Abstract
Mucin secretion is regulated by extracellular signaling molecules emanating from local, neuronal, or endocrine sources. Quantifying the rate of this secretion is important to understanding how the exocytic process is regulated, and also how goblet/mucous cells synthesize and release mucins under control and pathological conditions. Consequently, measuring mucins in a quantitatively accurate manner is the key to many experiments addressing these issues. This paper describes procedures used to determine agonist-induced mucin secretion from goblet cells in human bronchial epithelial (HBE) cell cultures. It begins with primary epithelial cell culture, offers methods for purifying MUC5AC and MUC5B mucins for standards, and describes five different microtiter plate binding assays which use various probes for mucins. A polymeric mucin-specific antibody is used in standard and sandwich ELISA formats for two assays while the others target the extensive glycosylated domains of mucins with lectin, periodate oxidation, and antibody-based probes. Comparing the data derived from the different assays applied to the same set of samples of HBE cell cultures indicates a qualitative agreement between baseline and agonist stimulated mucin release; however, the polymeric mucin-specific assays yield substantially lower values than the assays using nonspecific molecular reporters. These results indicate that the more non-specific assays are suitable to assess overall secretory responses by goblet cells, but are likely unsuited for specific measurements of polymeric mucins, per se.
Keywords: Mucin, Secretion, Exocytosis, Human bronchial epithelial cell culture, Goblet cell
1. Introduction
Polymeric mucins are a crucial component of the mucus that lines the normal airway epithelium and provides a first line of defense for the lungs against inspired aerosols, particulates, and pathogens (1). Their exocytic release from goblet cells and submucosal glands in the airways marks the beginning of the maturation of mucins into luminal mucus. The mucins are heavily glycosylated proteins (~80–90% carbohydrate), with monomeric molecular weights of ~2 MDa, and polymeric molecular weights measured in the 10–100 s of MDa (2). Mucins are synthesized and dimerized in the ER and glycosylated and oligomerized in the Golgi complex. They are packaged in, and released from, the trans-Golgi network as large, 1-µm-sized secretory granules, which are stored in the apical pole of goblet or mucous cells until they are released by exocytosis following activation by agonist (3, 4). Essential to lung defense in normal physiology, mucins and mucus are overproduced in all of the airway inflammatory diseases, including asthma, chronic obstructive pulmonary disease, and cystic fibrosis, to such an extent that mucus plugging, gas trapping, and infection are major problems for patients and their pulmonologists (5). As a consequence, understanding the regulation of mucin biosynthesis and secretion is a long-sought goal, toward which substantial progress has been made in the past two decades. Our laboratory has been involved in the effort to delineate the regulation of mucin secretion from goblet cells (4) for most of this period, and in this chapter we share our current methods for studying mucin secretion from human bronchial epithelial (HBE) cell primary cultures.
2. Materials
2.1. Human Bronchial Epithelial Cell Culture
Bronchial epithelial growth medium (BEGM). LHC Basal Medium (Biosource, Camarillo, Cat. # P118-500), supplemented with the growth factors, antibiotics, and other ingredients detailed in the procedures and Tables 1 and 2 of ref. 6 (see Note 1).
Air–liquid interface (ALI) medium. 50:50 Mixture of LHC Basal Medium and DMEM-H (Gibco, Carlsbad, CA, Cat. # 11995-065), supplemented with the growth factors, antibiotics, and other ingredients detailed in the procedures and Tables 1 and 2 of ref. 6 (see Note 1).
Human airway epithelial cells. Cells may be harvested by proteolytic digestion of human turbinates, polyps, trachea, or bronchi obtained in conjunction with surgeons and/or pathologists, following the procedures detailed in ref. 6. Alternatively, they can be purchased from commercial sources, e.g., Cell Applications, Inc. (http://www.cellapplications.com), Lonza Group (formerly, Clonetics, Cambrex; http://www.lonza.com), or Lifeline Cell Technology (http://www.lifelinecelltech.com/docs/SPC_AirwayEpi.pdf; see Note 2).
Corning Costar Transwell® Clear (polyester) cell culture inserts, 12 or 24 mm diameter (see Note 3).
2.2. HBE Cell Culture Mucin Secretion Experiments
2.3. Preparing Mucin Standards
8 M GuHCl Buffer is prepared by adding guanidine hydrochloride (spectrophotometric grade, e.g., Acros Organics, Cat. # 120230010) in 10 mM phosphate buffer, containing 5 mM EDTA, pH 6.5. Add one mini protease inhibitor cocktail tablet (PICtab-mini) for each 10-mL volume used, just before use (see step 2).
Complete® protease inhibitor cocktail tablets (PICtab), Roche Applied Sciences, use 1 tablet/50 mL of solution (Cat. # 11873580001) or one mini tablet/10 mL (Cat. # 11836153001).
Spectra/Gel® Absorbent (Spectrum Laboratory Products, Cat. # 888–16582).
Optilab Interferometric Refractometer with a filter at 680 nm (Wyatt, CA, USA).
Astra 4.9 (or later) data acquisition and processing software (Wyatt, CA, USA).
Sepharose CL2B (Sigma, MO, USA) or Sephacryl 1000 (GE Bio-sciences Uppsala Sweden) (12 × 2.5 cm, ~12 mL) gel-permeation chromatography column.
7 Port V7 manual injection valve (GE Bio-sciences Uppsala Sweden).
Pump, Rheos 2000 (Flux instruments, Thermo Scientific, USA) or equivalent solvent delivery pump.
Running buffer: 200 mM NaCl, 10 mM Tris, 10 mM EDTA, pH 7.0. Filter and degas by sonication (for longer storage, add 0.1% w/v Na azide).
2.4. Microtiter Plate Mucin Assays
Flat Bottom, High Binding, 96-well microtiter plates (Costar, Cat. # 3590).
Phosphate-buffered saline (PBS).
Wash buffer: PBS containing 0.05% v/v Tween-20 (PBST).
Blocking solution: 5% w/v milk, 1% w/v BSA, or 0.1% w/v gelatin, as specified, in PBST.
Phosphate citrate buffer: Citric acid 3.36 g, sodium phosphate monobasic 9.88 g, adjust pH to 5.0, bring to 1 L. Store at 4°C; alternatively, use phosphate-citrate buffer tablet, Sigma, Cat. # P4809.
