots were probed with antibodies 111.3 and 419 for detection of apoptin and LT, respectively. In cases where only Western blot analysis was required, supernatants of cell lysates were added immediately to Laemmli sample buffer, fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electroblotted onto polyvinylidene difluoride membranes. Blots were incubated with the antibody α-108-P exactly as described previously (38) to determine the phosphorylation state of apoptin when SV40 proteins were coexpressed; antibody 111.3 was then added to confirm the expression of basal apoptin. Horseradish peroxidase-conjugated goat antibody against rabbit or mouse immunoglobulin G, i.e., GαR-HRP or GαM-HRP (Amersham BioSciences), was used as a secondary antibody. Detected signals were visualized by enhanced chemiluminescence according to the manufacturer's protocol (Amersham BioSciences).
In vitro kinase assay.
The in vitro kinase assays were carried out essentially as described by Rohn et al. (38). Briefly, MEF cells infected with retroviral vector pBabe-LT or pBabe-eGFP were lysed 4 days postinfection by repeated freeze-thawing three times in kinase buffer (20 mM HEPES [pH 7.4], 20 mM MgCl2, 150 mM Nacl, and standard inhibitors of proteases and phosphatases). Twenty micrograms of protein from clarified supernatant was added to 3 μg of substrate, the purified His-tagged apoptin protein VP3-His (22), and incubated at 30°C for 30 min in a final volume of 30 μl of kinase buffer containing 50 μM ATP. The reacted mixtures were subjected to Western blot analysis, followed by immunoprobing with antibody α-108-P to determine the phosphorylation of apoptin, with 111.3 to confirm the presence of His-tagged apoptin protein, and with 419 and mouse α-GFP (Clontech) to show the expression of SV40 LT and the control protein GFP, respectively.
RESULTS
Transient coexpression of SV40 LT in normal cells triggers the nuclear localization and apoptosis-inducting activity of apoptin.
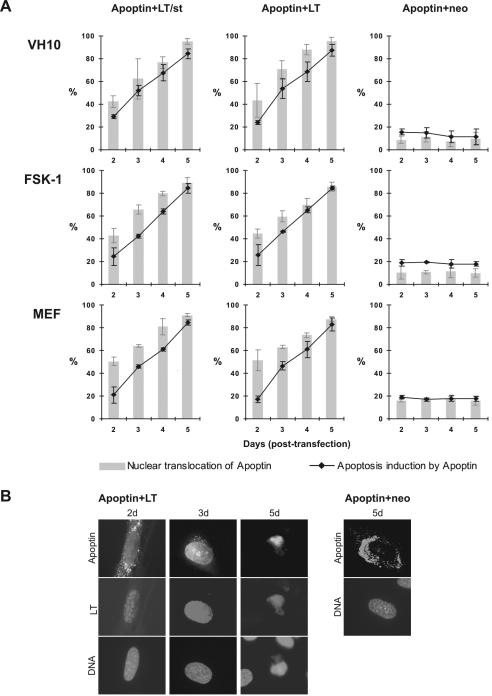
To address whether apoptin could be activated by the transient expression of the transforming SV40 viral oncoprotein in normal cells, we selected two human normal cell types, VH10 and FSK-1, and one murine cell type, MEF, and cotransfected them using DOTAP transfection with the apoptin construct pCMV-VP3 plus pRSV-SV40(LT/st) encoding both SV40 early region products LT and st (LT/st), pRSV-LT expressing wild-type LT alone, or empty vector pCMV-neo as a negative control. As seen in Fig. 2, apoptin was induced to migrate from the cytoplasm to the nucleus in all three cell types in the presence of SV40 LT/st or LT alone, with nuclear accumulation gradually increasing with time posttransfection from 45 to 50% at day 2 to 85 to 95% at day 5 (Fig. 2A), reaching the level of nuclear localization seen with apoptin in tumor or transformed cells (10, 52, 53). In parallel with this shift in apoptin's localization, apoptin was also activated to induce cell death (Fig. 2). This apoptotic activity, like the nuclear accumulation, increased with time posttransfection to 80 to 90% at day 5, a level equal to that habitually seen with tumor or transformed cells (10, 52, 53). In strong contrast, apoptin cotransfected with empty vector as a negative control did not induce apoptosis (Fig. 2A) and showed the expected predominantly cytoplasmic retention (Fig. 2B). To rule out cell type peculiarities, we repeated similar analyses of several other normal cell types, including VH25, and achieved similar results (data not shown). Taken together, these results show that transient coexpression of SV40 LT is sufficient to trigger two prominent activities, nuclear accumulation and apoptosis, that are characteristic of apoptin's tumor-specific behavior in multiple nontransformed cell types from two species.
FIG. 2.
Activation of apoptin by the SV40 early region gene. Three different cell types (primary human diploid fibroblasts [VH10], human epidermal keratinocytes [FSK-1], and primary MEFs) were cotransfected with the apoptin-encoding plasmid pCMV-VP3 plus the plasmid pRSV-SV40(LT/st) encoding both LT and st, pRSV-LT expressing LT only, or empty vector pCMV-neo as a negative control. The expression and localization of apoptin was determined by apoptin antibody α-VP3-C, and SV40 early region products (LT/st or LT alone) were probed with antibody 419. DAPI staining was used to determine the nuclear morphology of cells indicative of apoptosis. (A) Transfected cells were analyzed at 2, 3, 4, and 5 days posttransfection by immunofluorescence microscopy. Three independent experiments were performed. Mean values are presented with standard deviation bars. Gray bars indicate the percentage of cells in which apoptin is mainly nuclear; black lines indicate the percentage of death. (B) Representative immunofluorescent images of VH10 cells showing apoptin's nuclear translocation (left and middle panels) and apoptosis induction (right panel) in the presence of LT and apoptin's primarily cytoplasmic localization in the negative control (Apoptin+neo). DNA was stained with DAPI (bottom of each panel) to show the fate of the cells with apoptin plus LT or with apoptin plus the empty vector pCMV-neo.
Apoptin's activation by SV40 LT is not the result of binding.
