Abstract
The nuclear receptors CAR and PXR activate hepatic genes in response to therapeutic drugs and xenobiotics, leading to the induction of drug-metabolizing enzymes, such as cytochrome P450. Insulin inhibits the ability of FOXO1 to express genes encoding gluconeogenic enzymes. Induction by drugs is known to be decreased by insulin, whereas gluconeogenic activity is often repressed by treatment with certain drugs, such as phenobarbital (PB). Performing cell-based transfection assays with drug-responsive and insulin-responsive enhancers, glutathione S-transferase pull down, RNA interference (RNAi), and mouse primary hepatocytes, we examined the molecular mechanism by which nuclear receptors and FOXO1 could coordinately regulate both enzyme pathways. FOXO1 was found to be a coactivator to CAR- and PXR-mediated transcription. In contrast, CAR and PXR, acting as corepressors, downregulated FOXO1-mediated transcription in the presence of their activators, such as 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) and pregnenolone 16α-carbonitrile, respectively. A constitutively active mutant of the insulin-responsive protein kinase Akt, but not the kinase-negative mutant, effectively blocked FOXO1 activity in cell-based assays. Thus, insulin could repress the receptors by activating the Akt-FOXO1 signal, whereas drugs could interfere with FOXO1-mediated transcription by activating CAR and/or PXR. Treatment with TCPOBOP or PB decreased the levels of phosphoenolpyruvate carboxykinase 1 mRNA in mice but not in Car−/− mice. We conclude that FOXO1 and the nuclear receptors reciprocally coregulate their target genes, modulating both drug metabolism and gluconeogenesis.
Liver plays a major role in the metabolism of therapeutic drugs and environmental contaminants. It is endowed with a mechanism to induce hepatic enzymes, leading to increased detoxification and elimination of those xenobiotics. Drug induction is generally regulated by transcriptional activation of hepatic genes encoding drug-metabolizing enzymes, such as cytochrome P450s (CYPs) and specific transferases. Acting as the principal transcription factors which also form complexes with RXR, the nuclear receptors CAR and PXR play a central role in induction by binding to the phenobarbital (PB)- and xenobiotic-responsive enhancer modules PBREM and XREM (5, 6, 11, 12, 18, 46, 53), respectively, and activating transcription of their target genes, such as CYP genes, in response to a distinct but overlapping group of xenobiotics (23, 36, 50). However, induction is heavily influenced by endocrine conditions; glucocorticoid hormone, for example, augments CYP induction by PB (31). In contrast, insulin is known to repress the induction of drug-metabolizing activity by certain drugs and in diabetic livers (44, 49, 56). Insulin treatment either eliminated or significantly reduced PB induction of CYP2B in rat primary hepatocytes (17, 42, 58). Hepatic CYP2B, CYP3A, and CYP4A were increased in experimentally generated diabetic rats and mice and were reduced to normal levels by insulin treatment (37, 56). While insulin is involved in regulating drug metabolism, drug treatment influences insulin-responsive glucose metabolism. For instance, both PB and 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) decreased hepatic enzymes, such as phosphoenolpyruvate carboxykinase 1 (PEPCK1) and glucose-6-phosphatase (G6P) (1, 24, 50). Chronic PB treatment decreased plasma glucose and improved insulin sensitivity in diabetic patients (20). Here we investigated the molecular mechanism of insulin repression by focusing on whether CAR and/or PXR could be coregulated by insulin response transcription factor FOXO1 and vice versa.
A forkhead transcription factor, FOXO1, is an activator of gluconeogenic genes, such as PEPCK1, G6P, and insulin-like growth factor-binding protein 1 (29, 32, 38, 57). Insulin inhibits FOXO1 activity, leading to repression of these genes. In fact, the insulin receptor knockout mouse partially restored serum glucose to normal levels when it was crossed with a Foxo1+/− mouse (28). These gluconeogenic genes contain an insulin response sequence (IRS) to which FOXO1 can bind directly and activate in the absence of insulin (7, 32, 38, 51, 57). Insulin treatment triggers the phosphorylation of FOXO1 via a phosphatidylinositol 3-kinase (PI3K)-Akt pathway (7, 30, 35). Phosphorylation inactivates FOXO1 by decreasing the binding affinity of FOXO1 to IRS and/or exporting FOXO1 from the nucleus (2, 25, 34, 59). FOXO1 has been implicated as a coregulator for various nuclear receptors, coactivating or corepressing the estrogen, thyroid, and retinoid A receptors (39, 60), whereas glucocorticoid and progesterone receptors (60), hepatic nuclear factor 4 (HNF4) (9), and peroxisome proliferator-activated receptor γ (PPARγ) (4) are corepressed. In addition, the androgen receptor prevents the binding of FOXO1 to IRS, leading to a repression of FOXO1-IRS activity (21). Our yeast two-hybrid screening identified FOXO1 as a CAR-binding protein. These observations prompted us to investigate whether FOXO1 mediates the insulin-dependent repression of CAR and/or PXR-mediated trans-activation of drug-induced genes, such as CYP genes. Moreover, we also investigated whether CAR and/or PXR receptors reciprocally coregulate FOXO1-IRS activity.
By employing glutathione S-transferase (GST) pull-down, mammalian two-hybrid, and transient luciferase reporter assays and in vivo animal experiments, we demonstrate here that the nuclear receptors and FOXO1 cross-talk to coregulate their response elements. Drug and glucose metabolisms, which are two major liver functions that can be regulated independently, appeared to be coordinately regulated by this cross-talk.
MATERIALS AND METHODS
Materials.
5α-Androst-16-en-3α-ol (androstenol) was purchased from Steraloids, Inc. Insulin, insulin-like growth factor 1 (IGF1), TCPOBOP, and pregnenolone 16α-carbonitrile (PCN) were purchased from Sigma-Aldrich. Restriction endonucleases and DNA-modifying enzymes were purchased from New England Biolabs, Inc. [35S]methionine was purchased from Amersham. LY294002 was purchased from Calbiochem.
Plasmid constructions.
The following plasmids were kindly provided: XREM-3A4-Luc plasmid by Brian Goodwin at GlaxoSmithKline (5); rPKBα/pECE and rPKBαK179 M/pECE by Ushio Kikkawa at Kobe University; myr-rPKBα/pcDL-SRM by Kiyoshi Hidaka at the National Institute of Environmental Health Sciences. In all plasmids, m, r, and h in front of the insert denote mouse, rat, and human, respectively. The pGL3/1.8 kbp-2B6-Luc, pGL3/1.6 kbp-2B6-Luc (52), pGEX/mCAR, pCMX/hRXR (11), pCR3/mCAR (47), and pBIND/mCAR (55) plasmids were described previously. The CAR mutant T176V in pCR3 plasmid and more details of its characterization will be published elsewhere (A. Ueda, K. Matsui, Y. Yamamoto, L. Pedersen, T. Sueyoshi, and M. Negishi, unpublished data). Full-length PXR (18) was amplified from a mouse liver cDNA library and was cloned into pcDNA3-V5-His (Invitrogen) and pGEX-4T-1 (Stratagene) vectors designated pcDNA3/mPXR and pGEX/mPXR, respectively. cDNA encoding residues 425 to 652 was amplified from a FOXO1 clone selected from the yeast two-hybrid screening and was inserted into pACT (Promega) to generate pACT/mFOXO1Ct. Using proper sets of primers, the first and second exons were amplified from mouse genomic DNA using LA taq DNA polymerase with GC buffer (TaKaRa Shuzo, Otsu, Japan) and were cloned into pCR2.1-TOPO (Invitrogen). Inserts were cut out by double digestion with BamHI and EcoRI from the TOPO plasmids, ligated, and recloned into pcDNA3-V5-His to obtain the full-length cDNA of FOXO1, designated pcDNA3/mFOXO1. Using pcDNA3/mFOXO1 as a template, proper mutated primers, and the QuikChange mutagenesis kit (Stratagene), Thr24, Ser253, and Ser316 were mutated to alanine to generate pcDNA3/mFOXO13A. To clone the full-length FOXO1 cDNA into pGEX-4T-1, the cDNA was amplified to include an additional XhoI site at the 5′ end, digested with XhoI, and inserted at the SalI site of pGEX-4T-1 to create pGEX/mFOXO1. For the various Akt expression vectors, the full-length cDNAs of wild-type Akt (AktWt), kinase-negative Akt (AktKN), and constitutively activated Akt (AktCA) were amplified from rPKBα/pECE, rPKBαK179 M/pECE, and myr-rPKBα/pcDL-SRM, respectively. These cDNAs were cloned into pcDNA3-V5-His plasmids designated pcDNA3/AktWt, pcDNA3/AktKN, and pcDNA3/AktCA, respectively. To construct the pGL3/IRS-tk-Luc plasmid, a complementary pair of oligonucleotides containing three tandem copies of IRS (5′-TCGAGCAAGCAAAACAAGCTAGCAAAACAAGTACGCAAAACAAGTA-3′ from the human IGFBP1 gene) (48) was synthesized. The underlined sequence indicates an additional overhanging XhoI restriction site. Oligonucleotides were annealed and cloned into the XhoI-digested site of the pGL3-Basic vector containing a 160-bp thymidine kinase (tk) promoter (47), and a plasmid was selected containing six repeats of IRS. For amplification of FOXO1 from its genomic clone, Pfu Turbo DNA polymerase (Stratagene) was used. Insert sequences of newly constructed vectors were verified by DNA sequencing.