Substrate solution: O-phenylene diamine dihydrochloride (OPD; Pierce, Cat. # 34062) in citrate phosphate buffer (make fresh for each use), 40 mg OPD in 100 mL citrate phosphate buffer, add 40 µL 30% (v/v) hydrogen peroxide (Sigma, Cat. # 1009).
2.4.1. Conventional ELISA
Primary antibody, monoclonal antibody (H6C5, hybridoma supernatant) made against intact mucins purified from CF sputum ((7); see Note 5).
Secondary antibody, goat anti-mouse-HRP conjugated (Jackson ImmunoResearch, Cat. # 115-035-020), from which a stock, stored at −20°C, is made by diluting 1:1 in glycerol.
2.4.2. Mucin Subunit Ab ELISAs
The mucin subunit antibody is a rabbit, polyclonal antibody made against purified, reduced, and alkylated salivary MUC5B; however, as detailed in Note 5, the antibody appears to detect all vertebrate polymeric mucins.
Goat anti-rabbit-HRP (Jackson ImmunoResearch, Cat. # 111-035-003), from which a stock, stored at −20°C, is made by diluting 1:1 in glycerol.
Non-binding 96-well plates (Corning, Cat. # CLS3641) or 1-mL cluster tubes (Costar, Cat. # 4408).
0.1 M Tris buffer, pH 8.0.
Dithiothreitol (DTT; Sigma, Cat. # D9163), 10× stock, 100 mM, 15.42 mg/mL, in Tris buffer (make fresh for each use).
Iodoacetamide (Sigma, Cat. # I1149), 10× stock, 250 mM, 46.25 mg/mL, in Tris buffer (make fresh for each use).
2.4.3. WGA ELLA
Lectin from Triticum vulgaris (WGA), Peroxidase labeled, Sigma, Cat. # 3590, lyophilized powder reconstituted in sterilized PBS, 1 mg/mL.
2.4.4. Periodic Acid-Biotin-Hydrazide Assay
Sodium acetate buffer, 100 mM, EDTA, 5 mM, pH 5.5. Dissolve 13.61 g sodium acetate·3H2O and 1.46 g EDTA in 500 mL water. Adjust pH to 5.5 with acetic acid.
Periodic acid, 1 mM. Dissolve 2.28 mg periodic acid in 10 mL of acetate buffer (make fresh for each use).
Biotin hydrazide: Sigma, Cat. # B-7639, stock 50 mM in DMSO. Dissolve 12.9 mg of biotin hydrazide in 1 mL DMSO (need to warm for complete solubility). Store in 50-µL aliquots at −20°C; make a fresh working solution by adding a 50-µL aliquot to 15 mL acetate buffer.
Streptavidin–HRP, Sigma, Cat. # 3892. Supplied as a 1 mg/mL solution.
3. Methods
3.1. HBE Cell Culture
HBE cell culture techniques that yield good-quality primary cultures with a degree of differentiation that mimics adult airway epithelia have been available for several years. These cultures have proven quite useful for the study of regulated mucin secretion. However, for laboratories just engaging in the practice, it must be pointed out that good primary culture techniques are essential to ensure high-quality results. The techniques are considerably more laborious than those used for the growth and maintenance of cell lines; shortcuts are few. That said, the techniques required are outside the scope of this review with its focus on techniques for using the cultures in secretion experiments. Consequently, we offer a brief overview, but otherwise refer the reader to ref. 6 which offers both a historical perspective and detailed procedures of the culture system and to Chapter 15.
HBE cell cultures are primary cultures that begin, ideally, with epithelial cells digested proteolytically from airway tissues (see Note 6). When these cells are seeded on plastic or the type of permeable insert used for epithelial cell culture, only the basal cells adhere—the occasional ciliated and/or goblet cell that might be observed in such cultures the first few days after seeding has been introduced into the culture as a clump that includes basal cells (see Note 7). The seeded cells proliferate for 2–3 days in BEGM medium to form a confluent monolayer, at which point the luminal liquid is removed and the culture medium switched from BEGM to ALI, the classic conditions of “ALI” cultures (see Note 8). Under ALI conditions in which the cells are fed with ALI medium, selectively, at their basolateral surfaces three times per week, the cells differentiate over a 4- to 6-week period to assume a mucociliary phenotype.
3.2. HBE Cell Culture Mucin Secretion Experiments
The methods described here for determining the mucins secreted from HBE cell cultures evolved over several years of trial and error in our laboratory. Despite extensive previous experience with mucin secretion assays using SPOC1 and other cell lines (8), and with HBE cell cultures in airway biology experiments (9– 11), our initial experience in HBE cell culture mucin secretion experiments was so disappointing that our first published results used HBE cells grown instead in tracheal xenografts, in the backs of nude mice (7). The problems we experienced using HBE cell cultures were reflected in poor secretory responses, or a complete lack of response, to purinergic agonists (ATP, UTP, ATP γS). In subsequent trouble-shooting, we found from periodic acid-Schiff (PAS)-stained cultures that goblet cell mucin stores were discharged during the wash procedure that preceded agonist exposure, likely resulting from mechanical stimulation associated with mis handling the cultures. A serious complicating requirement in these experiments is that several successive washes are required to remove accumulated mucins from culture surfaces in order to be able to detect those freshly secreted—one or two washes are unsatisfactory, as shown below. As a consequence, we developed the following protocol to preserve HBE mucins stores during preparation for experiments. It is based on an experimental paradigm of a wash procedure to remove extracellular mucins from HBE cell cultures, followed by shorter baseline and agonist secretion periods. One way to emphasize the importance of a gentle wash procedure is to note that it consumes ~4.5 h of time, relative to a typical experimental period of ~1 h.
3.2.1. Prewash, 48–72 h Prior to the Beginning of an Experiment
6- or 12-well cluster plates bearing cell cultures are removed from the incubator and placed individually in foam pads (see Note 4). The lumen (top) of each culture is washed, gently, with PBS, and the ALI medium in the basal (bottom) compartment is replaced. At this time, Dermabond® is used to glue the inserts in place by applying a small drop between the rim of each cell culture insert and the top of the respective well (see Note 3). The cultures, in their foam pads, are placed, unstacked, in the incubator (see Note 9).
3.2.2. Careful Wash Procedure, Four Washes, Total Time ~4.5 h
In all subsequent handling steps, plates are prepared one at a time. A foam pad bearing a culture is carefully picked up and removed from the incubator, carried to the hood without any sudden, jarring motions, and gently placed on the deck—placement on the deck is best done, before sitting, by placing the rear edge (toward the back of the hood) of the foam pad down first at a slight angle, and then lowering the pad slowly to rest on the deck surface.