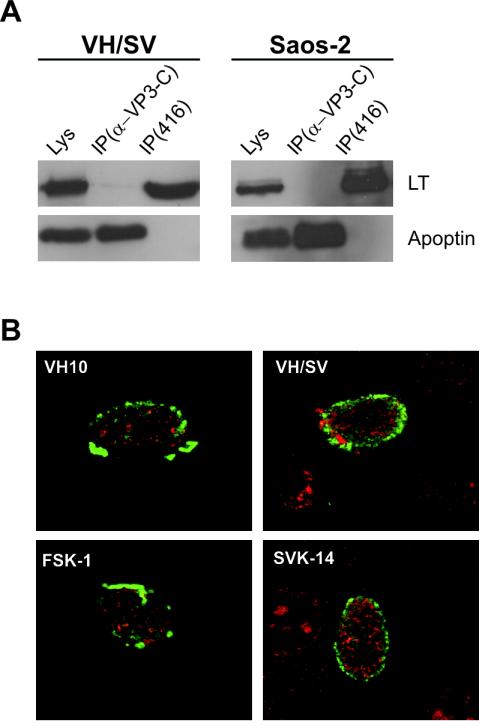
The vast majority of SV40 LT in SV40-transformed cells are known to be located in the cell nuclei (14), a cellular compartmentalization also seen in our experiments (Fig. 1C). Given that SV40 LT activates apoptin, we examined whether apoptin binds directly or indirectly to LT protein. To do so, we transfected pCMV-VP3 into SV40-transformed cell line VH/SV or cotransfected Saos-2 tumor cells with pCMV-VP3 and pRSV-LT with FuGENE 6. Two days after transfection, mild lysates of the transfected cells were subjected to coimmunoprecipitation (co-IP) and Western blot analysis. As seen in Fig. 3A, when either ectopically expressed alone in SV40-transformed cells or simultaneously coexpressed with LT, apoptin did not co-IP with LT with either apoptin antibody α-VP3-C or LT antibody 416. Confocal immunofluorescence microscopy showed that when coexpressed in the primary human cells VH10 and FSK-1, apoptin did not colocalize with SV40 LT in the nucleus or in the nucleus of two SV40-transformed cell lines, VH/SV and SVK-14 (Fig. 3B). These data suggest that the activation of apoptin did not occur via association with SV40 LT but was instead mediated by some transient transforming signal induced by SV40 LT.
FIG. 3.
Apoptin does not associate with SV40 LT. (A) No co-IP between apoptin and LT. pCMV-VP3 was transfected in SV40-stably transformed VH/SV cells or cotransfected with pRSV-LT in Saos-2 cells. Co-IP and Western blot assay were carried out 2 days posttransfection with apoptin antibody α-VP3-C or SV40 LT antibody 416. Lysates (Lys) without IP were used as control to show the coexpression of apoptin and SV40 LT. (B) No colocalization in the nucleus between apoptin and LT. Confocal immunofluorescence microscopy was carried out with apoptin- and LT-cotransfected primary human normal cell types VH10 and FSK-1 and with apoptin-transfected SV40 stably transformed cell types VH/SV and SVK-14. Apoptin was probed with α-VP3-C (green), and SV40 LT with 419 (red).
The minimal apoptin-activating domain resides in the N terminus of SV40 LT.
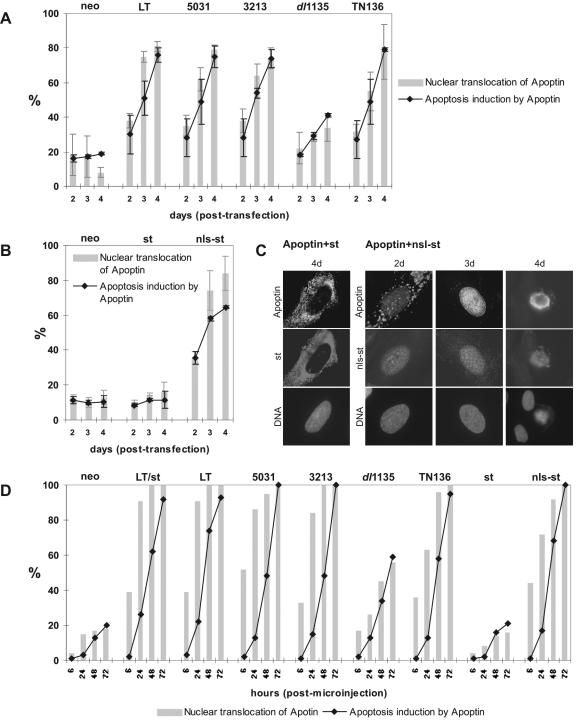
In an effort to identify the minimal domain in LT that stimulates the transient intracellular pathway sufficient to activate apoptin, we performed a set of cotransfection experiments in VH10 primary human diploid fibroblasts with a collection of LT mutants. The LT mutants 3213, 5031, dl1135, and TN136 were selected because each of them is known to alter at least one of the three functioning domains of SV40 LT (see Materials and Methods and Fig. 1A for details). As shown in Fig. 4A, when either the p53 binding-defective LT protein 5031 or the pRb binding-defective LT protein 3212 was coexpressed, apoptin was still activated as effectively as by wild-type LT, as assessed by nuclear localization and apoptosis activity, indicating that association of LT with either of these cellular tumor suppressors was not required for these effects. In contrast, however, the activation of apoptin by the J domain-deleted mutant dl1135 was significantly reduced relative to that of wild-type LT (Fig. 4A). These experiments suggest that the transforming ability of LT executed by the J domain is important for LT to activate apoptin. To confirm that apoptin's activation was mediated by the J domain, another LT mutant, TN136, which expresses only the first 136 N-terminal aa of wild-type LT and contains the J domain, a functional pRb binding domain, and the NLS, was tested. As shown in Fig. 4A, the TN136 mutant was indeed sufficient to activate apoptin's nuclear accumulation and apoptotic induction to a level comparable to that of wild-type LT. Given that the Rb binding domain was dispensable for apoptin's activation, these results together support the hypothesis that, in addition to being necessary, the first 82 aa of LT (the J domain) seem to be sufficient to signal the activation of apoptin.
FIG. 4.