Yeast two-hybrid screen.
The ligand-binding domain (residues 87 to 358) of the mouse CAR T176V mutant was cloned into the pGBKT7 plasmid (Clontech). This plasmid was cotransfected with the mouse liver MATCHMAKER cDNA library (Clontech) into the Y190 yeast strain. Positive yeast colonies were selected in the presence of TCPOBOP (1 μM), and the binding activity was measured by β-galactosidase assay according to the manufacturer's instructions. One-hundred five yeast colonies were isolated from 2.2 × 106 primary transformants. The plasmids encoding the activation domain fusion proteins were recovered from these yeast transformants, and the inserts were sequenced. Three of these inserts were found to encode FOXO1 and were cloned as a fusion protein in frame with the GAL4 activation domain.
GST pull-down assay.
The GST fusion proteins GST-mCAR, GST-mPXR, and GST-mFOXO1 were expressed in Escherichia coli strain BL21 cells and were purified with glutathione-Sepharose. The cDNAs of FOXO1, CAR, and PXR in pcDNA3-V5-His were in vitro translated in the presence of [35S]methionine using the TNT-coupled reticulocyte lysate system (Promega) according to the manufacturer's instructions. GST pull-down assays were carried out as described previously (19).
Cell culture and transfection.
HepG2 cells were cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml in an atmosphere of 5% CO2 at 37°C. Primary hepatocytes were prepared from male Cr1:CD-1(ICR)BR mice (Charles River Laboratories) as described previously (13). HepG2 cells plated in 24 wells at 50 to 60% confluence were transfected with lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. pRL-CMV (10 ng) (Promega) was used as an internal control for all transfection assays. Mouse primary hepatocytes were cotransfected with pGL3/1.8 kbp-2B6-Luc (10 μg) and pRL-CMV (5 μg) by the electroporation method (16). For experiments with RNAi, SMARTpool (M-003006-01-05; Dharmacon) for human FOXO1 or an unrelated RNA (5′-NNUGGUAGGCAGGAUGAGUAC-3′) as nonspecific control was transfected into HepG2 cells using lipofectamine 2000. After 24 h of transfection the cells were treated with drug for an additional 48 h before harvest.
Animal treatment.
Cr1:CD-1(ICR)BR male mice (7 to 8 weeks old) maintained on a 12-h light and 12-h dark cycle were randomly divided into two groups that were intraperitoneally injected with TCPOBOP (0.3 mg/kg of body weight) or dimethyl sulfoxide (DMSO). Twenty-four hours after injection, mice were fed until sacrificed to prepare liver RNAs. The original background strain of Car−/− mice used in a previous work (50) was changed to C3H/HeNCrlBR mice (Charles River) by repeated backcrossing until microsatellite analysis showed that mice contained 98% of the C3H markers. Car−/− and Car+/+ male mice were treated with a single injection of 90 mg of diethylnitrosamine/kg, followed by chronic treatment with PB in drinking water (500 ppm) for 32 weeks. These treatments were originally for investigating liver tumor promotion. Livers and blood samples were collected and used for analyses.
Real-time PCR.
TRIZOL reagent (Invitrogen) was used to extract total RNAs from HepG2 cells, mouse livers, and primary mouse hepatocytes, and cDNAs were prepared from total RNAs using Super-Script II reverse transcriptase (Invitrogen). Real-time PCR was performed with an ABI prism 7700 sequence detection system (Applied Biosystems) with the following primers and probes: Hs00231106_m1 (Applied Biosystems) for the human FOXO1 gene; 5′-6FAM-CATCACGGCCAATGTTATCTGCTCCA-TAMRA-3′, 5′-ACCCCACGTTCCTCTTCCA-3′, and 5′-CAGCAGGCGCAAGAACTGA-3′ for CYP2B10 (14); 5′-6FAM-ACAACTGTTGGCTGGCTCTCACTGACC-TAMRA-3′, 5′-GTGTCATCCGCAAGCTGAAG-3′, and 5′-TTCGATCCTGGCCACATCTC-3′ for the mouse Pepck1 gene. The TaqMAN rodent GAPDH control reagent (Applied Biosystems) was used as internal control.
Gel shift assay.
CAR, RXR, PXR, and FOXO1 proteins were produced by in vitro translation as described above. For probes, three sets of double-stranded oligonucleotides containing NR1 sequence (47), wild-type IRS (5′-GATCGCTAGATGCAAAACAACTTGTGACGATC-3′), and mutant IRS (5′-GATCGCTAGATGCAACAGAACTTGTGACGATC-3′) were synthesized and end-labeled by using [32P]dATP and DNA polymerase Klenow fragment (New England Biolab). Gel shift assays were performed as described previously (11). For competition or antibody supershift assays, unlabeled oligonucleotide, rabbit immunoglobulin G (Santa Cruz), or FOXO1 antibody (Santa Cruz) was added 15 min before adding radioactive oligonucleotide to start the binding reaction.
RESULTS
FOXO1 as a CAR-binding protein.
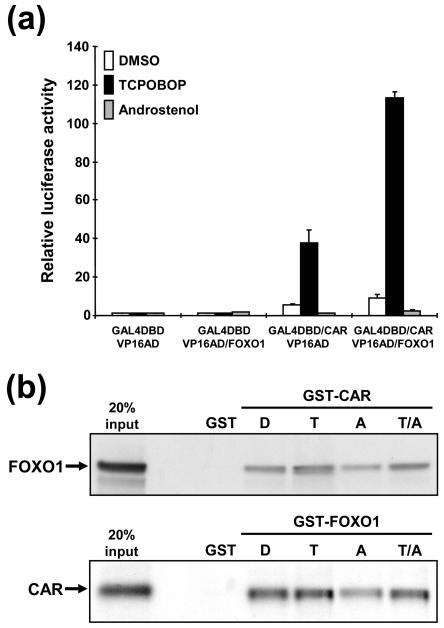
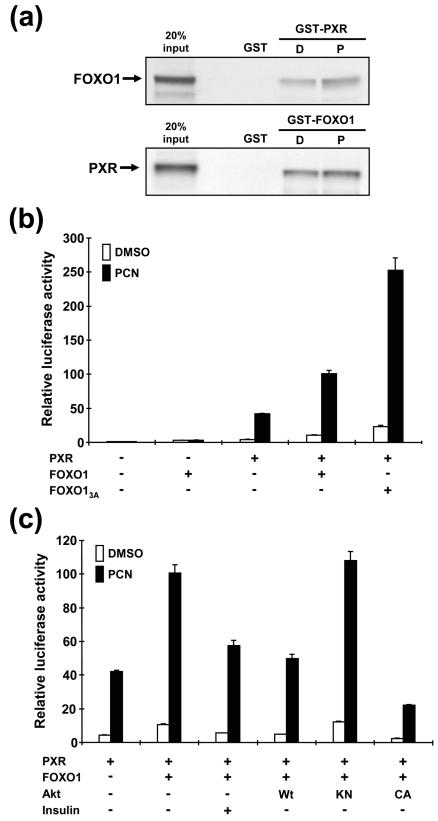
Yeast two-hybrid screening of a mouse liver cDNA library was performed using the CAR ligand-binding domain as bait. Of the total 105 positive clones isolated, 18 were RXRα and five were RXRβ. Both receptors are known to form heterodimers with CAR, indicating that the screening was effective. The forkhead transcription factor FOXO1 was also one of the positive clones, and the deduced amino acid sequences from three independent clones contained 228 residues of the C-terminal region of FOXO1. Subsequently, a mammalian two-hybrid assay was employed to examine the functional interaction between CAR and FOXO1 in which pBind/mCAR (CAR fused to the GAL4 DNA-binding domain) and VP16AD/mFOXOCt (cloned VP16 activation domain in front of FOXO1Ct) were cotransfected into HepG2 cells. Although CAR is constitutively active in HepG2 cells, its fusion with GAL4DBD decreased constitutive activity and conferred the capability of being activated by TCPOBOP (Fig. 1a). Cotransfection of VP16AD/mFOXOCt resulted in a 3.0-fold coactivation in the presence of TCPOBOP, while no such coactivation was observed in the presence of the CAR repressor androstenol (Fig. 1a). VP16AD/mFOXOCt alone did not enhance the reporter activity. We performed a GST pull-down assay to investigate the direct binding of CAR with FOXO1 by using an in vitro-translated full-length FOXO1 and a bacterially expressed recombinant GST-mCAR fusion protein or vice versa. Binding of GST-CAR (or GST-FOXO1) to the 35S-labeled FOXO1 (or CAR) was decreased in the presence of androstenol and was recovered by the addition of TCPOBOP (Fig. 1b). These results unequivocally showed that CAR binds to FOXO1 in a ligand-dependent manner.