Plates are subjected to a gentle wash, defined as follows: raising the rear edge of the foam pad to an ~45° angle and holding it there with a foam block or other device, the lid is removed, gently, the pipette tip, held vertically, is inserted into the front corner of the cell culture to slowly remove and add luminal liquid, following which the pad is relowered gently to the deck surface. These actions allow surface liquids to flow slowly over culture surfaces to, and from, an isolated corner during removal and addition, respectively. “Gently” cannot be overemphasized in all movements involving the HBE cell culture wash procedure.
Gentle wash 1: Add warm (37°C) DMEM (400 µL for 12-mm inserts, 1 mL for 24-mm inserts) to culture lumens, gently reposition the dish and pad to the horizontal, and replace in the incubator for 10 min.
Gentle wash 2–4: Collect and save the surface liquid, add a new volume of warm DMEM, and return to incubator for 1 h (see Note 10).
3.2.3. Baseline Secretion Period
Procedure is the same as in gentle wash 2–4, but during this incubation the mucins secreted and collected at the end of the 1-h period are taken to represent those released at baseline (see sample results at the end of Subheading 3).
3.2.4. Experimental Period
Most experiments have an experimental procedure during the next and generally final incubation. The mucins released, collected at the end of the period, are frequently normalized to those released during the baseline secretion period. In the sample results illustrated below, ATP γS was used to stimulate regulated mucin secretion via P2Y2 purinoceptors (4), and we suggest this condition as a good control for most experiments.
3.2.5. Illustrative Data
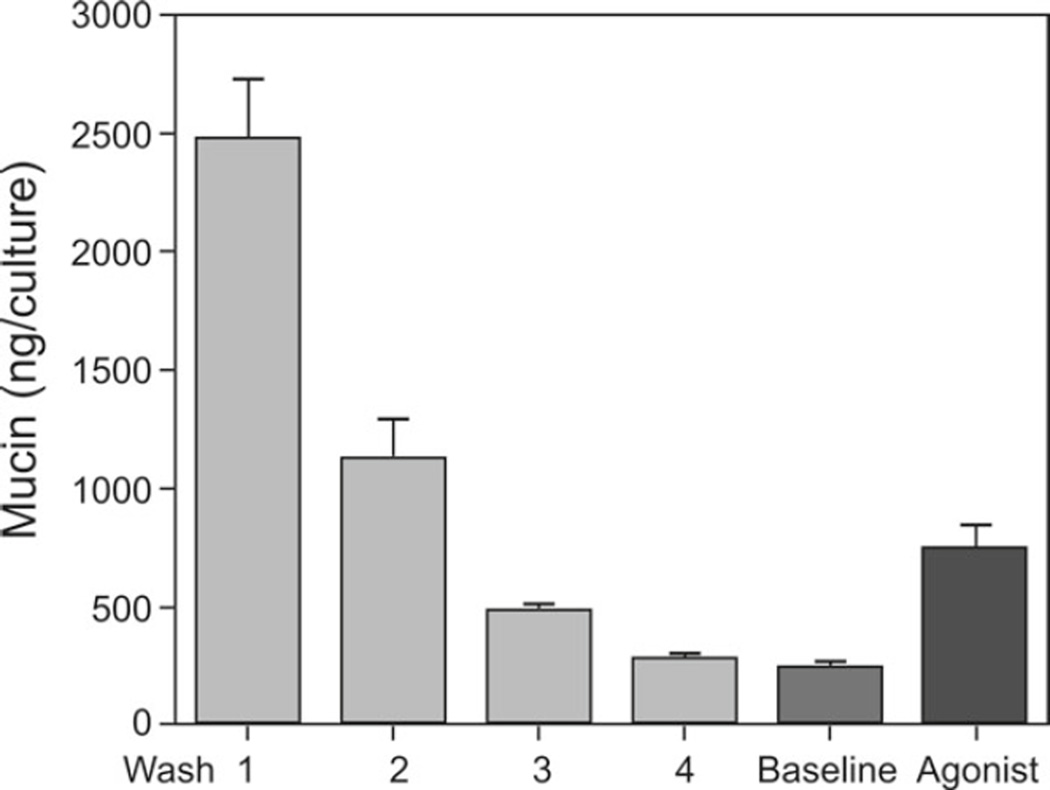
Figure 1 shows the results of a typical agonist secretion experiment with HBE cell cultures grown on 12-mm Transwell Clears using ATP γS. During the wash procedure, ~4,300 ng of mucin was removed from the cultures. While the majority of this mucin was removed during the initial 10-min wash period, significant quantities remained that were removed during the next 3 h-long washes. Note that the mucins secreted during the baseline period were quantitatively similar to those from the final wash period. In other experiments (not shown), in which a second baseline period follows the first instead of an incubation with agonist, the mucins from both baseline periods were similar to those from the final wash period; i.e., with this procedure, a steady-state release of mucins at baseline is achieved. The approximately three-fold stimulation of mucin secretion by a maximal concentration of ATP γS is typical; the multiplicity (agonist/baseline) in our hands ranges from ~2 to 4.
Fig. 1.
Mucins recovered from HBE cell cultures 12-mm inserts) during a careful wash procedure, and the subsequent baseline and agonist (ATPγS, 100 µM) secretion periods (mean ± SE, n = 6). The conventional mucin subunit ELISA was used for these assays.
3.3. Preparing Mucin Standards
Ready sources of mucus from which mucins may be purified for use as standards are HT29 cell cultures (3), saliva (12), and HBE cell cultures (13), which secrete, respectively, MUC5AC, MUC5B, and both MUC5AC (~20%) and MUC5B (~80% (13); see Note 11). Saliva also contains MUC7, but this smaller, nonpolymeric “contaminant” is easily removed by size-exclusion chromatography on Sepharose CL2B, as described below. Mucins are purified according to their buoyant density using density-gradient centrifugation in CsCl, according to the technique first described in 1983 by Carlstedt and Sheehan ((14); see Note 12).
HT29 cell mucus collection. HT-29-A1 cells, or another subclone that secretes MUC5AC as the only polymeric mucin detectable at the glycoprotein level, are cultured in RPMI 1640 medium with L-glutamate containing 10% (v/v) fetal calf serum. The media removed from the cultures during feeding and/or passaging is saved by adding PICtab (1 tablet/50 mL), and pooled (4°C) until a sufficient quantity is achieved (1 L is a reasonable minimum). The pooled material is concentrated in dialysis bags using Specta/Gel® to ~1/3 its original volume, and then GuHCl is added to a final concentration of 4 M.