Apoptin is activated by an N-terminal determinant of LT. (A) Differential activation of apoptin by a collection of SV40 LT mutants. pCMV-VP3 was cotransfected in VH10 cells with pRSV-LT (wild-type LT), pRSV-3213 (pRb binding mutant), pRSV-5031 (p53 binding mutant), dl1135 (J domain deletion), and pRSV-TN136 (truncated LT containing N-terminal 136 aa), and pCMV-neo as a negative control. Three separate cotransfection experiments were performed. Cells were analyzed 2, 3, and 4 days posttransfection. Immunofluorescence analysis was performed as described in the text, except that the expression of dl1135 was determined by antibody KT3. (B) Activation of apoptin by the NLS-tagged SV40 st (nls-st). Cotransfection experiments were carried out with VH10 cells with pCMV-VP3 and pRSV-st encoding st, pRSV-nls-st expressing nls-st, and pCMV-neo as a control. Two separate experiments were performed. (C) Immunofluorescence images showing apoptin inactive in the presence of cytoplasmic st (first panel) but active in nuclear translocation (second and third panel) and cell killing (fourth panel) triggered by nuclear nls-st. (D) Reproducibility of the effect of SV40 proteins (wild type and mutant) on the activation of apoptin, confirmed by using an alternative DNA transduction strategy, microinjection. Comicroinjected VH10 cells were analyzed 6, 24, 48, and 72 h postmicroinjection.
Although LT does not require the coexpression of st to activate apoptin (Fig. 2A), the sequence encoding the J domain also occurs in the st protein (3). Therefore, we also addressed whether apoptin could be activated by coexpression of SV40 st alone. In both VH10 and FSK-1 cells cotransfected with pCMV-VP3 and pRSV-st encoding st alone, we found that the cytoplasmically localized st was not sufficient to activate apoptin (Fig. 4B; data for FSK-1 not shown), despite the fact that st shares with LT the key first 82 aa of the J domain. However, we noticed that st expressed by pRSV-st was present mostly in the cytoplasm, due to its lack of an NLS sequence. Whereas dl1135 was significantly less effective in the activation of apoptin because of the deletion in J domain, TN136 with an intact J domain and an NLS sequence nevertheless localized to the nucleus and functioned as effectively as the wild-type LT. These observations suggested an association between the effectiveness of J domain in its apoptin-activating function and its nuclear location. In other words, the J domain might need a nuclear target to initiate a cellular situation required for inducing or sufficient to induce apoptin's activation. Therefore, we created a version of st containing an NLS (nls-st) and tested it for its ability to activate apoptin. VH10 cells were cotransfected with pCMV-VP3, pRSV-nls-st, pRSV-st, and pCMV-neo as a negative control, using the FuGENE 6 method. Whereas st remained in the cytoplasm together with inactive apoptin (Fig. 4B and C), nls-st carrying the J domain entered the nucleus (Fig. 4C) and subsequently triggered apoptin's nuclear translocation and apoptosis induction in a manner that increased with time posttransfection (Fig. 4B and C). This activation was similar to that observed with the truncated TN136 (Fig. 4A), as well as the wild-type LT (Fig. 2 and Fig. 4A). This result suggests that the J domain must indeed reside in the nucleus to activate apoptin.
To confirm the reproducibility of the effects of SV40 proteins described above on apoptin activation, we used another DNA delivery strategy, microinjection, which yielded similar results (Fig. 4D), except that the effect of SV40 proteins on apoptin activation was much quicker, likely due to the accelerated gene expression inherent in this DNA delivery technique. As seen in Fig. 4D, apoptin's nuclear translocation was visible as early as 6 h postmicroinjection. Additional microinjection experiments with pCMV-VP3 plus pRSV-nls-st or pRSV-st were carried out with another normal cell type, CD31-negative fibroblasts, which also gave similar results (data not shown).
Transient expression of SV40 LT in normal cells activates apoptin kinase both in vivo and in vitro.
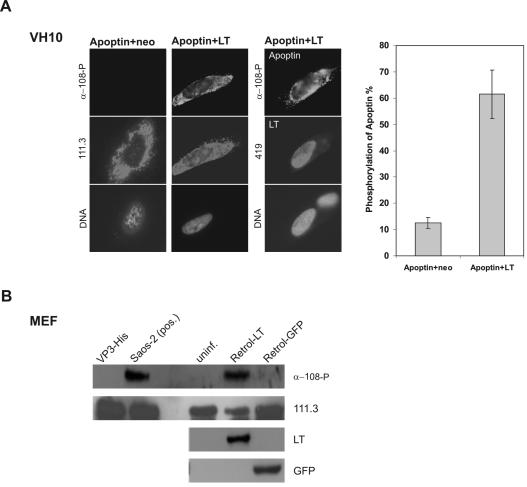
It has been shown recently that apoptin is regulated by an as-yet-unidentified tumor-specific kinase activity both in vivo and in vitro, which phosphorylates apoptin on position of Thr108 and thereby activates it (38). Particularly pertinent to our study here, apoptin phosphorylation has been confirmed in two SV40-transformed cell lines, VH/SV and SVK-14 (38). Therefore, our observation that transient coexpression of LT in the counterpart normal fibroblasts (VH10) and keratinocytes (FSK-1) could activate apoptin suggested a possible link between the LT-established transient signaling pathway and apoptin's tumor-specific kinase. To address this hypothesis, we set out to determine whether transient expression of LT constructs could lead to the phosphorylation of apoptin in normal cells. First, we comicroinjected (i) pCMV-VP3 plus pRSV-LT and (ii) pCMV-VP3 plus pCMV-neo as a negative control into the nucleus of live VH10 cells. In our experience, the protein expression from injected DNA is generally detectable 3 h after injection. At 6 h postinjection, cells were fixed and double stained with antibody α-108-P to detect phosphorylated apoptin and with antibody 111.3 to determine the basal apoptin, regardless of the state of phosphorylation. In parallel samples, antibody 419 was used to confirm the coexpression of SV40 LT. We have shown previously that, at a population level (e.g., in a cell lysate), apoptin phosphorylation is negligible or absent after transfection in low-passage nontransformed cells (38). However, when assessed at the single-cell level in immunofluorescence assays, certain cells in the culture manifest transient kinase activity, translating to 5 to 20% of cells harboring α-108-P-positive apoptin protein at any given time (J. L. Rohn, unpublished data). In contrast, in tumor cell lines, 80 to 100% of apoptin-positive cells stained positively for α-108-P, suggestive of constitutive kinase activity (Rohn, unpublished). Consistent with these observations, the percentage of phosphorylated apoptin to basal apoptin (i.e., the ratio of α-108-P-positive cells to 111.3-positive cells) in VH10 cells coinjected with apoptin and empty vector was 14% (Fig. 5A). In contrast, in the cells coinjected with pCMV-VP3 and pRSV-LT, apoptin was phosphorylated at 6 h after injection at a significantly higher level of 62% (Fig. 5A), indicating that even transient expression of LT induces apoptin's phosphorylation on Thr108. Moreover, double staining of apoptin with α-108-P and 111.3 frequently displayed phosphorylated apoptin in the cytoplasm (Fig. 5A), suggesting that the phosphorylation of apoptin in the transient transforming situation was an early event, possibly preceding apoptin's nuclear translocation and apoptosis induction. This contrasts with the situation in tumor cells, where some phosphorylated apoptin could be detected in the cytoplasm, while the majority was always found in the nuclear pool (38).
