Abstract
Most effects of the messenger molecule nitric oxide (NO) are mediated by cGMP, which is formed by NO-sensitive guanylyl cyclase (GC) and degraded by phosphodiesterases (PDEs). In platelets, NO elicits a spike-like cGMP response and causes a sustained desensitization. Both characteristics have been attributed to PDE5 activation caused by cGMP binding to its regulatory GAF domain. Activation is paralleled by phosphorylation whose precise function remains unknown. Here, we report reconstitution of all features of the NO-induced cGMP response in human embryonic kidney cells by coexpressing NO-sensitive GC and PDE5. The spike-like cGMP response was blunted when PDE5 phosphorylation was enhanced by additional overexpression of cGMP-dependent protein kinase. Analysis of PDE5 activation in vitro revealed a discrepancy between the cGMP concentrations required for activation (micromolar) and reversal of activation (nanomolar), indicating the conversion of a low-affinity state to a high-affinity state upon binding of cGMP. Phosphorylation even increased the high apparent affinity enabling PDE5 activation to persist at extremely low cGMP concentrations. Our data suggest that the spike-like shape and the desensitization of the cGMP response are potentially inherent to every GC- and PDE5-expressing cell. Phosphorylation of PDE5 seems to act as memory switch for activation leading to long-term desensitization of the signaling pathway.
INTRODUCTION
The nitric oxide (NO)/cGMP signaling cascade plays an important role in the regulation of a variety of physiological responses such as smooth muscle relaxation, inhibition of platelet aggregation, and synaptic plasticity. The messenger molecule NO exerts its effects by the stimulation of NO-sensitive guanylyl cyclase (GC). Activation of NO-sensitive GC leads to enhanced production of the intracellular messenger cGMP, which in turn regulates several target enzymes. Most of the known cGMP effects are mediated by the activation of cGMP-dependent protein kinases (PKGs), cGMP-regulated ion channels or by the regulation of cGMP-dependent phosphodiesterases (PDEs). The increase in cGMP is terminated by the action of cGMP-degrading phosphodiesterases.
NO-sensitive GC consists of two different subunits, α and β, and contains a prosthetic heme group that mediates NO stimulation (Friebe and Koesling, 2003). Two isoforms of NO-sensitive GC (α1β1 and α2β1) have been identified differing in tissue distribution and subcellular localization. For the α2-containing isoform, a role in synaptic transmission is conceivable because this protein was shown to be mainly expressed in brain and to be targeted to synaptic membranes (Russwurm et al., 2001). In contrast, the α1β1 heterodimer is more widely distributed with prominent expression in vascularized tissues (Mergia et al., 2003).
cGMP is degraded by PDEs; of the 11 PDE families known to date, three PDEs specifically hydrolyze cGMP (PDE5, PDE6, and PDE9), whereas others degrade both cGMP and cAMP, e.g., PDE1 and PDE2 (Juilfs et al., 1999; Francis et al., 2000). In many cell types, PDE5 plays a major role for cGMP degradation. The enzyme has been described as cGMP-binding cGMP-specific PDE because at least one of its two regulatory GAF domains (GAF-A and GAF-B) was known to bind cGMP (McAllister-Lucas et al., 1993). Recently, PDE5 has been shown to be directly activated by cGMP binding to the regulatory GAF-A domain (Okada and Asakawa, 2002; Corbin et al., 2003; Mullershausen et al., 2003; Rybalkin et al., 2003). In intact cells, activation of PDE5 is usually paralleled by phosphorylation at a conserved serine residue by PKG.
Stimulation of intact cells with NO leads to a cGMP response determined by the ratio of cGMP-forming and -degrading activities. In platelets, the α1β1 isoform of NO-sensitive GC and PDE5 control intracellular cGMP levels. The NO-induced cGMP response in platelets is characterized by a transient spike-like elevation of cGMP with a fast increase and a subsequent decline of cGMP to resting levels in 40 s. Aortic smooth muscle displays a comparable NO-induced cGMP response with a somewhat broader profile. In both cell types, restimulation with NO elicits a reduced cGMP response, demonstrating desensitization of the NO/cGMP pathway (Mullershausen et al., 2001). Although the mechanism of the cGMP-induced activation of PDE5 seems to explain the characteristic spike-like shape and the desensitization of the cGMP response, it is not entirely clear whether other so far unknown factors are involved.