FIG. 1.
Direct binding of CAR to FOXO1. (a) Mammalian two-hybrid assay. The pG5-Luc reporter plasmid was cotransfected with various combinations of pBIND, pACT, pBIND/mCAR, and pACT/mFOXO1Ct into HepG2 cells. The amount of each plasmid was 0.2 μg, and the total amount of plasmids was adjusted by pcDNA3-V5-His. At 24 h after transfection, cells were treated with 0.1% DMSO, TCPOBOP (250 nM), or androstenol (10 μM) for an additional 24 h. Subsequently, cells were harvested and cell extracts were prepared for dual-luciferase assays. Relative activities were calculated by taking the activity obtained from the GAL4DBD- and VP16AD-transfected cells in the presence of DMSO as one. Bars indicate means ± standard deviation. (b) GST pull-down assay. In vitro-translated 35S-labeled FOXO1 and CAR were incubated with bacterially expressed GST-mFOXO1 and GST-mCAR fusion proteins, respectively, in the presence of 0.1% DMSO, 1 μM TCPOBOP (T), 10 μM androstenol (A), or both (T/A). GST was used as a negative control for binding. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography were done as described in Materials and Methods.
FOXO1 as a CAR coactivator.
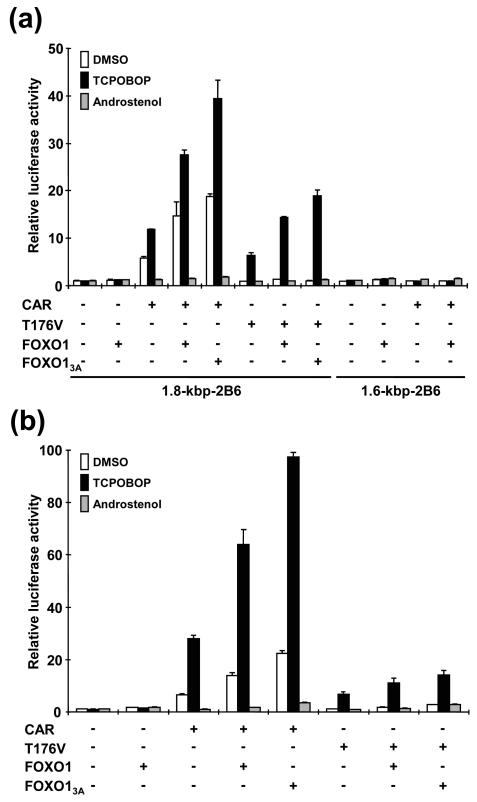
Given the fact that FOXO1 is a CAR-binding protein, a series of transient transfection assays were performed to investigate whether FOXO1 coregulated the trans-activation of the CAR target gene CYP2B6. For this purpose, the 1.8-kbp 5′-flanking DNA of the CYP2B6 gene that contains a 51-bp CAR-binding site, called PBREM, was placed in front of a Luc reporter gene. FOXO1 coactivated the 1.8 kbp-2B6-Luc reporter 2.5-fold when CAR was coexpressed in the presence and absence of TCPOBOP, whereas FOXO1 alone could not activate the reporter (Fig. 2a). FOXO1 contains three Akt phosphorylation sites (Thr-24, Ser-253, and Ser-316). The FOXO13A mutant, in which all three sites were replaced by alanine, should result in an increased retention and activity of FOXO1 in the nucleus (7, 38). The nuclear-retaining FOXO13A mutant was more effective in coactivating the reporter as indicated by an approximately 4-fold increase instead of the 2.5-fold increase for FOXO1 (Fig. 2a). To examine whether coactivation by FOXO1 required the CAR-binding site, the 1.6-kbp 5′-flanking DNA of the CYP2B6 gene that lacked the PBREM was used in the reporter assay. FOXO1 did not coactivate the 1.6 kbp-2B6-Luc reporter (Fig. 2a). Moreover, similar to the 1.8 kbp-2B6-Luc reporter, the PBREM-tk-Luc reporter gene was also coactivated by FOXO1 (data not shown). Thus, FOXO1 coactivated CAR-mediated PBREM activity. Although the fact that coactivation by FOXO1 and FOXO13A was repressed by androstenol suggested coactivation occurred when CAR was active, determining whether the coactivation required direct activation of CAR by TCPOBOP was problematic because CAR was constitutively active in transfected HepG2 cells. Therefore, the CAR mutant T176V that possessed low constitutive activity and the capability of being activated by TCPOBOP was also used for these assays. In these experiments, coactivation by FOXO1 occurred only in the presence of TCPOBOP (Fig. 2a). Because CAR activates XREM of the CYP3A gene that contains multiple receptor-binding sites (43), we also tested whether FOXO1 could coactivate the XREM (Fig. 2b). CAR-mediated XREM activity was upregulated by FOXO1 and FOXO13A 2.5- and 3.5-fold, respectively. Furthermore, this upregulation occurred only in the presence of TCPOBOP when T176V was used for assays. These results clearly indicated that FOXO1 acts as a coactivator to regulate CAR-mediated trans-activation of multiple targets.
FIG. 2.
Coactivation of CAR by FOXO1 in HepG2 cells. (a) Transient transfection assays were performed by cotransfecting pCR3/mCAR, pcDNA/T176V, pcDNA3/mFOXO1, or pcDNA3/mFOXO13A with 1.8 kbp-2B6-Luc or 1.6 kbp-2B6-Luc reporter plasmid into HepG2 cells. (b) XREM-3A4-Luc plasmid was used as the reporter. The amount of each plasmid was 0.2 μg, and the total amount of plasmid in an assay was adjusted by using an empty plasmid. Cells were treated as described in the legend to Fig. 1. Relative activities were calculated by taking the activity of the cells that were transfected by only the reporter plasmid in the presence of DMSO as one. Bars indicate means ± standard deviations.
Akt as a signal of FOXO1-CAR coactivation.
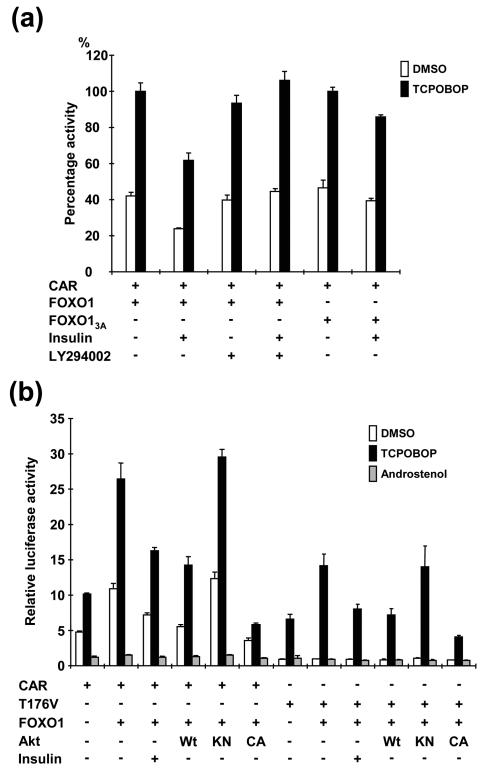
FOXO1 is an insulin response transcription factor that is regulated by the PI3K-Akt pathway. In response to insulin, PI3K inactivates FOXO1 by phosphorylation. If this inactivation was conserved in HepG2 cells, insulin treatment should decrease FOXO1-CAR activity to a lesser extent than the FOXO13A-CAR activity. Consistent with this hypothesis, insulin treatment decreased FOXO1 coactivation of CAR by about 40%, but coactivation with FOXO13A was decreased by only 14% (Fig. 3a). This differential effect of FOXO1 and FOXO13A was significantly pronounced in the presence of PI3K inhibitor LY294002. In fact, insulin did not reduce FOXO1 activity in LY294002-treated HepG2 cells (Fig. 3a). To provide further evidence that supports the direct involvement of FOXO1 in coactivating CAR, coexpression of Akt or its functional mutants should affect the coactivation accordingly. To examine this hypothesis, AktWt and its kinase-negative and constitutively activated mutants (AktKN and AktCA, respectively) were coexpressed with FOXO1, CAR, and the 1.8-kbp Luc reporter in HepG2 cells. Following insulin treatment, FOXO1 activity was decreased by 40% (Fig. 3b). Similar to this insulin response, coexpression of Akt decreased FOXO1-dependent coactivation in the presence or absence of TCPOBOP, whereas, as expected from the lack of kinase activity, AktKN did not affect the coactivation of CAR. Coexpression of AktCA appeared to effectively inactivate FOXO1, reducing the coactivation by over 80%. Neither FOXO1 coactivation of CAR nor its repression by AktWt was observed in the presence of androstenol. When the corresponding experiments were performed with the T176A mutant, AktWt and its mutants had an effect only in the presence of TCPOBOP (Fig. 3b). These results indicate that an insulin-PI3K-Akt pathway regulates FOXO1-dependent coactivation of CAR.