Saliva collection. Collect (whole) saliva by chewing pieces of Parafilm® and expectorating the saliva into ice-cold 8 M GuHCl buffer, to which PICtab (1 tablet/50 mL) is freshly added, until the GuHCl is diluted to 4 M (150 mL is a reasonable minimum). This material is subjected to a crude separation by chromatography on a Sepharose CL2B column (see Note 13) eluted with 4 M GuHCl; the void volume containing MUC5B is collected while the included volume containing other proteins and glycoproteins, such as MUC7 and DMBT1, is discarded.
HBE cell culture mucus collection. Beginning ~7–10 days following confluence, HBE cell cultures secrete significant quantities of MUC5AC and MUC5B in a ratio of ~1:5 (13). These mucins are collected at the times of culture feeding by placing 0.5 or 1.0 mL of PBS in the lumens of 12- or 24-mm Transwell cultures, gently pipetting it up and down 2–3 times, then removing the material, adding PICtab (1 tablet/50 mL), and pooling it (4°C) until a sufficient quantity is achieved (250 mL is a reasonable minimum). GuHCl is added to a final concentration of 4 M (see Note 14).
Purification of mucins by cesium chloride density gradient (see Note 15). To any of the above mucin-rich materials in 4 M GuHCl, add CsCl to a density of 1.4 g/mL using the equation: x = v (1.347 ρ − 0.0318M − 1.347), where x is CsCl (g), v is the volume of material, ρ is the final density (1.4 g/mL), and M is the molarity of GuHCl (14). The material is centrifuged to equilibrium at 38,000 rpm at 15°C in a Beckman 50.2 Ti rotor (131,242 × g) for 3 days, the tubes fractionated into 2.5-mL volume, and each fraction is analyzed for density (by accurately weighing 1 mL), absorbance at 260 nm (DNA) and 280 nm (protein), and PAS staining (mucins; by slot blotting, (15)). The mucin-rich, PAS-positive fractions, which should have densities of ~1.4–1.5 g/mL, are pooled and dialyzed exhaustively against 0.2 M GuHCl, 5 mM EDTA in 10 mM phosphate buffer, pH 6.5. Next, the material in 0.2 M GuHCl is prepared for the second centrifugation in CsCl, centrifuged, the tubes fractionated, and fractions analyzed exactly as the first time. The mucin-rich, PAS-positive fractions are pooled and dialyzed against PBS for 3–4 days with two buffer changes each day.
Determination of mucin concentrations in the standards (see Note 16). Refractive index is used to measure the absolute concentration of mucins that are being used as standards. The refractive index of a solution receives contributions from the solvent and the solute. The contribution from the solute is generally a linear function of the concentration and is described as the refractive index increment, d n/dc, which for mucins is 0.165 mL/g. Using a gel filtration column with large exclusion media (Sepharose CL2B or Sephacryl S1000), mucins are separated from other proteins and can be quantified in a single step as the eluant from a size-exclusion chromatography column is simply passed through a refractive index detector.
Precondition the column with at least two-column volumes of running buffer or until getting a stable baseline.
Dilute a sample of the purified mucin stock solution 10–20 times with the running buffer, and inject 500 µL of the diluted standard to the column.
Elute the sample at a 0.5 mL/min flow rate and collect data from the in-line Optilab refractometer for 40 min with 5-s collection intervals.
Using ASTRA software, define the mucin peak at the void (Vo) volume, and use the d n/dc ratio of 0.165 mL/g to calculate the absolute quantitation of mucin in the injected volume. Multiply by the dilution factor to calculate mucin concentration in the stock standard solution.
Store purified mucin in aliquots at −20°C.
3.4. Microtiter Plate Mucin Assays
Below, we offer five different microtiter plate assays for detecting mucins in secretions from HBE cell cultures obtained during experiments in which the luminal surfaces are carefully prewashed to remove mucus that may have accumulated prior to the experiment. Illustrative data are presented below from an agonist challenge experiment for each of the assays using each assay on the same set of samples.
3.4.1. Enzyme-Linked Immunosorbent Assay
These are conventional microtiter plate binding assays that use a primary mucin-specific antibody for detection and a secondary antibody conjugated to horseradish peroxidase (HRP) as a reporter. We offer three enzyme-linked immunosorbent assay (ELISA) variants: (1) conventional ELISA, which uses a conventional antibody, monoclonal, in this case; (2) mucin Subunit ELISA, which uses the “mucin subunit” antibody which requires the mucins to be reduced with DTT for detection (see Note 5); and (3) mucin subunit sandwich ELISA, which uses the same mucin subunit antibody in a sandwich format.
3.4.2. WGA Enzyme-Linked Lectin Assay (see Note 17)
Lectins are carbohydrate-binding proteins, some of which are useful for detecting mucins. Because lectins are glycan specific, they are not mucin specific and their rigorous use therefore requires validation (e.g., 16–18). Our laboratory uses WGA, specific for GlcNAc β1–4GlcNAc β1–4GlcNAc and Neu5Ac (sialic acid), to detect mucins from HBE cell cultures and mouse tracheas.
3.4.3. Periodic Acid-Biotin-Hydrazide Assay
This assay (7) is based on the same principle as the PAS stain for mucins; however, it uses a biotin conjugate of hydrazide, instead of Schiff’s reagent, to react with the ketones formed from the periodate oxidation of the vicinal diols of susceptible sugars—which in mucins are typically terminal sialic acid residues. The advantage of periodic acid-biotin-hydrazide (PABH) over PAS is that the staining is amplified by streptavidin-conjugated HRP, increasing the sensitivity of the assay many fold.
3.4.4. Mucin Binding Assays
Because these assays have many steps in common, the procedure that follows, for brevity, is offered as a linear list of steps, each declared as being common to all the assays or specific to a particular one.
Techniques Common to All Assays (see Note 18)
The dilution factor used for preparation of samples and standards depends on the relative concentrations of mucins; consequently, it is therefore determined empirically in preliminary assays (see Note 19).
All incubations are done in a humidified chamber(s).
All washes are with PBST, 200 µL/well; multiple washes are indicated by an “x” factor, e.g., wash = single wash; wash 2x = 2 washes.