FIG. 5.
Activation of apoptin kinase by SV40 LT in normal cells both in vivo and in vitro. (A) In vivo phosphorylation of apoptin in VH10 cells. VH10 cells were comicroinjected with pCMV-VP3 and pRSV-LT and with pCMV-VP3 and pCMV-neo as negative control. 6 h postmicroinjection, coinjected cells were analyzed with the apoptin phosphospecific antibody α-108-P for phosphorylated apoptin (top), 111.3 for basal apoptin (middle images in the first and second panel), and 419 for coexpressed LT (middle image in the third panel). In addition, the bottom images of each panel show nuclear DNA staining with DAPI. The graph shows the percentage of phosphorylated apoptin as a ratio of α-108-P-positive cells to 111.3-positive-cells. (B) In vitro phosphorylation of apoptin in MEF cells. Using purified apoptin protein VP3-His as substrate, in vitro kinase assays were performed with MEF cells infected with LT-expressing retrovirus (Retrol-LT) or control GFP retrovirus (Retrol-GFP) 4 days postinfection, uninfected MEF (uninf.) as an additional negative control, and Saos-2 tumor cells as a positive control [Saos-2 (pos.)]. The blot was probed with antibody α-108-P for the phosphorylated apoptin, with 111.3 for the addition of apoptin-His protein, and with 419 and mouse α-GFP for the expression of SV40 LT and the control protein GFP, respectively.
We next set out to determine whether this increase in phosphorylated apoptin was correlated in a concomitant induction or increase of apoptin kinase activity when assessing the lysates of cells which transiently express LT. To analyze similar aberrant versus counterpart normal cells at a genetic level, we infected primary MEF cells with an ecotropic SV40 LT-encoding retrovirus or with a control GFP-encoding retrovirus, comparing these infected MEF cells to uninfected MEF cells for an additional negative control. Cells were then harvested, and lysates were produced for an in vitro kinase assay with purified apoptin protein VP3-His as a substrate and assayed for specific phosphorylation on Thr108 by α-108-P Western blotting. Strikingly, while there was virtually no kinase activity detectable in the uninfected MEF cells or in MEF cells infected with GFP control virus, the SV40 LT-expressing retrovirus induced very strong activation of apoptin kinase, comparable to the level of constitutive kinase activity in the positive control Saos-2 tumor cells (Fig. 5B). These results further support our model that SV40 LT leads to rapid activation of apoptin's (regulatory and/or activating) tumor-specific kinase.
The domain on SV40 LT responsible for the activation of apoptin kinase is also restricted to the N-terminal J domain.
Because we had mapped the domain capable of activating apoptin to aa 1 to 82 of the J domain, we next tested whether this region was also necessary and sufficient to activate apoptin kinase and subsequent phosphorylation of apoptin on Thr108. To address this question, we comicroinjected pCMV-VP3 plus pRSV-nls-st, pRSV-st (wild type), or pCMV-neo (negative control) into VH10 cells and tested the percentage of phosphorylated apoptin at 6 h postinjection, as already described. Compared to the negative control (13% basal phosphorylation), nls-st induced 63% phosphorylation, whereas wild-type st lacking the NLS did not induce phosphorylation significantly above background (data not shown). These results suggest that the J domain directed to the nucleus was sufficient to activate apoptin's kinase to a level comparable to that stimulated by the wild-type LT (Fig. 5A).
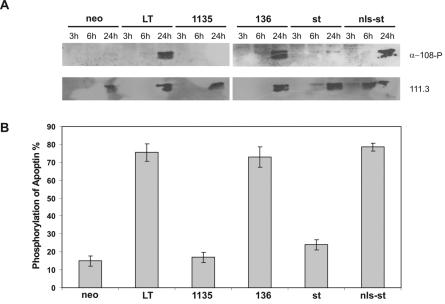
To follow up these results with a larger sample size, we decided to inspect the induction of apoptin phosphorylation on a population level in cells cotransfected with apoptin and various LT mutants, using α-108-P reactivity on Western blotting as a readout. To this end, we used AMAXA nucleofection to cotransfect CD31-negative primary human normal fibroblasts with the apoptin-expressing pCMV-VP3 plasmid plus the following SV40 protein-encoding pRSV-based plasmids: LT, dl1135, TN136, st, nls-st, or pCMV-neo as a negative control. We analyzed cells at 3, 6, and 24 h posttransfection for apoptin phosphorylation activity and protein expression (in our hands, the latter was not robustly detected until 24 h posttransfection) by Western blotting. As shown in Fig. 6A, all SV40 proteins previously shown to activate apoptin's ability to translocate to the nucleus and induce apoptosis in normal cells were also able to induce the phosphorylation of apoptin on Thr108. Specifically, cotransfection with LT, TN136, and nls-st plasmids resulted in a robustly phosphorylated apoptin protein (at 24 h), whereas cotransfection with dl1135 and wild-type st plasmids did not (Fig. 6A). Restaining the blots with 111.3 confirmed the similar levels of expression of basal apoptin at 24 h posttransfection in all cases (Fig. 6A). In parallel, immunofluorescence assays, as an independent assessment of apoptin phosphorylation, were performed 24 h posttransfection to score the ratio of α-108-P-positive cells to 111.3-positive cells at the single-cell level as described above. The results again correlated the ability of an SV40 protein to activate apoptin with its ability to phosphorylate apoptin; specifically, whereas cotransfections with LT, TN136, and nls-st plasmids showed more than 70% of all apoptin-containing cells harboring the phosphorylated form, the number was drastically lower for cells cotransfected with dl1135 (17%) and wild-type st (24%), which was only slightly higher than the background phosphorylation level in the negative control (15%) (Fig. 6B). Taken together, our results confirm that, along with nuclear localization and apoptosis induction, the SV40 J domain targeted to the nucleus is both necessary and sufficient to confer robust activation of apoptin kinase.