FIG. 3.
Regulation by rAkt of coactivation of CAR by FOXO1. Either pCR3/mCAR or pCR3/T176V was cotransfected with pcDNA3/mFOXO1, pcDNA3/rAktWt, pcDNA3/rAktKN, and/or pcDNA3/rAktCA into HepG2 cells. In addition, the 1.8 kbp-2B6-Luc reporter was also cotransfected. Except for the cases of insulin (500 nM) and LY294002 (20 μM), the drug concentrations of all treatments and cultures were the same as those described in the legend to Fig. 1. (a) Differential effects of insulin on the wild-type and mutant FOXO1-mediated CAR activities. The percentage of activity relative to the CAR- and FOXO1/FOXO13A-transfected cells in the presence of TCPOBOP is given as 100. Bars indicate means ± standard deviations. (b) Differential effects of the wild-type and mutated Akts on the FOXO1-mediated CAR activity. Relative activities were calculated by taking the activity of the cells that were transfected by only the reporter plasmid in the presence of DMSO as one.
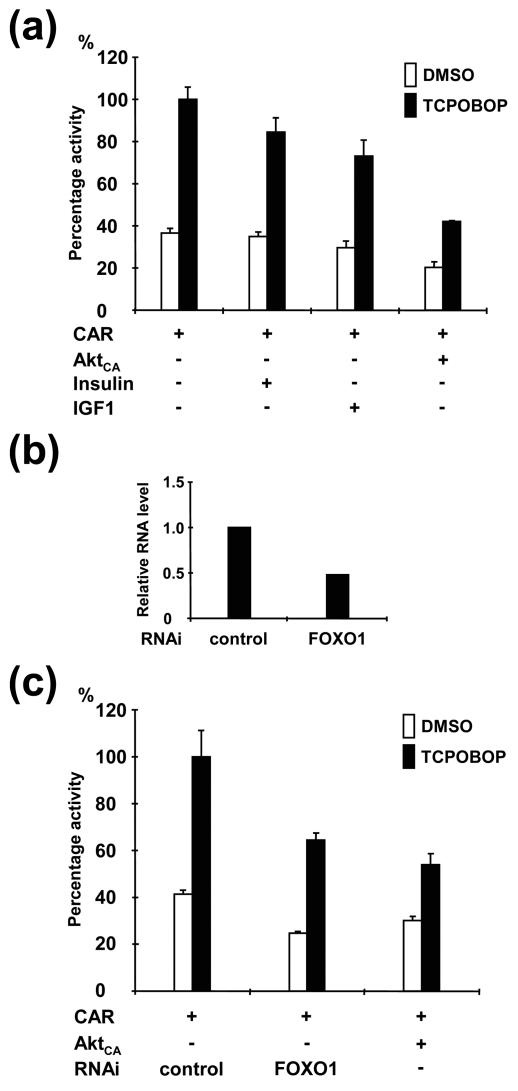
Given the fact that AktCA decreased the activity level of the 1.8 kbp-2B6-Luc reporter below the activity level observed without exogenous FOXO1 (Fig. 3b), we examined whether AktCA inactivated endogenous FOXO1 to decrease the reporter activity. For this experiment, HepG2 cells were treated with insulin and IGF1, and subsequently CAR-mediated reporter activity was measured. Although IGF1 was slightly more effective, both insulin and IGF1 reduced the activity by 15 to 25%, suggesting that AktCA inactivated endogenous FOXO1 in HepG2 cells (Fig. 4a). If, in fact, the endogenous FOXO1 was directly involved in the regulation of CAR activity via Akt, the removal of Akt should eliminate the activity. To test this hypothesis, RNAi of FOXO1 was cotransfected into HepG2 cells. Real-time PCR analysis indicated an approximately 50% reduction of endogenous FOXO1 mRNA (Fig. 4b). This reduction of endogenous FOXO1 mRNA was correlated with the repression of CAR activity (Fig. 4c). In fact, the reporter activity derived from CAR with RNAi of FOXO1 was decreased at a level equivalent to that observed by cotransfection of AktCA. These results suggested that endogenous FOXO1 could also be functional in regulating the CAR-mediated transactivation in HepG2 cells.
FIG. 4.
Regulation of CAR activity by endogenous FOXO1. (a) Transient transfection assays. The 1.8 kbp-2B6-Luc reporter and pCR3/mCAR were cotransfected with or without pcDNA3/rAktCA into HepG2 cells. Cells were cultured and treated with insulin, IGF1, and/or TCPOBOP as described in the legend to Fig. 1. Relative fold activities were calculated by taking the activity of the cells that were transfected with the pCR3/mCAR and 1.8 kbp-2B6-Luc plasmids in the presence of TCPOBOP as 100. Bars indicate means ± standard deviations. (b) Real-time PCR. HepG2 cells were transfected with FOXO1 RNAi (50 pmol) or control oligonucleotides (50 pmol) in MEM supplemented with 10% fetal bovine serum for 48 h and MEM containing 5% charcoal dextran-treated fetal calf serum for another 24 h. Total RNA was extracted for real-time PCR analysis with specific probes for FOXO1 mRNA. (c) Transient transfection assay. pCR3/mCAR and 1.8 kbp-2B6-Luc plasmids were cotransfected in the presence of FOXO1 RNAi or control RNAi. Cells were cultured and treated as described above. Relative activities were calculated by taking the activity of the cells that were transfected with the pCR3/mCAR and 1.8 kbp-2B6-Luc plasmids in the presence of TCPOBOP and control RNAi as 100.
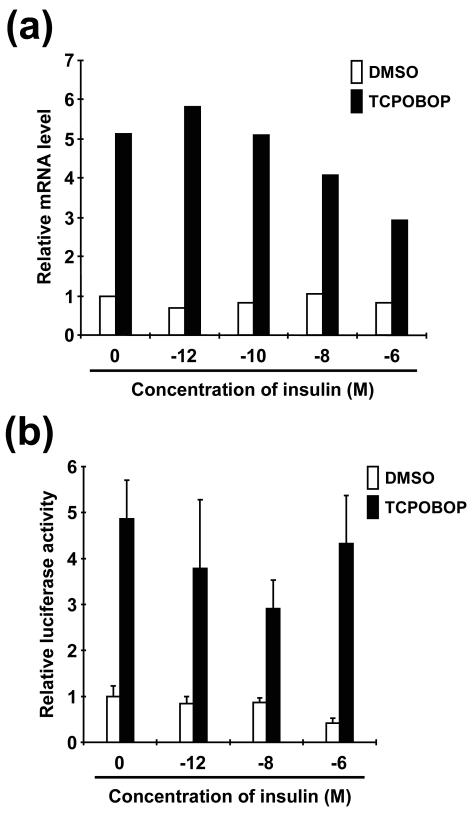
Although insulin repression of PB induction was reported for rat primary hepatocytes (17, 42), it has not been demonstrated in mouse primary hepatocytes. Upon finding that FOXO1 is a key coactivator of CAR in HepG2 cells, we examined whether insulin repressed the induction of the Cyp2b10 gene by TCPOBOP in mouse primary hepatocytes (Fig. 5a). TCPOBOP induced CYP2B10 mRNA levels fivefold without insulin in the culture medium. The induction was slightly augmented by 10−12 M insulin, followed by a gradual decrease in mRNA levels as insulin concentration increased. The rate of induction by TCPOBOP at 10−6 M insulin was only threefold compared to the maximum eightfold induction at 10−12 M insulin. Under the culture conditions used, insulin clearly repressed the induction of the endogenous Cyp2b10 gene, suggesting that, acting as a CAR coactivator in response to insulin, FOXO1 can regulate Cyp2b10 induction in vivo in hepatocytes. In attempting to correlate the induction of the Cyp2b10 gene with CAR-mediated 1.8 kbp-2B6-Luc reporter activity, the reporter plasmid was transfected into mouse primary hepatocytes, followed by insulin treatment. While insulin at 10−6 M was somewhat less effective in repressing CAR activity, the luciferase reporter activity was decreased as insulin concentration increased up to 10−8 M (Fig. 5b). These results support the hypothesis that insulin regulates CAR activity to repress the Cyp2b10 gene in primary hepatocytes.
FIG. 5.