To elucidate whether NO-sensitive GC and PDE5 are sufficient for the characteristics, i.e., shaping and desensitization of the NO-induced cGMP response, we stably expressed these enzymes in human embryonic kidney (HEK) cells. The spike-like shape of the NO-induced cGMP response as well as the desensitization in these cells was reminiscent of the features observed in platelets and smooth muscle. The role of PDE5 phosphorylation within the NO/cGMP response was assessed using a phosphorylation site mutant PDE5(S102A) and by enhancing phosphorylation by coexpression of PKG. The experiments show that phosphorylation is not required for the shaping of the NO-induced cGMP response but serves to stabilize the activated state of PDE5 most likely by increasing the affinity of the GAF-A domain for cGMP.
MATERIALS AND METHODS
Reagents
Antibody against phosphorylated PDE5 was prepared by immunizing rabbits with the phosphorylated peptide C-TRKIS(PO3)ASEFDR coupled to keyhole limpet hemocyanin. Affinity purification was carried out by sequential adsorption to the peptides C-TRKISASEFDR and C-TRKIS(PO3)ASEFDR immobilized on SulfoLink Coupling Gel (Pierce Chemical, Rockford, IL) according to the manufacturer's instructions. Polyclonal antisera against β1-GC and PDE5 were raised by immunizing rabbits with an N-terminal fragment of 40 kDa (bovine β1-GC) and 50 kDa (human PDE5A1), respectively, fused to glutathione S-transferase (GST); antiserum against PKG-Iα was raised using the bovine PKG-Iα holoenzyme fused to GST (Biogenes, Berlin, Germany). Affinity-purified antibodies to bovine α1 GC were raised as described previously (Russwurm et al., 2001). [α-32P]GTP was from Amersham Biosciences (Piscataway, NJ), and purified PKG-Iα was purchased from Calbiochem (San Diego, CA). S-Nitrosoglutathione (GSNO) and YC-1 were from Alexis (Läufelfingen, Switzerland). All other reagents, including the protease inhibitor cocktail, were purchased from Sigma-Aldrich (St. Louis, MO).
Cloning of PDE5 and Generation of PDE5(S102A)
Human PDE5A1 was cloned by standard procedures from human placenta and verified by sequencing. Mutation of serine 102 to alanine was performed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's protocol. The mutation was confirmed by DNA sequencing. The cDNA coding for bovine PKG-Iα was a kind gift from Drs. Robert Feil and Franz Hofmann (Institut für Pharmakologie und Toxikologie Technische Universität München, München, Germany). All cDNAs were cloned into the vector pcDNA3 (Invitrogen, Carlsbad, CA).
Cell Culture of HEK Cells
HEK 293 cells were cultured in DMEM supplemented with 5% heat-denatured fetal calf serum and 1% penicillin/streptomycin at 37°C in a humidified 5% CO2 atmosphere.
Generation of Cell Lines
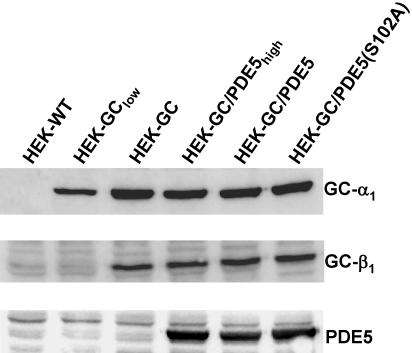
cDNA fragments of the bovine α1 and β1 subunits of NO-sensitive GC and the human PDE5 were cloned into the pcDNA3 vectors bearing the resistance against G418, hygromycin, and zeocin, respectively (Invitrogen). Consecutive transfection of these cDNAs into HEK 293 cells was performed using Fu-GENE 6 transfection reagent (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's standard protocol. Cells stably expressing the respective protein were selected using the above-mentioned antibiotics (200 μg/ml). Individual clones were screened by Western blot and determination of GC or PDE activity, respectively. The cellular GC content was unaffected by subsequent transfections as controlled by Western blot shown in Figure 1. The GC activity of clone HEK-GClow and HEK-GC were ∼34 and 300 pmol cGMP/s/mg, respectively, with 106 cells corresponding to 0.2 mg of protein (Bradford).