All blocks are incubations with 0.1% (w/v) or 1% (w/v) gelatin, 5% (w/v) milk, or 1% (w/v) BSA, in PBST, as specified below, 200 µL/well, 1 h at 37°C.
Mucin Subunit Antibody, Sandwich ELISA
For the Sandwich ELISA, this is the first step; skip for all other assays.
Coat plates with WGA lectin (10 µg/mL) in PBS (see Note 17), 100 µL/well. Incubate overnight at 4°C or 2 h at 37°C; wash 4×.
-
Block in 1% gelatin and wash 2×, or block, store in blocking buffer overnight at 4°C; wash 4×.
Since the mucin subunit antibody detects mucins whose disulfide bonds have been reduced, the samples must be reduced and alkylated prior to detection (see Note 20); however, because reduction harms WGA binding, this step needs to occur before sample plating for the sandwich ELISA. Reduction and alkylation can be done on samples distributed on separate nonbinding microtiter plates or in cluster tubes.
To each sample and standard (1–100 µL; see Note 18), add Tris buffer such that the total volume = 180 µL (e.g., for a 20 µL sample, add 160 µL Tris buffer).
Add 20 µL DTT stock (final concentration, 10 mM); mix and incubate for 15–20 min at 37°C.
Add 25 µL iodoacetamide stock (final concentration ~25 mM; iodoacetamide should be ~2.5-fold molar excess over DTT); mix and incubate, in the dark, for 30 min at RT.
Common Procedures: Plate and Wash
For all assays:
Plate samples and standards diluted in PBS, as necessary, 100 µL/well (see Note 18). Incubate for 2 h at 37°C or overnight at 4°C.
Wash 4×.
Blocking and Detection: Assay Specific
- Conventional ELISA (H6C5 or other antibody).
- Block with 5% w/v milk in PBST; wash 2×.
- Incubate with H6C5 or other primary antibody (generally, 1:1,000 in 1% w/v milk in PBST), 100 µL/well, overnight at 4°C or 2 h at 37°C; wash 4×.
- Incubate with an appropriate secondary, HRP-conjugated antibody (generally, 1:1,000 in PBSt), for 1 h at 37°C.
- WGA ELLA (see Note 21):
- Block with 0.1% gelatin in PBST; wash 2×.
- Incubate with WGA: HRP (2.5 µg/well) in PBST, 100 µL/well, 1 h at 37°C.
- Mucin subunit Ab-specific ELISAs, conventional and sandwich formats: Prior to blocking for the conventional mucin subunit ELISA, the mucins need to first be reduced and alkylated (see Note 20); for the sandwich ELISA, skip steps (a)–(c), as the samples were reduced and alkylated prior to plating.
- Add DTT stock diluted 1:10, 100 µL/well (final concentration 10 mM), and incubate for 10–15 min at RT; pour off the DTT solution (invert and shake plate over sink).
- Add iodoacetamide stock diluted 1:10, 100 µL/well (final concentration 25 mM), incubate in the dark for 30 min at RT.
- Wash 4×.
- Block with 1% BSA in PBST; wash.
- Incubate with mucin subunit antibody (1:5,000 in 1% BSA in PBST), 100 µL/well, 2 h at 37°C or overnight at 4°C; wash 4×.
- Incubate with an appropriate secondary, HRP-conjugated antibody (1:2,000 in PBST), for 1 h at 37°C.
- PABH assay:
- Incubate with 1 mM periodic acid (100 µL/well) at RT for 10 min in the dark.
- Add biotin hydrazide solution (50 µL/well), mix well, and incubate for 45 min at RT.
- Wash 4× with 5-min incubations between each wash (to more completely remove the DMSO from the biotin hydrazide solution), and incubate with streptavidin–HRP in PBST, 100 µL/well, for 20 min.
Common Procedures: Wash and Develop
For all assays:
Wash 4×.
Develop with OPD substrate, 150 µL/well, 10–15 min at room temperature; stop OPD reaction with 4 M H2SO4, 50 µL/well.
Determine ODs at 490 nm in plate reader (e.g., Spectra Max Plus, Molecular Devices) and use an appropriate software program to construct standard curve and calculate sample values.
Sample Data
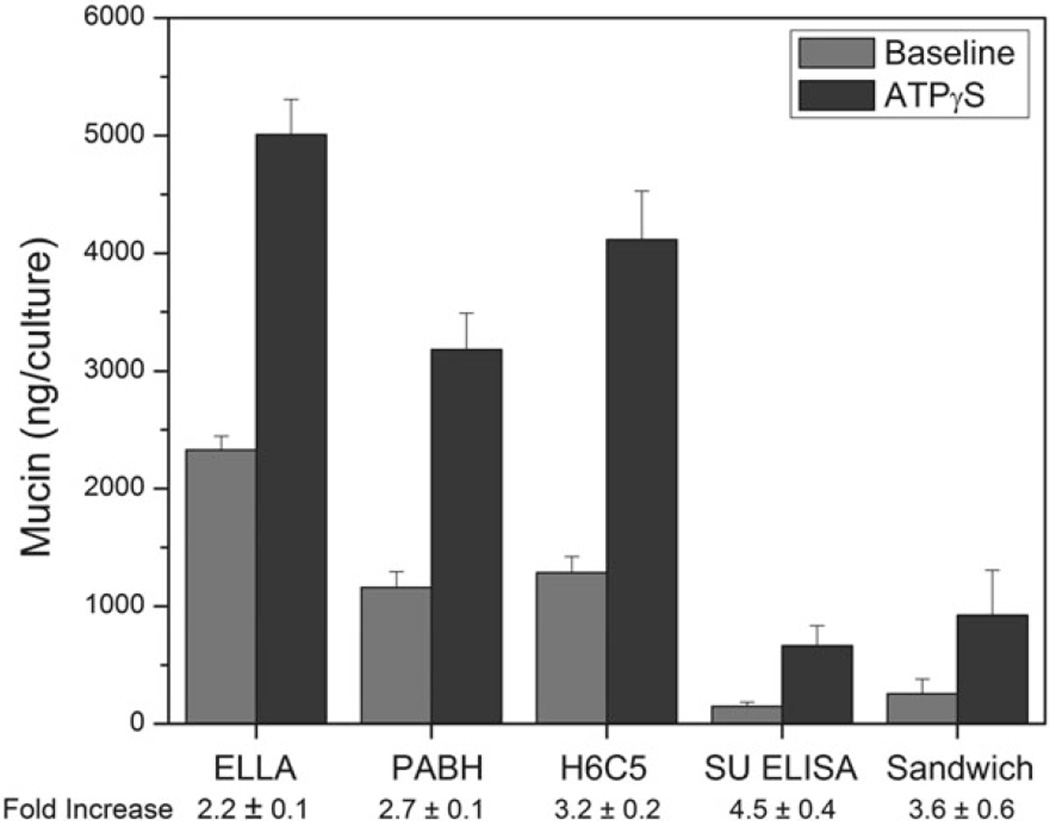
Figure 2 shows data for baseline and agonist exposure periods from an experiment with HBE cell cultures, with the samples being analyzed by each of the five assays described above. Note that the two mucin subunit antibody-based assays (conventional and sandwich ELISAs) yielded similar data, with baseline- and agonist-stimulated mucins of ~200 and ~700 ng/culture, as well as multiplicities (fold increases) >3.5. The other assays, in contrast, had values several fold higher, with baseline- and agonist-stimulated values >1,000 and >3,000 ng/culture, and proportionately lower multiplicities. These differences most likely reflect probe specificities: WGA, PABH, and the H6C5 antibody bind indiscriminately to mucins of all types, as well as to other glycan-bearing compounds, whereas the mucin subunit antibody is specific to polymeric mucins, MUC5AC and MUC5B in this case. Nonetheless, the less specific assays do yield results qualitatively similar to the more specific, so their use may be justified for situations, where the intent lies more in studying whether or not a goblet cell secretory response is initiated or modulated. For questions relating to the release of polymeric mucins, per se, mucin-specific probes, such as the mucin subunit antibody, are required.