Repression of the Cyp2b10 gene by insulin in mouse primary hepatocytes. (a) Mouse primary hepatocytes were prepared and treated with 0.1% DMSO or 250 nM TCPOBOP in the presence of various concentrations of insulin ranging from 10−12 to 10−6 M. At 8 h after treatment, total RNA was extracted and subjected to real-time PCR analysis with specific probes for the Cyp2b10 gene. Relative levels were expressed by taking the level in DMSO-treated hepatocytes without insulin treatment as one. (b) Luciferase activity was measured 24 h after electroporation with pGL3/1.8 kbp-2B6-Luc (10 μg), pRL-CMV (5 μg), and drug treatment. Relative activities were calculated by taking the level in DMSO-treated hepatocytes without insulin treatment as one. Bars indicate means ± standard deviations.
PXR as another FOXO1 target.
PXR activates the same enhancer sequences as CAR. Therefore, we examined whether FOXO1 could regulate PXR activity in the same way it can with coactivated CAR. A GST pull-down assay showed that in vitro-translated FOXO1 bound to GST-mPXR, which was increased by the specific PXR activator pregnenolone PCN (Fig. 6a). PCN activated the PXR-dependent XREM-3A4-Luc promoter in transfection assays in the absence of FOXO1 10-fold (Fig. 6b). In the presence of exogenous FOXO1 and FOXO13A, the promoter activity was further enhanced 2.5- and more than 5-fold, respectively (Fig. 6b). Similar to results obtained with CAR, the coactivation by FOXO1 was repressed by insulin treatment and coexpression of AktWt, whereas inactive AktKN did not affect the coactivation (Fig. 6c). The constitutively active AktCA abolished the coactivation completely and decreased the promoter activity to a level below that without FOXO1. These results confirmed that FOXO1 also coactivates PXR via an insulin-PI3K-Akt pathway in the same way that CAR activity is regulated.
FIG. 6.
Regulation of PXR activity by FOXO1. (a) GST pull-down assay. In vitro-translated 35S-labeled FOXO1 and PXR were incubated with bacterially expressed GST-mFOXO1 and GST-mPXR, respectively, in the presence of 0.1% DMSO (D) or 50 μM PCN (P). (b) Transient transfection assay. XREM-3A4-Luc reporter was cotransfected with pcDNA3/mFOXO1 (or pcDNA3/mFOXO13A) and/or pcDNA3/mPXR into HepG2 cells. Cells were treated with drugs, and the activity was calculated as described in the legend to Fig. 1. In the case of PCN treatment, 10 μM was used to activate PXR. (c) Transient transfection assays. XREM-3A4-Luc reporter was cotransfected with pcDNA3/mPXR and pcDNA3/mFOXO1 into HepG2 cells. In some cases, cells were additionally transfected with pcDNA3/rAktWt, pcDNA3/rAktKN, or pcDNA3/rAktCA. PCN (10 μM) and insulin (500 nM) were used to treat cells. Relative activities were calculated by taking the activity of the cells that were transfected by only the reporter plasmid in the presence of DMSO as one. Bars indicate means ± standard deviations.
CAR and PXR as FOXO1 corepressors.
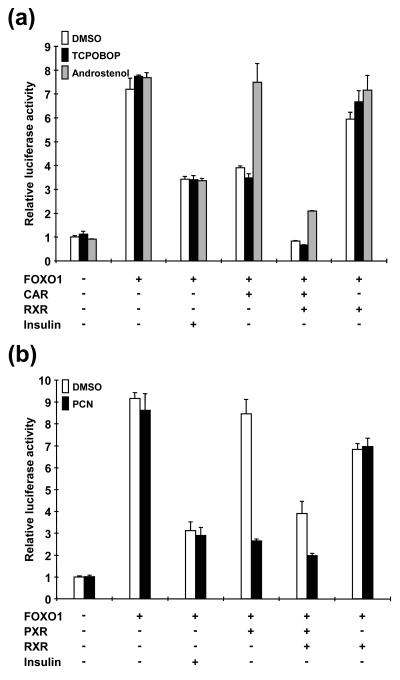
Whether CAR and/or PXR could repress FOXO1-mediated trans-activation of the IRS was investigated, because other nuclear receptors, such as ERα and PPARγ, are known to repress IRS activation. When FOXO1 alone was cotransfected with the IRS-tk-Luc reporter into HepG2 cells, it activated the IRS approximately eightfold (Fig. 7a). This IRS activity was not affected by the presence of either the CAR activator TCPOBOP or the repressor androstenol. As expected, insulin treatment decreased IRS activity by 50%. Coexpression of CAR repressed IRS activity to a level similar to that obtained by insulin treatment. Consistent with its constitutive active nature, CAR repressed the IRS activity in the absence of TCPOBOP. However, the fact that the activity was retained in the presence of androstenol suggested that the receptor must be in an active form to repress the IRS. Additional coexpression of RXR with CAR eliminated FOXO1-IRS activity in which CAR, not RXR, was the primary responsible factor, because the activity was retained when RXR alone was coexpressed. During overexpression of RXR, CAR has a stronger ability to repress the IRS. Similar to CAR, when PXR was activated by PCN the receptor could also repress FOXO1-IRS activity (Fig. 7b). RXR overexpression also increased the capability of PXR to repress the activity even in the absence of PCN. These results clearly indicate that both CAR and PXR repress FOXO1-IRS activity in cotransfected HepG2 cells. The repression was strictly activator dependent in the absence of coexpressed RXR, while it lost some degree of dependency in the presence of RXR.
FIG. 7.
Repression by CAR and PXR of FOXO1-IRS activity. The IRS-tk-Luc reporter and pcDNA3/mFOXO1 were cotransfected with pCR3/mCAR (a) or pcDNA3/mPXR (b) into HepG2 cells. In some cases, pCMX/hRXR was additionally transfected. Relative activities were calculated by taking the activity of the cells that were transfected by only the reporter plasmid in the presence of DMSO as one. Bars indicate means ± standard deviations.
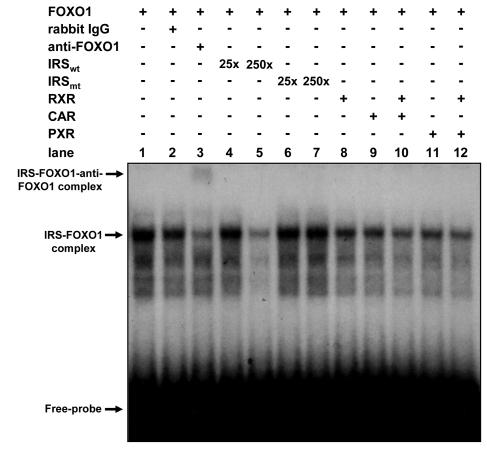
Subsequently we performed gel shift assays using the in vitro-translated proteins and an oligonucleotide probe containing IRS (Fig. 8). One major and multiple minor band shifts were formed with in vitro-translated FOXO1 (lane 1). Only the major one was specifically supershifted by anti-FOXO1 antibody (lane 2 versus 3) and was competed with 250× cold oligonucleotide to approximately 33% of the FOXO1-IRS binding (lane 1) but not with mutated oligonucleotide (lanes 4 and 5 versus lanes 6 and 7), indicating that this represented a FOXO1-IRS complex. Formation of the complex was also reduced in the presence of RXR (lane 8), CAR (lane 9), and PXR (lane 11). When both CAR (or PXR) and RXR were present in the assay (lanes 10 and 12), the complex formation was further reduced to 48% of the amount shown in lane 1, which was similar to the degree of reduction by the cold competitor. Thus, both CAR and PXR became more effective in reducing the FOXO1-IRS complex by heterodimerizing with RXR. The degree of complex reduction by the receptors correlated with that of the inhibition by them of FOXO1-IRS activity (Fig. 7). Thus, the nuclear receptors CAR and PXR directly bind to FOXO1 and reduce formation of the FOXO1-IRS complex, repressing its activity.
FIG. 8.
CAR and PXR inhibit FOXO1 binding to IRS. In vitro-translated FOXO1, CAR, hRXR, and PXR were incubated with the radiolabeled oligonucleotides for wild-type (w) and mutant (m) IRS. For antibody supershift assays, unlabeled oligonucleotides, rabbit immunoglobulin G, and hFOXO1 antibody were cotreated (lane 2 and 3). Excess amounts of unlabeled wIRS and mIRS (25× and 250×) were used for competitive assays (lanes 4 to 7). Gel shift assays were performed as described in Materials and Methods. Band intensity of the IRS-FOXO1 complex was measured with Gene Tools (Hitachi Software Engineering Co., Ltd.).
Apparent role of CAR in vivo.