Figure 1.
Western blot detection of stably expressed proteins. The α1 and β1 subunits of NO-sensitive GC and human PDE5 were detected in HEK 293 cells after stable transfection of the respective cDNAs. Untransfected control cells (HEK) are shown in the first lane; lanes 2-6 contain the cell lines used in this study. The β1 subunit of HEK-GClow cells is below the detection limit but becomes visible upon longer exposure.
Determination of cGMP Levels
HEK cells were seeded on 24-well plates and cultured to confluence for 24-48 h. Before the experiment, medium was exchanged with HEPES buffer (154 mM NaCl, 5.6 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 3.6 mM NaHCO3, 10 mM HEPES, pH 7.4, 5.6 mM glucose) and equilibrated for 15 min at 37°C. Synthesis of cGMP was induced by the addition of 100 μM GSNO. YC-1 was added 15 min before NO stimulation. Cells were lysed at various time points after the NO stimulus by exchanging the HEPES buffer with ice-cold 100 mM HCl. Determination of cGMP in the cell lysate was carried out using a radioimmunoassay as described previously (Friebe et al., 1998).
Western Blot Detection
HEK cells were seeded on 24-well plates and cultured to confluence for 24-48 h. After the respective treatment, the medium was removed and the reactions were stopped by addition of Laemmli-buffer and boiling for 5 min. SDS-PAGE and immunoblotting were performed as described previously (Mullershausen et al., 2003).
HPLC Detection of GTP
After stimulation of HEK-GC cells (100 μM GSNO; 5 min), the reactions were stopped by the addition of ice-cold 0.8 M HClO4 and frozen at -80°C. After thawing, samples were centrifuged (15 min; 20,000 × g; 4°C), and supernatants were adjusted to pH 12 with KOH and frozen at -80°C. After a second centrifugation step, supernatants were diluted into running buffer A (see below), and pH was adjusted to that of the running buffer. Samples were loaded onto a Mono-Q HR5/5 column (Amersham Biosciences) and eluted with a linear gradient (buffer A: 20 mM K2HPO4, pH 8.0; buffer B: 1 M NaCl, 20 mM K2HPO4, pH 8.0; 0-20% B, 240 min; flow rate 0.5 ml/min). Elution of nucleotides was monitored at 254 nm; GTP was identified by co-chromatography of [α-32P]GTP.
PDE Assay
PDE activity was measured by the conversion of [32P]cGMP into [32P]GMP as described previously (Mullershausen et al., 2001). All assays were performed at a substrate concentration of 0.1 μM cGMP at 37°C for 5 min in a total volume of 100 μl; total substrate hydrolysis was <6% in all incubations.
Determination of PDE5 Activity in HEK Cells after NO Stimulation
Aliquots (450 μl) of a suspension of HEK-GC/PDE5 and HEK-GC/PDE5(S102A) in HEPES buffer (2-2.5 × 106 cells/ml) were equilibrated at 37°C for 10 min and stimulated with GSNO. At the indicated time points, the incubations were terminated by the addition of 500 μl of an ice-cold protease inhibitor mix (2 μM pepstatin A, 0.4 μM benzamidine, 0.5 mM phenylmethylsulfonyl fluoride, 4 mM dithiothreitol [DTT] and 1 mg/ml bovine serum albumin [BSA]) and brief sonication (1 pulse; 5 s) on ice using a Branson sonifier B-12. After centrifugation (15 min; 20,000 × g; 4°C), PDE activity was determined in the supernatant.