Fig. 2.
Mucins sampled following baseline and agonist (ATPγS, 100 µM) periods, as measured, on the same set of samples, by the binding assays described above. SU = mucin subunit antibody, for both the ELISA and sandwich (ELISA) assays indicated. Results expressed as the mean ± SE, n = 3; fold increase = agonist stimulated/baseline.
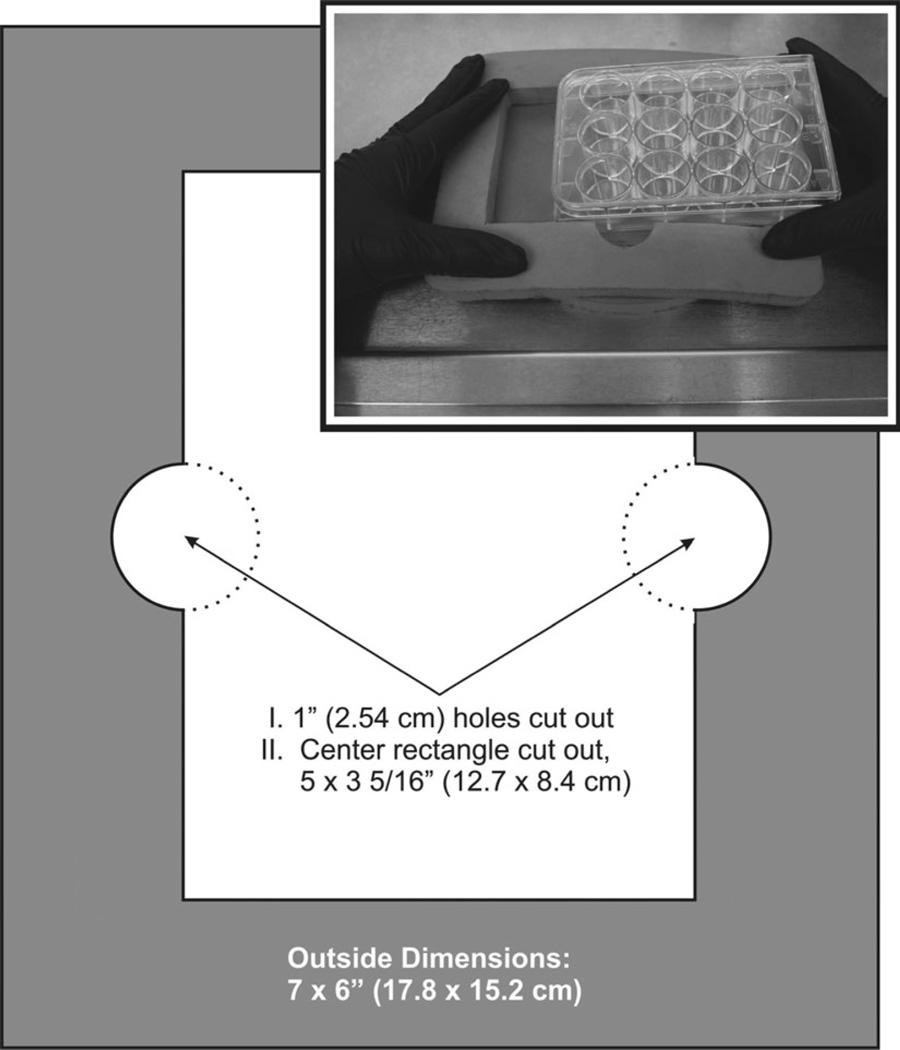
Fig. 3.
Foam pad, top piece, to hold 6- or 12-well cluster plates during HBE cell culture wash procedures. The center cutout should hold the plate quite firmly, i.e., it should be necessary to work the plate into the cutout by applying pressure to all sides of the plate as it is inserted. Inset: Image of a cluster plate being inserted into an assembled foam pad.
Acknowledgments
Studies in the authors’ laboratories which lead to the development of the techniques described were supported by National Institutes of Health grants, HL-063756 and HL084934, and grants from the Cystic Fibrosis Foundation.
Footnotes
The culture media used for HBE cell culture, BEGM, and ALI have a substantial history, which is outlined in ref. 6 along with detailed instructions on their compositions. This reference is the result of many years of experience in HBE cell culture by our UNC colleague, Dr. Scott Randell, who directs the operation of our Cell Culture Facility, and it should be read in detail by anyone contemplating HBE cell culture, at any scale.
Commercial sources for human epithelial cells appear to change dynamically, as companies are bought, sold, or traded by large corporations. Hence, if the Web links indicated do not work, we suggest searching the Web using the suppliers’ names (e.g., Cambrex).