FIG. 6.
Apoptin kinase is activated by an N-terminal determinant of LT. The CD31-negative human primary fibroblasts were cotransfected using nucleofection transfection with pCMV-VP3 plus pRSV-LT, pRSV-dl1135, pRSV-136, pRSV-st, pRSV-nls-st, or pCMV-neo as a negative control and analyzed at the given times posttransfection by Western blot assay and parallel immunofluorescence microscopy. (A) Western blots showing the phosphorylated apoptin probed with α-180-P and the basal apoptin reprobed with 111.3. (B) Percentage of phosphorylation of apoptin at 24 h after transfection, scored as described in the legend to Fig. 5A. Two independent experiments were performed.
DISCUSSION
Apoptin is a tumor-specific death protein, which is also activated in SV40-transformed human fibroblasts. In this study, we show that transient expression of SV40-components in normal cells was sufficient to confer upon apoptin three of its tumor-specific properties. Not only could LT rapidly induce the nuclear translocation and apoptosis induction characteristic of apoptin in tumor cells, but strikingly, it also upregulated the activity of the cellular kinase responsible for phosphorylating apoptin on Thr108, a modification known to activate apoptin (38). Furthermore, all three of these tumor-specific properties could be induced by a small N-terminal determinant of LT, the J domain, which has been shown to be one of the main transforming domains of LT (13, 46). The related protein st, which also carries the J domain, did not confer apoptin activation unless it was fused to the NLS domain and thus transferred to the nucleus. These results establish an important correlation between a transient transforming situation and a tumorigenic state in which apoptin is activated via the same cellular pathway. Moreover, our demonstration that an LT gene-encoded transformation domain activates apoptin kinase closes the link between the observations that apoptin is activated in transformed cells (10) and that apoptin is activated by phosphorylation (38). This hypothesis is strengthened by experiments with several alanine mutants of full-length apoptin that could not be phosphorylated and showed a significant impairment in the ability to induce apoptosis in human tumor cells, although apoptosis was not abolished completely (Rohn, unpublished). This result was not unexpected, because apoptin has two distinct apoptosis domains (12), of which only one (the C-terminal death domain) maps in the vicinity of the phosphorylation site. Indeed, when a mutant was prepared which lacked both the N-terminal death domain and the C-terminal phosphorylation site, apoptin's killing activity was abolished completely (Rohn, unpublished).
CAV infection is often associated with the coinfection of transforming avian viruses such as Marek's disease (30), which may upregulate the kinase of apoptin during cellular transformation, thereby offering an opportunistic advantage to CAV. Interestingly, it was shown that VP2, one of the other CAV proteins, can function as a dual-specific protein phosphatase in vitro (34). Although this work did not address in vivo substrates, it is consistent with the idea that phosphorylation pathways may be important for CAV's replication cycle and for apoptin's activity.
Cole and Tevethia have reported that N-terminal as well as the C-terminal LT segments sensitize rat embryo fibroblasts to apoptosis following genotoxic damage (8). Therefore, specific functions of LT and/or st may contribute to cell transformation or to the induction of apoptosis in the presence of certain factors. In our experiments, it was shown that transient expression of SV40 proteins did not induce apoptosis in human normal cells (Fig. 1C), which excluded the possibility that LT and/or st per se could sensitize the cells to apoptosis and further confirmed that the activity of apoptin was induced by the transient expression of SV40 components in normal cells.
It has been shown that full-length LT mutants incapable of binding either pRb or p53 were capable of relieving contact inhibition, a hallmark of transformation (40). Very recently, a study of growth of SV40-transformed human mesothelial cells has provided findings relevant to the process of SV40-mediated human cell transformation, an effect that cannot be accounted for solely by SV40 LT inhibition of pRb and p53 (2). These reports confirm that inhibition of pRb and p53 is insufficient for SV40 LT transformation. Interestingly, the experiments described in this report show that these same two properties are also dispensable for the activation of apoptin. In addition, LT was capable of activating apoptin effectively in p53-mutated cell types MEF (murine; p53−/−) and GM1492 (human; p53+/−) (data not shown), further suggesting that apoptin's activation did not result from the inactivation of the tumor suppressor protein p53 by SV40 LT.
Besides the pRb and p53 binding domains, the N-terminal J domain is one of the main regions implicated in transformation. This region has a conserved sequence homology with the J domain of the DnaJ family of molecular chaperones and plays an indispensable role in viral replication, transcription control, and virion assembly, as well as transformation (13, 20, 43, 46). Different J domain mutants display various degrees of penetration for transformation ability (46). The J domain mutant dl1135(▵17-27) fails to transform established cell lines and to immortalize primary cells (9, 25, 37, 48). In our hands, dl1135 brought about a significant reduction (almost 50%) in apoptin activation, suggesting an involvement of J domain-related function in the activation of apoptin. The incomplete abolishment of apoptin's activation by dl1135 could result from the multiple effects of other known and possibly unknown functional domains in LT. Subsequent experiments with the truncated mutant TN136 supported the hypothesis that the insufficient effect of dl1135 on apoptin's activation was due to the N-terminal J domain deletion. It has been reported that amino- and carboxy-terminal transforming activities of SV40 LT can act independently, as demonstrated in studies in which coexpression of two separate LT fragments (aa 1 to 147, containing the pRb binding domain, and aa 251 to 708, containing p53 binding domain) resulted in immortalization of primary cells at an efficiency comparable with that of full-length LT (47). TN136 contains an intact J domain, with a pRb binding functional domain and NLS, comprising 136 aa of the N terminus of LT. Possibly because of the amino-terminal transforming potential of LT as reported above, TN136 functioned as well as wild-type LT toward activating apoptin, supporting the hypothesis that the activation of apoptin is induced via the N-terminal fragment of LT. Taken together with the data that the pRb and p53 binding domains were not involved in apoptin's activation, as well as the fact that nls-st could activate apoptin but st alone could not, we conclude that an intact J domain is likely to be both necessary and sufficient for activating apoptin, provided that this J domain can access the nucleus.