Activation of PDE5 in HEK Cell Supernatants
PDE5-transfected HEK cells (1 × 107) were harvested and lysed in 800 μl of homogenization buffer (50 mM NaCl, 1 mM EDTA, 50 mM triethanolamine/HCl, pH 7.4, 2 mM DTT, and a 100-fold dilution of protease inhibitor cocktail (Sigma-Aldrich) by sonication (1 pulse; 5 s). Lysates were centrifuged (30 min; 20,000 × g; 4°C), and cytosolic fractions were used for activation experiments. Before all experiments, cytosols were diluted with homogenization buffer to yield a PDE activity of 1 nmol GMP/min/ml and treated with 40 U/ml alkaline phosphatase (10 min; 37°C) to degrade any ATP present. Activation of PDE5 was induced by preincubation of the cytosols (5 min; 30°C) with the indicated concentration of cGMP in the presence of MgCl2 (10 mM); to induce phosphorylation, ATP (500 μM) and purified PKG (50,000 U/ml) were included. Preincubations were terminated by a 1000-fold dilution into ice-cold dilution buffer (0.5 mg/ml BSA, 3 mM DTT, 10 mM MgCl2, and 50 mM triethanolamine/HCl, pH 7.4); 10 μl of the diluted samples were used for determination of PDE5 activity. For determination of deactivation, preincubations were performed with 30 μM cGMP (with or without ATP and PKG) as described above. Samples were diluted 1000-fold into prewarmed (30°C) dilution buffer and further incubated at 30°C. At the indicated time points, aliquots were withdrawn and put on ice; 10 μl of the samples were used for determination of PDE5 activity. The portion of cGMP that was degraded during preincubation with 30 μM cGMP was determined as described previously (Mullershausen et al., 2003). For analysis of PDE5 phosphorylation, preincubations were terminated by the addition of Laemmli sample buffer and boiling of the samples (5 min).
Data Analysis
All data are expressed as mean ± SEM of at least three independent experiments assayed in duplicates. Western blots were taken from representative experiments.
RESULTS
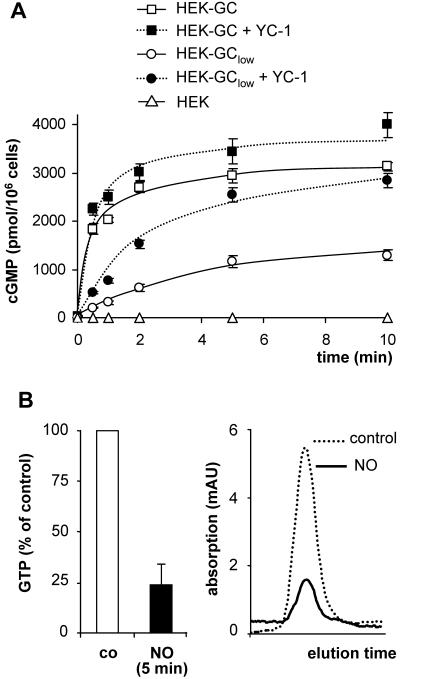
NO-sensitive GC, PDE5 and PKG are the key determinants of NO/cGMP signaling in many cells. For a better understanding of the contributions of the individual components, we sequentially reconstituted the NO/cGMP signaling pathway in intact cells. First, HEK 293 cells were stably transfected with the cDNAs of the α1 subunit and β1 subunit of NO-sensitive GC. Expression of both GC subunits was controlled by Western blot analysis; no NO-sensitive GC was detected in nontransfected control cells (Figure 1, lane 1). Two cell clones with different GC expression levels were selected for further investigation (Figure 1, lanes 2 and 3). Both clones were stimulated with GSNO (100 μM), and the resulting increases in cGMP are shown in Figure 2A. In nontransfected control cells, only a marginal increase in cGMP was detected after NO stimulation compared with the transfected cells. In the clone with higher GC expression (clone HEK-GC), cGMP levels rose to a plateau of ∼3000 pmol/106 cells within 2 min corresponding to an ∼200-fold cGMP increase. The development of a plateau in the transfected cells indicated a decreasing rate of cGMP formation that has been interpreted as desensitization of NO-sensitive GC (Bellamy et al., 2000). On the other hand, depletion of the substrate GTP has been shown to limit cGMP formation in platelets when cGMP degradation was inhibited (Mullershausen et al., 2001). Therefore, we determined the GTP content of HEK-GC cells before and after NO stimulation. As shown in Figure 2B, there was a drastic ∼80% reduction of GTP levels in HEK-GC cells after NO stimulation; this decrease in cellular GTP may limit cGMP formation.
Figure 2.
NO-induced cGMP accumulation in HEK cells stably expressing NO-sensitive GC. (A) cGMP response in untransfected control cells (HEK) and in cells with high (HEK-GC) and low (HEK-GClow) expression level of NO-sensitive GC after stimulation with GSNO (100 μM) in the absence or presence of YC-1 (100 μM). (B) Relative GTP content of HEK-GC cells 5 min after the incubation with or without GSNO (left). GTP was quantified by determination of area under the curve after chromatographic separation of nucleotides using a Mono-Q column. Right, original trace data of a representative elution profile.