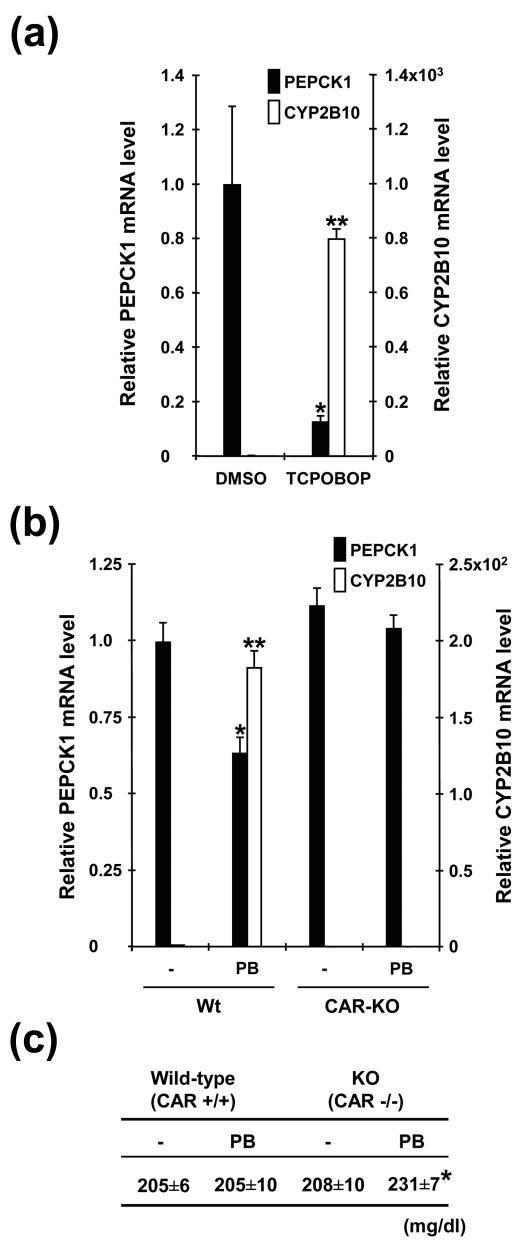
The results obtained from various binding and cell-based transfection assays indicated that activation of CAR may result in the repression of FOXO1-regulated genes in liver in vivo. Animal experiments were performed to investigate whether CAR could regulate gluconeogenesis. Because CAR is a stress response transcription factor, its functional role may depend on the liver's physiological state. Thus, to avoid the risk that these states could confuse the interpretation of the functional role of CAR in liver in vivo, two animal models were used for the investigations. First, Cr1:CD-1(ICR)BR male mice were treated with TCPOBOP, resulting in the repression of PEPCK1 mRNA, while CYP2B10 mRNA was induced (Fig. 9a), which was associated with a slight decrease of the serum glucose levels (168 ± 33.2 and 135.7 ± 11.9 mg/dl in the DMSO and TCPOBOP treatments, respectively). Second, Car+/+ and Car−/− mice were chronically treated with PB, in which PEPCK1 mRNA was decreased in only the Car+/+ mice (Fig. 9b), indicating that CAR mediated the decrease. In addition, we examined if this decrease altered serum glucose level in PB-treated Car+/+ and Car−/− mice, finding that the glucose level was only increased in the Car−/− mice. Thus, the lack of CAR-mediated repression appeared to prevent an increase in serum glucose level in response to the PB treatment. These results indicate that the CAR-FOXO1 pathway, in fact, can modulate serum glucose levels by regulating gluconeogenic enzymes, such as PEPCK1, and provide insight into understanding the multiple functional roles of CAR in animals.
FIG. 9.
CAR regulates the Pepck1 gene. Male mice were treated as described in Materials and Methods. (a) Liver RNA samples were individually prepared from three mice for each group of Cr1:CD-1(ICR)BR mice and were individually subjected to real-time PCR analysis with specific probes for PEPCK1 and CYP2B10 mRNAs, respectively. Relative mRNA levels were expressed by taking those with DMSO as one. Values are means ± standard errors of the means. An asterisk indicates a P value of <0.05 and a double asterisk indicates a P value of <0.0001 for PEPCK1 and CYP2B10 mRNAs, respectively, compared with those of the DMSO-treated mice. (b) Liver RNAs were prepared from 12 mice for each of the Car−/− and Car+/+ groups and were individually subjected to real-time PCR assay. Relative mRNA levels were expressed by taking those with no PB treatment as one (± standard errors of the mean). An asterisk indicates P < 0.02 and a double asterisk indicates P < 0.0001 for PEPCK1 and CYP2B10 mRNAs, respectively, compared with those of the mice not treated with PB. (c) Serum glucose levels of the Car−/− and Car+/+ mice. Serum was collected from 20 for each group of the Car−/− and Car+/+ mice. The glucose level was individually measured using the COBAS MIRA Plus CC Analyzer (Roche Diagnostics) and was averaged. KO, knockout.
DISCUSSION
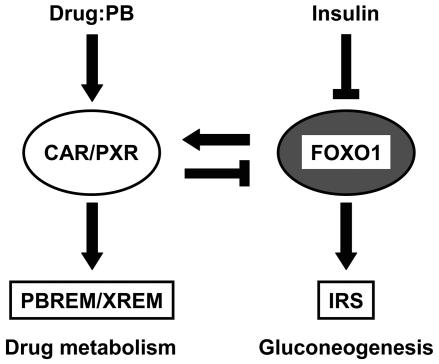
An increase in liver microsomal drug-metabolizing activity in alloxan-treated diabetic rats was first reported in the early 1960s when drugs such as barbiturates were also found to induce the same activity (3, 33). It was later confirmed that the increased drug metabolism activity was due to the induction of enzymes, such as CYP2B and CYP3A. For example, P450b (presently called CYP2B1) was increased 25- to 30-fold in the liver microsomes of alloxan-treated rats, and insulin treatment restored P450b to normal levels (56). In the present study, we have demonstrated that the regulatory mechanisms of the insulin response and of the drug-induced transcription cross paths through direct interaction between FOXO1 and the nuclear receptors CAR and PXR. Accordingly, drug metabolism and gluconeogenesis could be reciprocally coregulated by the transcription factors in response to insulin and/or drugs (Fig. 10).
FIG. 10.
Schematic representation of cross-talk. Arrows indicate activation and coactivation, while stop bars indicate repression and corepression.
Our study first showed that FOXO1 binds directly to CAR. While both FOXO1- and insulin-nonresponsive FOXO13A coactivated CAR activity, insulin effectively repressed FOXO1 coactivation but not FOXO13A coactivation. AktCA, but not AktKN, inhibited FOXO1-CAR activity. Finally, insulin represses the induction of CYP2B10 mRNA in TCPOBOP-treated mouse primary hepatocytes. CAR accumulates in the nucleus after treatment with its activators, such as PB, TCPOBOP, and estradiol (15, 16). FOXO1 is in a position to coactivate CAR when the receptor accumulates in the nucleus. By virtue of the PI3K-Akt pathway, insulin inactivates FOXO1 by exporting it from the nucleus to the cytoplasm (2, 34, 59). When in the nucleus, FOXO1 upregulates CAR activity, augmenting the expression of CAR target genes, such as CYP2B genes. Insulin, on the other hand, downregulates the receptor by exporting its coactivator FOXO1 from the nucleus, which could result in the repression of the CAR-regulated genes. Thus, coregulation of CAR by FOXO1 provides insulin with a regulatory mechanism to repress hepatic drug metabolism. In addition to CAR, FOXO1 also activates PXR, suggesting that insulin repression of drug metabolism via FOXO1 may be a general pathway. A gel shift assay with the CAR-binding sequence NR1 as a probe showed that binding of CAR-RXR to NR1 was decreased in the presence of FOXO1, suggesting that CAR interacted with FOXO1 (data not shown). However, a supershift band that represented a FOXO1-CAR-RXR complex was not detected under the experimental conditions used. While one can argue that the ternary complex is simply too large in size to enter into a gel or is not stable enough to be detected, failing to detect it would also pose the question of whether FOXO1 forms a stable complex with CAR-RXR-NR1 to activate it. If so, how could FOXO1 activate CAR or RXR? These molecular mechanisms remain elusive and will be an interesting research objective for future investigations.