A recent in vivo study has shown that coexpression of the SV40 early region (LT/st), the gene encoding the telomerase catalytic subunit, and an oncogenic allele of the H-ras gene in various human cell types conferred upon these cells the ability to form tumors in mice, emphasizing that in addition to the ability of LT to disable pRb and p53, the additional perturbation of protein phosphatase 2A by the C terminal unique to st was required for complete transformation of human cells (19), although interestingly, not the J domain. Moreover, a requirement for nuclear localization of st was not seen in these experiments. These results suggest that the effects mediated by st are complex; further study will be required to determine whether the apoptin-activating pathway described in this report is involved in in vivo tumor growth.
Our experiments demonstrated that the J domain of SV40 LT is necessary and sufficient not only for activating apoptin's nuclear translocation and apoptosis induction but also for upregulating apoptin's cellular kinase activity. Our LT and st derivatives resulting in the activation of these three processes share the N-terminal 82 aa that are now known to exhibit DNA J-domain function and not the protein phosphatase 2A binding domain (39). However, we cannot exclude other unknown activities within the N-terminal 1 to 82 aa shared by LT and st to be also of importance. Because no evidence of association or colocalization of LT with apoptin was detected, it seems likely that kinase activity is upregulated downstream of a nuclear pathway issuing from the J domain of LT and/or nls-st. Phosphorylation of apoptin seems to be an immediate event, as it occurs in the cytoplasm soon after coexpression of LT, prior to apoptin's nuclear translocation and apoptosis induction. Indeed, our previous work showed that a phospho-mimic apoptin mutant, T108E, could translocate to the nucleus of normal cells and kill them, further supporting the notion that phosphorylation of apoptin is an early rather than a late event in the activation process (38). Because the J domain of LT can confer upon apoptin the ability to act in a tumor-specific manner, we speculate that apoptin has evolved to be an opportunistic substrate of a pathway common to most transformed and tumorigenic cells and possibly essentially so. Further studies should determine whether and how novel kinase cascades are involved in SV40-J-domain-activated apoptin phosphorylation, which may in turn shed light on the complex process of (human) tumorigenesis.
The J domain of LT has also been shown to govern the interaction of LT with Hsc70, a member of a chaperone family containing a conserved domain structure with a large amino-terminal ATPase domain (13, 46). It has been suggested that the chaperone activity of SV40 LT is responsible for the first step in the process of cellular immortalization in vitro: the extension of life span inherent in most transformed cells (21). Because the J domain associates with Hsc70 and because apoptin's activation is J-domain dependent, we speculate that the molecular chaperones of LT and Hsc70 may well play role(s) in LT's initiation of the transient transforming situation capable of activating apoptin kinase and subsequent activities. Interestingly, LT is actively involved in regulating the activation states of its targets, such as the Rb-related proteins p130 and p107 (13, 23, 24, 44, 45) and cellular kinases (49), by phosphorylation. Further investigation into how apoptin's kinase is activated by the J domain may address this hypothesis.
Taken together, our data support a model whereby a cellular pathway triggered by the N-terminal determinant of SV40 LT rapidly induces apoptin kinase, which in turn activates apoptin to translocate to the nucleus and induce apoptosis. Given the broad scope of apoptin's tumor-specific activity, this pathway is likely to be switched on in all transformed or tumorigenic cells.
Acknowledgments
We thank A. G. Jochemsen for donating MEFs and the wild-type SV40 protein-expressing plasmids, B. Klein for VH/SV cells, J. M. Pipas for the LT mutant plasmids, K. Rundell for the st-expressing plasmid, and N. Henriquez and L. Van Dijk for technical assistance.
This work was supported by grants from The Netherlands Ministry of Economic Affairs, The Hague, The Netherlands, and Schering AG, Berlin, Germany.