The NO-induced cGMP response of the cell clone with lower GC expression (clone HEK-GClow) was characterized by slower cGMP accumulation, leading to a lower plateau level of ∼1500 pmol/106 cells (Figure 2A). Apparently, endogenous PDE activity was sufficient to counteract the low cGMP-forming activity in these cells. Accordingly, the nonspecific PDE inhibitor and GC sensitizer YC-1 raised the NO-induced cGMP plateau to the same level as in the cells with the high GC content (Figure 2A). This plateau seems to represent an ultimate level of cGMP, which is limited by the availability of the substrate GTP. Consistently, there was only a slight effect of YC-1 on NO-induced cGMP accumulation in the HEK-GC cells (high GC content).
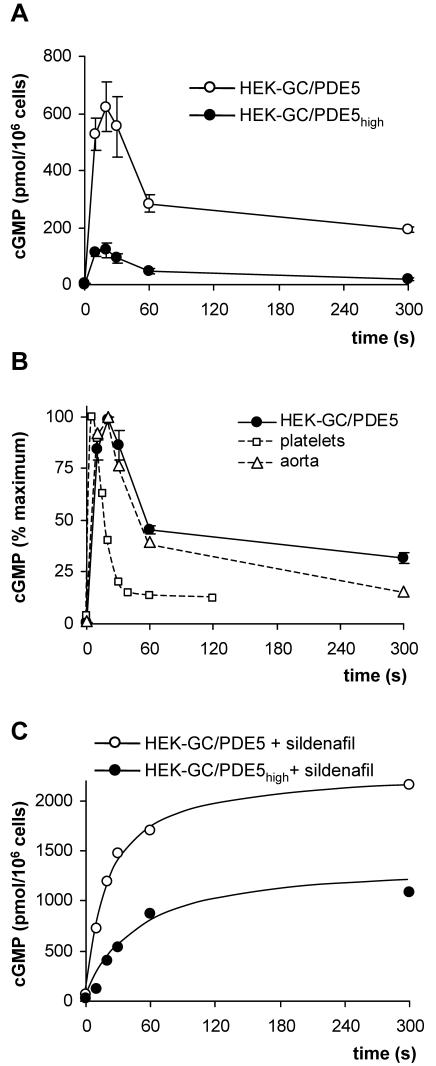
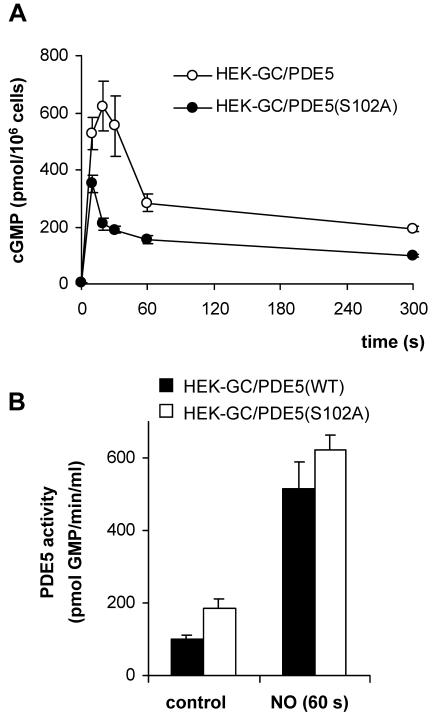
HEK-GC cells were then stably transfected with the cDNA coding for human PDE5. Again, two cell clones (clones HEK-GC/PDE5 and HEK-GC/PDE5high) were chosen for further investigation. Stimulation of these cells with NO resulted in a spike-like cGMP response, i.e., an initial increase and a subsequent decline of cGMP in ∼1 min (Figure 3A). This spike-like response is reminiscent of the one observed in platelets and very similar to aortic smooth muscle (Figure 3B). Thus, the spike-like shape of the NO-induced cGMP response can be reconstituted with only the two enzymes, NO-sensitive GC and PDE5. The peak concentration of cGMP in these cells was determined by the ratio of cGMP formation by NO-sensitive GC and PDE5 activity. In clone HEK-GC/PDE5high PDE5 activity was 1.5-fold higher than in cell clone HEK-GC/PDE5 as determined in cell lysates (120 and 80 pmol/s/mg protein, by using 1 μM cGMP substrate). To analyze GC activity, the NO-induced cGMP formation was determined in the presence of the PDE5 inhibitor sildenafil in intact cells (Figure 3C). Despite a comparable expression level of GC subunits in both clones (Figure 1), the initial rate of NO-induced cGMP formation in clone HEK-GC/PDE5high was reduced compared with clone HEK-GC/PDE5 (∼30 and ∼100 pmol/s/106 cells, respectively, corresponding to ∼150 and ∼500 pmol/s/mg protein). Accordingly, the ratio of GC activity and PDE5 activity in clone HEK-GC/PDE5high is ∼fivefold lower than in clone HEK-GC/PDE5 and is reflected by the reduced cGMP maximum. Qualitatively, however, the spike-like cGMP responses were similar in both clones. Clone HEK-GC/PDE5 was used in the following experiments. The ratio of GC versus PDE5 activity in this clone (GC, 500 pmol/s/mg protein; PDE5, 80 pmol/s/mg protein, using 1 μM cGMP substrate) was approximately twofold higher than in platelets (GC, 50 pmol/s/mg protein; PDE5, 17 pmol/s/mg protein, by using 1 μM cGMP substrate).