Our studies with CAR-null mice suggested that the receptor regulated the PB-mediated decrease of PEPCK1 mRNA. Our present investigations revealed the possible regulatory mechanism for PB-induced repression of gluconeogenic genes (Fig. 10). Following activation by PB, CAR binds to FOXO1 and prevents its binding to the IRS, which could result in transcriptional repression of the genes that are regulated by IRS, such as PEPCK1. In addition, future investigations may find the other gluconeogenic enzymes, including G6P and also key enzymes such as pyruvate dehydrogenase kinase 4 in glycolysis, to be regulated by CAR-FOXO1. This repression mechanism of FOXO1 by CAR is consistent with previous findings that nuclear hormone receptors (e.g., estrogen and androgen receptors, retinoid A receptor, and HNF4) bind to the DNA-binding domain of FOXO1 (21, 39) and that FOXO1 binding to IRS is decreased in the presence of the androgen receptor in a gel shift assay (21). CAR and PXR have now extended the list of nuclear receptors (e.g., ER, AR, GR, TR, RAR, HNF4, and PPARγ) that interact with and coregulate FOXO1. In addition to cell proliferation (22, 26, 40), glucose metabolism (29, 30), and adipogenesis (27), drug metabolism becomes a novel target of coregulation by FOXO1 cross-talking with these nuclear receptors. Because the constitutively active AktCA inhibited CAR activity in HepG2 cells, endogenous FOXO1 could be functional in coactivating the receptor. This functionality was further substantiated by the concomitant reduction of endogenous FOXO1 by RNAi and the repression of CAR activity. FOXO1 is the first factor to coregulate the nuclear receptors in response to an endogenous signal among various coregulators, such as SRC-1, NCoR, SMRT, and SHP, that are already known to regulate CAR and PXR (8, 10, 54). PGC1 is another signal-responsive coregulator that can activate both CAR and PXR (41). Whether it regulates the receptor in a signal-dependent manner has not been demonstrated.
While our present study concerned the mouse nuclear receptors and FOXO1, we also examined and confirmed that FOXO1 coactivated human CAR while the human receptor repressed FOXO1 (data not shown). It has been known for many years that diabetes elevates the activity of hepatic drug metabolism in humans. Moreover, PB was clinically used in diabetic patients to decrease their plasma glucose (20). The CAR and PXR response elements, such as PBREM and XREM, are conserved in many human genes, such as CYP2B6 (47), CYP3A4 (5), and UGT1A1 (45). Although a physiological implication of this cross-talk regulation remains only speculative at the moment, one possible scenario may be to balance NADPH and NADH consumption. Insulin acts to decrease NADPH consumption by drug metabolism, while drugs act to repress gluconeogenesis to increase NADPH supply for drug metabolism. NADPH is an essential electron donor for cytochrome P450-dependent monooxygenase activity. The concept of reciprocal coregulation by CAR or PXR and FOXO1 may have clinical implications in the understanding of disease prevention, drug-drug interactions, and the development of better drug treatments.
REFERENCES
- 1.Argaud, D., S. Halimi, F. Catelloni, and X. M. Leverve. 1991. Inhibition of gluconeogenesis in isolated rat hepatocytes after chronic treatment with phenobarbital. Biochem. J. 280:663-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96:7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon, R. L., L. G. Hart, and J. R. Fouts. 1961. The metabolism of drugs by liver microsomes from alloxan-diabetic rats. J. Pharmacol. Exp. Ther. 133:7-11. [PubMed] [Google Scholar]
- 4.Dowell, P., T. C. Otto, S. Adi, and M. D. Lane. 2003. Convergence of proxisome proliferator-activated receptor-γ and Foxo1 signaling pathway. J. Biol. Chem. 278:45485-45491. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin, B., M. R. Redinbo, and S. A. Kliewer. 2002. Regulation of cyp3a gene transcription by the pregnane x receptor. Annu. Rev. Pharmacol. Toxicol. 42:1-23. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin, B., E. Hodgson, and C. Liddle. 1999. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol. Pharmacol. 56:1329-1339. [DOI] [PubMed] [Google Scholar]
- 7.Guo, S., G. Rena, S. Cichy, X. He, P. Cohen, and T. Unterman. 1999. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J. Biol. Chem. 274:17184-17192. [DOI] [PubMed] [Google Scholar]
- 8.Handschin, C., and U. A. Meyer. 2003. Induction of drug metabolism; the role of nuclear receptors. Pharmacol. Rev. 55:649-673. [DOI] [PubMed] [Google Scholar]
- 9.Hirota, K., H. Daitoku, H. Matsuzaki, N. Araya, K. Yamagata, S. Asada, T. Sugaya, and A. Fukamizu. 2003. Hepatocyte nuclear factor-4 is a novel downstream target of insulin via FKHR as a signal-regulated transcriptional inhibitor. J. Biol. Chem. 278:13056-13060. [DOI] [PubMed] [Google Scholar]
- 10.Honkakoski, P., and M. Negishi. 2000. Regulation of cytochrome P450 (CYP) genes by nuclear receptors. Biochem. J. 347:321-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honkakoski, P., I. Zelko, T. Sueyoshi, and M. Negishi. 1998. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol. Cell. Biol. 18:5652-5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honkakoski, P., R. Moore, K. A. Washburn, and M. Negishi. 1998. Activation by diverse xenochemical of the 51-base pair phenobarbital-responsive enhancer module in the CYP2B10 gene. Mol. Pharmacol. 53:597-601. [DOI] [PubMed] [Google Scholar]
- 13.Honkakoski, P., R. Moore, J. Gynther, and M. Negishi. 1996. Characterization of phenobarbital-inducible mouse cyp2b10 gene transcription in primary hepatocytes. J. Biol. Chem. 271:9746-9753. [DOI] [PubMed] [Google Scholar]
- 14.Kakizaki, S., Y. Yamamoto, A. Ueda, R. Moore, T. Sueyoshi, and M. Negishi. 2003. Phenobarbital induction of drug/steroid-metabolizing enzymes and nuclear receptor CAR. Biochim. Biophys. Acta 1619:239-242. [DOI] [PubMed] [Google Scholar]
- 15.Kawamoto, T., S. Kakizaki, K. Yoshinari, and M. Negishi. 2000. Estrogen activation of the nuclear orphan receptor CAR (constitutive active receptor) in induction of the mouse Cyp2b10 gene. Mol. Endocrinol. 14:1897-1905. [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto, T., T. Sueyoshi, I. Zelko, R. Moore, K. Washburn, and M. Negishi. 1999. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol. Cell. Biol. 19:6318-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamura, A., Y. Yoshida, N. Kimura, H. Oda, and A. Kakinuma. 1999. Phosphorylation/dephosphorylation steps are crucial for the induction of CYP2B1 and CYP2B2 gene expression by phenobarbital. Biochem. Biophys. Res. Commun. 264:530-536. [DOI] [PubMed] [Google Scholar]
- 18.Kliewer, S. A., J. T. Moore, L. Wade, J. L. Staudinger, M. A. Watson, S. A. Jones, D. D. McKee, B. B. Oliver, T. M. Willson, R. H. Zetterstrom, T. Perlmann, and J. M. Lehman. 1998. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92:73-82. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi, K., T. Sueyoshi, K. Inoue, R. Moore, and M. Negishi. 2003. Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol. Pharmacol. 64:1069-1075. [DOI] [PubMed] [Google Scholar]
- 20.Lahtela, J. T., A. J. Arranto, and E. A. Sotanaieni. 1985. Enzyme inducers improve insulin sensitivity in no-insulin-dependent diabetic subjects. Diabetes 34:911-916. [DOI] [PubMed] [Google Scholar]
- 21.Li, P., H. Lee, S. Guo, T. G. Unterman, G. Jenster, and W. Bai. 2003. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol. Cell. Biol. 23:104-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machida, S., E. E. Spangenburg, and F. W. Booth. 2003. Forkhead transcription factor FoxO1 transduces insulin-like growth factor's signal to p27Kip1 in primary skeletal muscle satellite cells. J. Cell. Physiol. 196:523-531. [DOI] [PubMed] [Google Scholar]
- 23.Maglich, J. M., C. M. Stoltz, B. Goodwin, D. Hawkins-Brown, J. T. Moore, and S. A. Kliewer. 2002. Nuclear pregnane X receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol. Pharmacol. 62:638-646. [DOI] [PubMed] [Google Scholar]
- 24.Manenti, G., T. A. Dragani, and G. Della Porta. 1987. Effects of phenobarbital and 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene on differentiated functions in mouse liver. Chem. Biol. Interact. 64:83-92. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki, H., H. Daitoku, M. Hatta, K. Tanaka, and A. Fukamizu. 2003. Insulin-induced phosphorylation of FKHR (FOXO1) targets to proteosomal degradation. Proc. Natl. Acad. Sci. USA 100:11285-11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modur, V., R. Nagarajan, B. M. Evers, and J. Milbrandt. 2003. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J. Biol. Chem. 277:47928-47937. [DOI] [PubMed] [Google Scholar]