We use Transwell® inserts because they hang from the rims of the wells in a cluster plate. This configuration allows them to be mechanically stabilized by gluing them in place with Dermabond® just before an experiment. Other brands of hanging, polyester or PTFE inserts, should work as well. If Millicel® standing inserts are desired, we have found that the 10-mm PTFE inserts can be placed snugly into (recycled) 12-mm Transwell units after removing the membrane. Dermabond® is packaged in “single-use” plastic capsules with an applicator at one end—the adhesive is contained in an internal, thin-walled glass capsule, from which it is generally released for use by crushing the sides of the nested capsules. We instead remove the adhesive by cutting through the capsule walls with scissors, just below the applicator, and pouring it off into a microfuge tube in which it is stored until consumed. Dermabond is applied in 3 µL droplets to the Transwell® in three spots, placing the tip of the pipette to the grove between the underside of the insert rim and the top of the well to allow the adhesive to wick into the space.
The foam pads are essential for successful mucin secretion experiments. During an experiment, they provide a convenient means of handling plates, they reduce significantly the mechanical stimuli that can elicit secretion by cushioning the stresses caused by handling, and they provide insulation against temperature changes while plates are removed from an incubator. Unfortunately, those used in our laboratory (Fig. 3, inset) are no longer available and we have been unable to find commercial replacements of equal quality. It is quite reasonable, however, to make them from EPDM foam rubber, adhesive backed, 0.5″thick, 6″wide, 25′ long (~1.2 × 15.2 cm × 8 m long) cut into 7″(17.5 cm) lengths. Half of the foam pieces are used, as is, as bottom pieces. The others, which form the top pieces, first have 2.1″(2.54 cm) holes cut through with a cylindrical cutter (e.g., a cork borer), and then a 5 × 3 5/16″(12.7 cm × 8.4 cm) center rectangle is cut out, as indicated in Fig. 3. The adhesive faces of the two pieces are placed/glued together, with careful positioning, after removing the adhesive backings. The adhesive material on the bottom piece, exposed in the center of the top cutout, can be coated with an innocuous powder.
The conventional ELISA is suitable for most mucin antibodies that are likely to be used. For the mucin subunit ELISA, we use a polyclonal antibody made originally against purified cervical mucins that had been reduced and alkylated (19), a procedure that not only opens up all the D-domains and Cys-domains, but also depolymerizes the mucins. The antibody binds to epitopes exposed by disulfide reduction, and it has proved to detect all the polymeric mucins from every vertebrate species against which we have tested it, hagfish included (Abdullah, LH, CW Davis, and JK Sheehan, unpublished observations). To improve specificity for the Cys-rich and/or D domain peptides exposed upon reduction, the antibody is preabsorbed against intact, nonreduced salivary MUC5B, 10 µg mucin/100 µL antibody in nondiluted serum, and incubated overnight at 4°C (the proportions may need to be adjusted for other lots of antibody). The solution is centrifuged at 80,000 rpm in a Beckman TL-1000 tabletop ultracentrifuge for 2.5 h, and the supernatant harvested. The pre-absorbed antibody preparation has a functional lifetime of ~2 months. Note that the requirement for mucins to be reduced for detection with this antibody offers an unusual element of specificity, in that nonreduced samples may be used as a specificity control.
Airway cells received from commercial sources may, or may not, be derived by proteolysis of bronchial tissues. The original system described by Lechner and LaVeck harvested cells grown out from explanted airways tissue (20), and if this method is used commercially, the cells received will have experienced several series of cell division prior to seeding in the investigator’s laboratory. If this is the case, the cultures derived may be phenotypically quite different from those described using the methods described in ref. 6.
Basal cells are not only the primordial cell of the superficial airway epithelium in the large airways of humans (21), but they also serve as the anchor for columnar cells. Both ciliated and goblet cells possess basal protrusions which contact the basement membrane; however, their anchorage is actually to basal cells (22).
HBE cells grown in culture media other than the BEGM/ALI system have been studied for various aspects of airway epithelial biology (see ref. 6); however, regulated mucin secretion has been studied nearly exclusively from cells grown under BEGM/ALI culture conditions.
HBE cultures exposed to maximal doses of ATP (100 µM) for 30–40 min typically secrete all of their mucin stores; the stores take a minimum of 48 h to recharge fully (L.H. Abdullah and C.W. Davis, unpublished observation). Hence, the goblet cells should be fully charged when the cultures are removed for experimentation, 48–72 h later.
Mucin contents in the luminal washes are determined along with the baseline and experimental period samples, as part of good data quality assurance.
Purified mucins from HBE cell cultures contain both MUC5AC and MUC5B, but are useful nonetheless as a mucin standard when using the mucin subunit antibody since it recognizes both mucins or when using nonspecific assays, such as an ELLA or the PABH binding assay.
Other methods for purifying mucins, e.g., size-exclusion chromatography on Sepharose CL2B, are unsuitable for the preparation of standards because they result in dilution, rather than concentration, of the mucins during purification.
“Crude separation” is used to mean a low-resolution Sepharose CL2B chromatography procedure, in which up to 10% of the total column volume is loaded onto the column. It is used merely to separate the polymeric MUC5B from the monomeric MUC7.
HBE cell cultures may be established solely for the purpose of mucus collection (e.g., see ref. 23), as they maintain a good mucin production for 2–3 months after confluence. In such cases, the larger, 24-mm culture inserts are a better choice than the smaller ones.
The reader is referred to refs. 14, 24 for a full description of mucin purification by double isopycnic density-gradient centrifugation in CsCl. In the first centrifugation, in 4 M GuHCl, mucins and DNA (if present) comigrate to densities of ~1.4–1.5 g/mL. When the GuHCl is reduced to 0.2 M for the second centrifugation, DNA reassociates into a double-stranded form, which makes it migrate to lighter, final densities of ~1.2–1.3, whereas the mucins still migrate to a final density of ~1.4–1.5 g/mL.
An alternative to the use of refractive index in quantifying mucins is to use gravimetric analysis, in which a small volume of sample is dialyzed extensively against water and 100 µL is dried completely in an 80°C oven and weighed to the nearest µg. Though simpler to perform, both conceptually and mechanistically, this method is not preferred for it is subject to a variety of artifacts, including incomplete drying, and absorption of moisture as the sample is cooled and weighed.
Two styles of multichannel pipette are essential for good mucin binding assays on microtiter plates. First, a “single-stepper,” multichannel pipette enhances the transfer of test samples and standards to the microtiter plate, accurately measuring and transferring the solutions from a set of nonbinding cluster tubes (Costar 8-strip tubes, Cat. # 4408) arranged in the same pattern as the microtiter plate (we use either a 2.5–25 µL Brand Transferpette-8® or a 20–200 µL Costar 8-Pette®). Second, a “multistepper,” multichannel pipette allows wash solutions and other reagents to be applied rapidly to an entire plate (we use an Ovation BioNatural Pipette).