Figure 3.
NO-induced cGMP response in HEK cells stably expressing NO-sensitive GC and PDE5. (A) HEK-GC cells with low (HEK-GC/PDE5) or high (HEK-GC/PDE5high) expression level of PDE5 were stimulated with GSNO (100 μM), and cGMP levels were measured at the indicated time points. (B) For comparison, the NO-induced cGMP responses in human platelets (open squares) and rat aortic strips (open triangles) are shown in the same graph as the response in HEK-GC/PDE5; note that these data are expressed as percentage of maximum cGMP. (C) HEK-GC/PDE5 and HEK-GC/PDE5high cells were incubated with sildenafil (100 μM) for 10 min and then stimulated with GSNO (100 μM); cGMP levels were determined at the indicated time points.
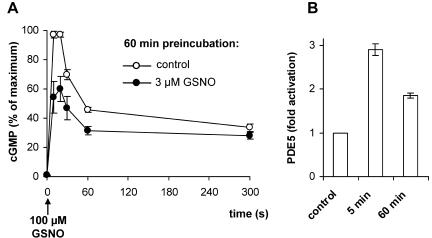
Recently, we have shown that NO induces a long-term desensitization of the cGMP response in platelets, which was caused by a sustained activation of PDE5. To assess the respective feature in the reconstituted signaling cascade, we performed the corresponding experiment in HEK-GC/PDE5 cells. After a short exposure (3 μM GSNO; 5 min), NO was removed by buffer exchange and cells were further incubated in the absence of NO for 60 min before a maximally activating NO stimulus was applied. Figure 4A shows the resulting cGMP response of the cells pretreated with or without NO, respectively. Even 60 min after short-term NO exposure, the NO-induced cGMP response was still reduced. By this time, cGMP had reached resting levels of ∼2 pmol/106 cells (not depicted), and analysis of cytosolic PDE5 activity revealed a 1.8-fold activation compared with a threefold activation observed directly after NO exposure (Figure 4B). Thus, as seen in platelets, NO induces a long-lasting desensitization of the cGMP response in HEK-GC/PDE5 cells that is caused by a sustained activation of PDE5.
Figure 4.
NO-induced desensitization of the cGMP response. (A) HEK-GC/PDE5 cells were incubated with either 0 μM (control) or 3 μM GSNO for 5 min, washed with buffer to remove NO, and further kept at 37°C. After 60 min, cells were restimulated with GSNO (100 μM), and cGMP levels were measured at the indicated time points; the resulting cGMP response was reduced compared with control. In each experiment, data were normalized to the maximal cGMP level measured under control conditions. (B) HEK-GC/PDE5 cells were incubated with or without 3 μM GSNO for 5 min, washed with buffer to remove NO, and further kept at 37°C for 60 min. PDE5 activity was determined in supernatant fractions before incubation (control), directly after incubation with 3 μM GSNO (5 min), and 60 min after removal of NO (60 min).