REFERENCES
- 1.Abrahams, P. J., A. Houweling, and A. J. Van der Eb. 1992. High levels of enhanced reactivation of herpes simplex virus in skin fibroblasts from various hereditary cancer-prone syndromes. Cancer Res. 52:53-57. [PubMed] [Google Scholar]
- 2.Bocchetta, M., L. Miele, H. I. Pass, and M. Carbone. 2003. Notch-1 induction, a novel activity of SV40 required for growth of SV40-transformed human mesothelial cells. Oncogene 22:81-89. [DOI] [PubMed] [Google Scholar]
- 3.Brodsky, J. L., and J. M. Pipas. 1998. Polymavirus T antigen: molecular chaperones for multiprotein complexes. J. Virol. 72:5329-5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butel, J. S. 2000. Simian virus 40, poliovirus vaccines, and human cancer: research progress versus media and public interests. Bull. W. H. O. 78:195-198. [PMC free article] [PubMed] [Google Scholar]
- 5.Butel, J. S., and J. A. Lednicky. 1999. Cell and Molecular biology of simian virus 40: implications for human infections and disease. J. Natl. Cancer Inst. 91:119-134. [DOI] [PubMed] [Google Scholar]
- 6.Carbone, M., P. Fisso, and H. I. Pass. 1997. Simian virus 40, poliovaccines and human tumors: a review of recent developments. Oncogene 15:1877-1888. [DOI] [PubMed] [Google Scholar]
- 7.Castellino, A. M., P. Cantalupo, I. M. Marks, J. V. Vartikar, K. W. Peden, and J. M. Pipas. 1997. trans-Dominant and non-trans-dominant mutant simian virus 40 large T antigen show distinct responses to ATP. J. Virol. 71:7549-7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole, S. L., and M. J. Tevethia. 2002. Simian virus 40 large T antigen and two independent T-antigen segments sensitize cells to apoptosis following genotoxic damage. J. Virol. 76:8420-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, B. S., and J. M. Pipas. 1995. T antigen encoded by replication-defective simian virus 40 mutants dl1135 and 5080. J. Biol. Chem. 270:15377-15384. [DOI] [PubMed] [Google Scholar]
- 10.Danen-van Oorschot, A. A. A. M., D. Fischer, J. M. Grimbergen, B. Klein, S.-M. Zhuang, J. H. F. Falkenburg, C. Backendorf, P. H. A. Quax, A. J. Van der Eb, and M. H. M. Noteborn. 1997. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc. Natl. Acad. Sci. USA 94:5843-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danen-van Oorschot, A. A. A. M., A. J. van der Eb, and M. H. M. Noteborn. 2000. The chicken anemia virus-derived protein Apoptin requires activation of caspases for induction of apoptosis in human tumor cells. J. Virol. 74:7072-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danen-van Oorschot, A. A. A. M., Y.-H. Zhang, S. R. Leliveld, J. L. Rohn, M. C. M. J. Seelen, M. W. Bolk, A. Van Zon, S. J. Erkeland, J.-P. Abrahams, D. Mumberg, and M. H. M. Noteborn. 2003. Importance of nuclear localization of Apoptin for tumor-specific induction of apoptosis. J. Biol. Chem. 278:27729-27736. [DOI] [PubMed] [Google Scholar]
- 13.DeCaprio, J. A. 1999. The role of the J domain of SV40 large T in cellular transformation. Biologicals 27:23-28. [DOI] [PubMed] [Google Scholar]
- 14.Deppert, W., and R. Schirmbeckt. 1995. The nuclear matrix and virus function. Int. Rev. Cytol. 162A:485-537. [DOI] [PubMed] [Google Scholar]
- 15.De Ronde, A., C. J. Sol, A. Van Strien, J. Ter Schegget, and J. Van der Noordaa. 1989. The SV40 small t antigen is essential for the morphological transformation of human fibroblasts. Virology 171:260-263. [DOI] [PubMed] [Google Scholar]
- 16.Fischer, D., F., S. Gibbs, P. Van de Putte, and C. Backendorf. 1996. Interdependent transcription control elements regulate the expression of the SPRR2A gene during keratinocyte terminal differentiation. Mol. Cell. Biol. 16:5365-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geissler, E. 1990. SV40 and human brain tumor. Prog. Med. Virol. 37:211-222. [PubMed] [Google Scholar]
- 18.Gorman, C., R. Padmanahan, and B. H. Howard. 1983. High efficiency DNA-mediated transformation of primate cells. Science 221:551-553. [DOI] [PubMed] [Google Scholar]
- 19.Hahn, W. C., S. K. Dessain, M. W. Brooks, J. E. King, B. Elenbaas, D. M. Sabatini, J. A. DeCaprio, and R. A. Weinberg. 2002. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol. 22:2111-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley, W. L., and S. J. Landy. 1994. Chaperone power in a virus? Trends Biochem. Sci. 19:277-278. [DOI] [PubMed] [Google Scholar]
- 21.Krøll, J. 2002. Molecular chaperones and the process of cellular immortalization in vitro. Biogerontology 3:183-185. [DOI] [PubMed] [Google Scholar]
- 22.Leliveld, S. R., Y.-H. Zhang, L. R. Rohn, M. H. M. Noteborn, and J. P. Abrahams. 2003. Apoptin induces tumor-specific apoptosis as globular multimer. J. Biol. Chem. 278:9042-9051. [DOI] [PubMed] [Google Scholar]
- 23.Ludlow, J. W., J. A. DeCaprio, C.-M. Huang, W.-H. Lee, E. Paucha, and D. M. Livingston. 1989. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell 56:57-65. [DOI] [PubMed] [Google Scholar]
- 24.Ludlow, J. W., J. Shon, J. M. Pipas, D. M. Livingston, and J. A. DeCaprio. 1990. The retinoblastoma susceptibility gene product undergoes cell cycle-dependent dephosphorylation and binding to and release from SV40 large T. Cell 60:387-396. [DOI] [PubMed] [Google Scholar]
- 25.Michalovitz, D., L. Fischer-Fantuzzi, C. Vesco, J. M. Pipas, and M. Oren. 1987. Activated Ha-ras can cooperate with defective simian virus 40 in the transformation of nonestablished rat embryo fibroblasts. J. Virol. 61:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titer retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noteborn, M. H. M. 2002. Apoptin as an anticancer therapy, p. 275-287. In N. B. La Thangue and L. R. Bandara (ed.), Targets for cancer chemotherapy. Humana Press, Totowa, N.J.