Sample and standard volumes applied to microtiter plates need to be adjusted according to the quantity of mucins they contain. As a guide, samples from HBE cell cultures grown in 12-mm Transwell inserts, in our hands, contain 50–150 ng/mL mucin at baseline, so for the subunit ELISA, our primary assay system, we use standards with the following dilution series: 500, 250, 125, 62.5, 31.3, 15.6, and 7.8 ng/mL HBE mucin.
Wheat germ agglutinin, WGA, is commonly used to bind sialic acid residues. In this sandwich ELISA, it is used to coat the microtiter plate surfaces and it functions by binding the mucins to the surface via the mucin-glycosylated repeat domains (as well as any other sialic acid glycoproteins or glycolipids present). Hence, in the end, the mucins are “sandwiched” between WGA and the detecting reagent, the mucin subunit antibody in this case.
Alkylation blocks reduced Cys residues, preventing disulfide reassociation when DTT is removed. Because the iodoacetamide consumes the excess DTT, the resulting mixture of sample and reagents/reactants can be applied safely to WGA-coated plates in the sandwich ELISA. Important: Do not wash before alkylation, as Tween-20 interferes in the reaction.
The blocking step is optional for the WGA ELLA. In our experience, skipping the step has no effect on the outcome of the assay; however, some laboratories may feel more comfortable including the step.
References
- 1.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2007;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 3.Sheehan JK, Kirkham S, Howard M, Woodman P, Kutay S, Brazeau C, Buckley J, Thornton DJ. Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J. Biol. Chem. 2004;279:15698–15705. doi: 10.1074/jbc.M313241200. [DOI] [PubMed] [Google Scholar]
- 4.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu. Rev. Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 5.Danahay H, Jackson AD. Epithelial mucus-hypersecretion and respiratory disease. Curr. Drug Targets. Inflamm. Allergy. 2005;4:651–664. doi: 10.2174/156801005774912851. [DOI] [PubMed] [Google Scholar]
- 6.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol. Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 7.Conway JD, Bartolotta T, Abdullah LH, Davis CW. Regulation of mucin secretion from human bronchial epithelial cells grown in murine hosted xenografts. Am. J. Physiol Lung Cell Mol. Physiol. 2003;284:L945–L954. doi: 10.1152/ajplung.00410.2002. [DOI] [PubMed] [Google Scholar]
- 8.Davis CW. Regulation of mucin secretion from in vitro cellular models. Novartis. Found. Symp. 2002;248:113–125. [PubMed] [Google Scholar]
- 9.Matsui H, Davis CW, Tarran R, Boucher RC. Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia. J. Clin. Invest. 2000;105:1419–1427. doi: 10.1172/JCI4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 11.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J. Clin. Invest. 1998;102:1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thornton DJ, Khan N, Mehrotra R, Howard M, Veerman E, Packer NH, Sheehan JK. Salivary mucin MG1 is comprised almost entirely of different glycosylated forms of the MUC5B gene product. Glycobiology. 1999;9:293–302. doi: 10.1093/glycob/9.3.293. [DOI] [PubMed] [Google Scholar]
- 13.Holmen JM, Karlsson NG, Abdullah LH, Randell SH, Sheehan JK, Hansson GC, Davis CW. Mucins and their O-Glycans from human bronchial epithelial cell cultures. Am. J. Physiol Lung Cell Mol. Physiol. 2004;287:L824–L834. doi: 10.1152/ajplung.00108.2004. [DOI] [PubMed] [Google Scholar]
- 14.Carlstedt I, Lindgren H, Sheehan JK, Ulmsten U, Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem. J. 1983;211:13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton DJ, Carlstedt I, Sheehan JK. Identification of glycoproteins on nitrocellulose membranes and gels. Methods Mol. Biol. 1994;32:119–128. doi: 10.1385/0-89603-268-X:119. [DOI] [PubMed] [Google Scholar]
- 16.Abdullah LH, Davis SW, Burch L, Yamauchi M, Randell SH, Nettesheim P, Davis CW. P2u purinoceptor regulation of mucin secretion in SPOC1 cells, a goblet cell line from the airways. Biochem. J. 1996;316:943–951. doi: 10.1042/bj3160943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson A, Kemp P, Giddings J, Sugar R. Development and validation of a lectin-based assay for the quantitation of rat respiratory mucin. Novartis. Found. Symp. 2002;248:94–105. [PubMed] [Google Scholar]
- 18.Clancy SM, Yeadon M, Parry J, Yeoman MS, Adam EC, Schumacher U, Lethem MI. Endothelin-1 inhibits mucin secretion from ovine airway epithelial goblet cells. Am. J. Respir. Cell Mol. Biol. 2004;31:663–671. doi: 10.1165/rcmb.2003-0331OC. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan JK, Boot-Handford RP, Chantler E, Carlstedt I, Thornton DJ. Evidence for shared epitopes within the ‘naked’ protein domains of human mucus glycoproteins. A study performed by using polyclonal antibodies and electron microscopy. Biochem. J. 1991;274:293–296. doi: 10.1042/bj2740293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechner JF, LaVeck MA. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J. Tiss. Cult. Meth. 1985;9:43–48. [Google Scholar]
- 21.Roomans GM. Tissue engineering and the use of stem/progenitor cells for airway epithelium repair. Eur. Cell Mater. 2010;19:284–299. doi: 10.22203/ecm.v019a27. [DOI] [PubMed] [Google Scholar]
- 22.Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp. Lung Res. 2001;27:401–415. doi: 10.1080/019021401300317125. [DOI] [PubMed] [Google Scholar]
- 23.Matsui H, Verghese MW, Kesimer M, Schwab UE, Randell SH, Sheehan JK, Grubb BR, Boucher RC. Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J. Immunol. 2005;175:1090–1099. doi: 10.4049/jimmunol.175.2.1090. [DOI] [PubMed] [Google Scholar]
- 24.Thornton DJ, Sheehan JK, Lindgren H, Carlstedt I. Mucus glycoproteins from cystic fibrotic sputum. Macromolecular properties and structural ‘architecture’. Biochem. J. 1991;276:667–675. doi: 10.1042/bj2760667. [DOI] [PMC free article] [PubMed] [Google Scholar]