- 27.Nakae, J., T. Kitamura, Y. Kitamura, W. H. Biggs III, K. C. Arden, and D. Accili. 2003. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell. 4:119-129. [DOI] [PubMed] [Google Scholar]
- 28.Nakae, J., W. H. Biggs III, T. Kitamura, W. K. Cavenee, C. V. E. Wright, K. C. Arden, and D. Accili. 2002. Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor FOXO1. Nat. Genet. 32:245-253. [DOI] [PubMed] [Google Scholar]
- 29.Nakae, J., T. Kitamura, D. L. Silver, and D. Accili. 2001. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Investig. 108:1359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakae, J., B.-C. Park, and D. Accili. 1999. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a wortmannin-sensitive pathway. J. Biol. Chem. 274:15982-15985. [DOI] [PubMed] [Google Scholar]
- 31.Pascussi, J. M., Z. Dvorak, S. Gerbal-Chaloin, E. Assenat, P. Maurel, and M. J. Vilarem. 2003. Pathophysiological factors affecting CAR gene expression. Drug. Metab. Rev. 35:255-268. [DOI] [PubMed] [Google Scholar]
- 32.Puigserver, P., J. Rhee, J. Donovan, C. J. Walkey, J. Cliff Yoon, F. Oriente, Y. Kitamura, J. Altomonte, H. Dong, D. Accili, and B. M. Spiegelman. 2003. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423:550-555. [DOI] [PubMed] [Google Scholar]
- 33.Remmer, H., and H. J. Merker. 1963. Drug-induced changes in the liver endoplasmic reticulum; association with drug-metabolizing enzymes. Science 142:1657-1658. [DOI] [PubMed] [Google Scholar]
- 34.Rena, G., A. R. Prescott, S. Guo, P. Cohen, and T. G. Unterman. 2001. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targeting. Biochem. J. 354:605-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rena, G., S. Guo, S. C. Cichy, T. G. Unterman, and P. Cohen. 1999. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J. Biol. Chem. 274:17179-17183. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfield, J. M., R. Vargas, W. Xie, and R. M. Evans. 2003. Genetic profiling defines the xenobiotic gene network controlled by the nuclear receptor pregnane X receptor. Mol. Endocrinol. 17:1268-1282. [DOI] [PubMed] [Google Scholar]
- 37.Sakuma, T., R. Honda, S. Magushi, H. Tamaki, and N. Nemoto. 2001. Different expression of hepatic and renal cytochrome P450s between the streptozotocin-induced diabetic mouse and rat. Xenobiotica 31:223-237. [DOI] [PubMed] [Google Scholar]
- 38.Schmoll, D., K. S. Walker, D. R. Alessi, R. Grempler, A. Burchell, S. Guo, R. Walther, and T. G. Unterman. 2000. Regulation of glucose-6-phosphatase gene expression by protein kinase Bα and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J. Biol. Chem. 275:36324-36333. [DOI] [PubMed] [Google Scholar]
- 39.Schuur, E. R., A. V. Loktev, M. Sharma, Z. Sun, R. A. Roth, and R. J. Weigel. 2001. Ligand-dependent interaction of estrogen receptor-α with members of the forkhead transcription factor family. J. Biol. Chem. 276:33554-33560. [DOI] [PubMed] [Google Scholar]
- 40.Seoane, J., H. V. Le, L. Shen, S. A. Anderson, and J. Massague. 2004. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117:211-223. [DOI] [PubMed] [Google Scholar]
- 41.Shiraki, T., N. Sakai, E. Kanaya, and H. Jingami. 2003. Activation of orphan nuclear constitutive androstane receptor requires subnuclear targeting by peroxisome proliferator-activated receptor γ coactivator-1α. A possible link between xenobiotic response and nutritional state. J. Biol. Chem. 278:11344-11350. [DOI] [PubMed] [Google Scholar]
- 42.Sidhu, J., and C. J. Omiecinski. 1999. Insulin-mediated modulation of cytochrome P450 gene induction profiles in primary rat hepatocyte cultures. J. Biochem. Mol. Toxicol. 13:1-9. [DOI] [PubMed] [Google Scholar]
- 43.Smirlis, D., R. Muangmoonchai, M. Edwards, I. R. Phillips, and E. A. Shephard. 2001. Orphan receptor promiscuity in the induction of cytochromes p450 by xenobiotics. J. Biol. Chem. 276:12822-12826. [DOI] [PubMed] [Google Scholar]
- 44.Sotaniemi, E. A., O. Pelkonen, A. J. Arranto, P. Tapanainen, A. Rautio, and M. Pasanen. 2002. Diabetes and elimination of antipyrine in man: an analysis of 298 patients classified by type of diabetes, age, sex, duration of disease and liver involvement. Pharmacol. Toxicol. 90:155-160. [DOI] [PubMed] [Google Scholar]
- 45.Sugatani, J., H. Kojima, A. Ueda, S. Kakizaki, K. Yoshinari, Q.-H. Gong, I. S. Owens, M. Negishi, and T. Sueyoshi. 2002. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by nuclear receptor CAR. Hepatology 33:1232-1238. [DOI] [PubMed] [Google Scholar]
- 46.Sueyoshi, T., and M. Negishi. 2001. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu. Rev. Pharmacol. Toxicol. 41:123-143. [DOI] [PubMed] [Google Scholar]
- 47.Sueyoshi, T., T. Kawamoto, I. Zelko, P. Honkakoski, and M. Negishi. 1999. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J. Biol. Chem. 274:6043-6046. [DOI] [PubMed] [Google Scholar]
- 48.Suwanickul, A., S. L. Morris, and D. R. Powell. 1993. Identification of an insulin-responsive element in the promoter of the human gene for insulin-like growth factor binding protein-1. J. Biol. Chem. 268:17063-17068. [PubMed] [Google Scholar]
- 49.Thummel, K. E., and J. B. Schenkman. 1989. Effects of testosterone and growth hormone treatment on hepatic microsomal P450 expression in the diabetic rat. Mol. Pharmacol. 37:119-129. [PubMed] [Google Scholar]
- 50.Ueda, A., H. K. Hamadeh, H. K. Webb, Y. Yamamoto, T. Sueyoshi, C. A. Afshari, J. M. Lehmann, and M. Negishi. 2002. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol. Pharmacol. 61:1-6. [DOI] [PubMed] [Google Scholar]
- 51.Vander Kooi, B. T., R. S. Streeper, C. A. Svitek, J. K. Oeser, D. R. Powell, and R. M. O'Brien. 2003. The three insulin response sequences in the glucose-6-phosphatase catalytic subunit gene promoter are functionally distinct. J. Biol. Chem. 278:11782-11793. [DOI] [PubMed] [Google Scholar]
- 52.Wang, H., S. Faucette, T. Sueyoshi, R. Moore, S. Ferguson, M. Negishi, and E. L. LeCluyse. 2003. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J. Biol. Chem. 278:14146-14152. [DOI] [PubMed] [Google Scholar]
- 53.Wei, P., J. Zhang, M. Egan-Hafley, S. Liang, and D. D. Moore. 2000. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature 407:920-923. [DOI] [PubMed] [Google Scholar]
- 54.Xie, W., and R. M. Evans. 2000. Orphan nuclear receptors: the exotics of xenobiotics. J. Biol. Chem. 276:37739-37742. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto, Y., T. Kawamoto, and M. Negishi. 2003. The role of the nuclear receptor CAR as a coordinate regulator of hepatic gene expression in defense against chemical toxicity. Arch. Biochem. Biophys. 409:207-211. [DOI] [PubMed] [Google Scholar]
- 56.Yamazoe, Y., N. Murayama, M. Shimada, K. Yamauchi, and R. Kato. 1989. Cytochrome P450 in livers of diabetic rats; regulation by growth hormone and insulin. Arch. Biochem. Biophys. 268:567-575. [DOI] [PubMed] [Google Scholar]
- 57.Yeagley, D., S. Guo, T. Unterman, and P. G. Quinn. 2001. Gene- and activation-specific mechanisms for insulin inhibition of basal and glucocorticoid-induced insulin-like growth factor binding protein-1 and phosphoenolpyruvate carboxykinase transcription. Roles of forkhead and insulin response sequences. J. Biol. Chem. 276:33705-33710. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida, Y., N. Kimura, H. Oda, and A. Kakinuma. 1996. Insulin suppresses the induction of the CYP2B1 and CYP2B2 gene expression by phenobarbital in adult rat cultured hepatocytes. Biochem. Biophys. Res. Commun. 229:182-188. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, X., L. Gan, H. Pan, S. Guo, X. He, S. T. Olson, A. Mesecar, S. Adam, and T. G. Unterman. 2002. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J. Biol. Chem. 277:45276-45284. [DOI] [PubMed] [Google Scholar]
- 60.Zhao, H. H., R. E. Herrera, E. Coronado-Heinsohn, M. C. Yang, J. H. Ludes-Meyers, K. J. Seybold-Tilson, Z. Nawaz, D. Yee, F. G. Barr, S. G. Diab, P. H. Brown, S. A. W. Fuqua, and C. K. Osborne. 2001. Forkhead homologue in rhabdomyosarcoma functions as a bifunctional nuclear receptor-interacting protein with both coactivator and corepressor functions. J. Biol. Chem. 276:27907-27912. [DOI] [PubMed] [Google Scholar]