- 28.Noteborn, M. H. M., A. A. A. M. Danen-van Oorschot, and A. J. Van der Eb. 1998. Chicken anemia virus: induction of apoptosis by a single protein of a single-stranded DNA virus. Semin. Virol. 8:497-504. [Google Scholar]
- 29.Noteborn, M. H. M., G. F. de Boer, D. J. van Roozelaar, C. Karreman, O. Kranenburg, J. G. Vos, S. H. M. Jeurissen, R. C. Hoeben, A. Zantema, G. Koch, H. van Ormondt, and A. J. van der Eb. 1991. Characterization of cloned chicken anemia virus DNA that contains all elements for the infectious replication cycle. J. Virol. 65:3131-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noteborn, M. H. M., and G. Koch. 1995. Chicken anemia virus infection: molecular basis of pathogenicity. Avian Pathol. 24:11-31. [DOI] [PubMed] [Google Scholar]
- 31.Noteborn, M. H. M., D. Todd, C. A. Verschueren, H. W. de Gauw, W. L. Curran, S. Veldkamp, A. J. Douglas, M. S. McNulty, A. J. van der Eb, and G. Koch. 1994. A single chicken anemia virus protein induces apoptosis. J. Virol. 68:346-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noteborn, M. H. M., A. J. van der Eb, G. Koch, and S. H. M. Jeurissen. 1993. VP3 of the chicken anemia virus (CAV) causes apoptosis, p. 299-304. In H. S. Ginsberg, F. Brown, R. M. Chanock, and R. A. Lerner (ed.), Vaccines 93: modern approach to new vaccines including prevention of AIDS. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Peden, K. W., A. Srinivasan, J. V. Vartikar, and J. M. Pipas. 1998. Effects of mutations within the SV40 large T antigen ATPase/p53 biding domain on viral replication and transformation. Virus Genes 16:153-165. [DOI] [PubMed] [Google Scholar]
- 34.Peters, M. A., D. C. Jackson, B. S. Crabb, and G. F. Browning. 2002. Chicken anemia virus VP2 is a novel dual specificity protein phosphatase. J. Biol. Chem. 277:39566-39573. [DOI] [PubMed] [Google Scholar]
- 35.Philips, B., and K. Rundell. 1988. Failure of simian virus 40 small t antigen to disorganize actin cables in nonpermissive cell lines. J. Virol. 62:768-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pipas, J. M., and A. J. Levine. 2001. Role of T antigen interactions with p53 in tumorigenesis. Semin. Cancer Biol. 11:23-30. [DOI] [PubMed] [Google Scholar]
- 37.Pipas, J. M., K. W. Peden, and D. Nathans. 1983. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol. Cell. Biol. 3:203-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohn, J. L., Y.-H. Zhang, R. I. J. M. Aalbers, N. Otto, J. den Hertog, N. V. Henriquez, C. J. H. van de Velde, P. J. K. Kuppen, D. Mumberg, P. Donner, and M. H. M. Noteborn. 2002. A tumor-specific kinase activity regulates the viral death protein Apoptin. J. Biol. Chem. 277:50820-50827. [DOI] [PubMed] [Google Scholar]
- 39.Rundell, K., and R. Parakati. 2001. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin. Cancer Biol. 11:5-13. [DOI] [PubMed] [Google Scholar]
- 40.Sachsenmeier, K., and J. M. Pipas. 2001. Inhibition of Rb and p53 is insufficient for SV40 T-antigen transformation. Virology 283:40-48. [DOI] [PubMed] [Google Scholar]
- 41.Sáenz-Robles, M. T., C. Sullivan, and J. M. Pipas. 2001. Transforming functions of simian virus 40. Oncogene 20:7899-7907. [DOI] [PubMed] [Google Scholar]
- 42.Smits, P. H., A. De Ronde, H. L. Smits, R. P. Minnaar, J. Van Der Noordaa, and J. Ter Schegget. 1992. Modulation of the human papillomavirus type 16 induced transformation and transcription by deletion of loci on the short arm of human chromosome 11 can be mimicked by SV40 small t. Virology 190:40-44. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan, A., A. J. McClellan, J. Vartikar, I. Marks, P. Cantalupo, Y. Li, P. Whyte, K. Rundell, J. L. Brodsky, and J. M. Pipas. 1997. The amino-terminal transforming region of simian virus 40 large T and small t antigen functions as a J domain. Mol. Cell. Biol. 17:4761-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stubdal, H., J. Zalvide, K. S. Campbell, C. Schweitzer, T. M. Roberts, and J. A. DeCaprio. 1997. Inactivation of pRb-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol. Cell. Biol. 17:4979-4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stubdal, H., J. Zalvide, and J. A. DeCaprio. 1996. Simian virus 40 large T antigen alters the phosphorylation state of the Rb-related proteins p130 and p107. J. Virol. 70:2781-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan, C. S., and J. M. Pipas. 2002. T antigen of simian virus 40: molecular chaperones for viral replication and tumorigenesis. Microbiol. Mol. Biol. Rev. 66:179-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tevethia, M. J., H. A. Lacko, and A. Conn. 1998. Two regions of simian virus 40 large T-antigen independently extend the life span of primary C75BL/6 mouse embryo fibroblasts and cooperate in immortalization. Virology 243:303-312. [DOI] [PubMed] [Google Scholar]
- 48.Yaciuk, P., M. C. Carter, J. M. Pipas, and E. Moran. 1991. Simian virus 40 large-T antigen expresses a biological activity complementary to the p300-associated transforming function of the adenovirus E1A gene products. Mol. Cell. Biol. 11:2116-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu, Y., and J. C. Alwine. 2002. Human cytomegalovirus major immediate-early proteins and simian virus large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and the cellular kinase Akt. J. Virol. 76:3731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, Y.-H., P. J. Abrahams, A. J. van der Eb, and M. H. M. Noteborn. 1999. The viral protein Apoptin induces apoptosis in UV-C-irradiated cells from individuals with various hereditary cancer-prone syndromes. Cancer Res. 59:3010-3015. [PubMed] [Google Scholar]
- 51.Zhang, Y.-H., S. R. Leliveld, K. Kooistra, C. Molenaar, J. L. Rohn, H. J. Tanke, J. P. Abrahams, and M. H. M. Noteborn. 2003. Recombinant apoptin multimers kill tumor cells but are nontoxic and epitope-shielded in a normal-cell-specific fashion. Exp. Cell. Res. 289:36-46. [DOI] [PubMed] [Google Scholar]
- 52.Zhuang, S.-M., J. E. Landegent, C. A. Verschueren, J. H. Falkenburg, H. van Ormondt, A. J. van der Eb, and M. H. M. Noteborn. 1995. Apoptin, a protein encoded by chicken anemia virus, induces cell death in various human hematologic malignant cells in vitro. Leukemia 9(Suppl. 1):S118-S120. [PubMed] [Google Scholar]
- 53.Zhuang, S.-M., A. Shvarts, H. van Ormondt, A. G. Jochemsen, A. J. van der Eb, and M. H. M. Noteborn. 1995. Apoptin, a protein derived from chicken anemia virus, induces p53-independent apoptosis in human osteosarcoma cells. Cancer Res. 55:486-489. [PubMed] [Google Scholar]