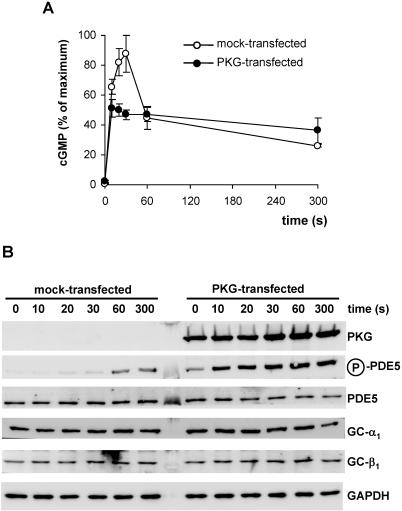
Phosphorylation of PDE5 at serine 102 has been reported and was shown to parallel activation by cGMP in intact cells (Mullershausen et al., 2001; Rybalkin et al., 2002; Shimizu-Albergine et al., 2003). Using a phospho-specific antibody, we assessed PDE5 phosphorylation during the observed cGMP response in HEK-GC/PDE5 cells. Concomitant to NO stimulation, weak phosphorylation of PDE5 was detected in HEK-GC/PDE5 cells (Figure 6B, second panel) suggesting the presence of an endogenous cyclic nucleotide-activated kinase. To be able to evaluate the role of PDE5 phosphorylation for the cGMP response in this cell model, we point-mutated PDE5(S102A) to prevent phosphorylation. PDE5(S102A) was then stably transfected into HEK-GC cells and the NO-induced cGMP response was analyzed. As shown in Figure 5A, these cells also showed a characteristic spike-like elevation of cGMP like the WT-PDE5-expressing cells. We conclude that phosphorylation of PDE5 is not required for the shaping of the cGMP response. In platelets, cGMP activation of PDE5 has been shown to be responsible for the rapid decline in cGMP levels. To confirm activation of the mutated PDE5(S102A), we determined the cytosolic activities of PDE5 and PDE5(S102A) after NO stimulation of intact cells. As shown in Figure 5B, NO stimulation of the respective cells caused activation of both WT-PDE5 and mutant PDE5(S102A). Even though cGMP levels had already been decreased at 60 s, activation of PDE5 persisted at this time. Remarkably, the basal activity in PDE5(S102A)-expressing cells was twofold higher and the NO-induced activation factor was lower than in clone HEK-GC/PDE5. We conclude that NO/cGMP-induced activation of PDE5, at least under the conditions tested, is independent of phosphorylation. To further investigate the impact of PDE5 phosphorylation, PKG was transiently transfected into GC/PDE5-expressing cells. Expression of PKG led to relevant phosphorylation of PDE5 already in the absence of NO; phosphorylation was further enhanced after addition of NO (Figure 6B). The NO-induced cGMP response in the PKG-expressing cells was changed dramatically as the cGMP spike was abolished. Apparently, phosphorylation facilitated PDE5 activation enabling PDE5 to outcompete NO-stimulated GC before higher cGMP levels were reached (Figure 6A). The fact that the same cGMP plateau developed in PKG-transfected and nontransfected cells indicates similar maximal PDE5 activities.
Figure 6.
NO-induced cGMP response in HEK-GC/PDE5 cells overexpressing PKG. (A) cGMP was determined after stimulation of the cells with GSNO (100 μM) at the indicated time points. In each experiment, data were normalized to the maximal cGMP value measured in the mock-transfected cells. The cGMP response was clearly blunted in cells expressing PKG. (B) Western blot detection of PKG and phosphorylated PDE5 after NO stimulation (100 μM GSNO) of mock-transfected (left) and PKG-transfected HEK-GC/PDE5 cells (right). Expression of PDE5, α1 and β1 subunits of NO-sensitive GC also is depicted; GAPDH is shown as control for equal loading of the lanes.
Figure 5.
(A) NO-induced cGMP response in HEK-GC cells stably expressing the phosphorylation site mutant PDE5(S102A). HEK-GC cells stably expressing PDE5 mutated at the phosphorylation site [HEK-GC/PDE5(S102A)] were stimulated with GSNO (100 μM), and cGMP was determined at the indicated time points. (B) NO/cGMP-mediated activation of wild-type (WT) or mutant PDE5. Intact HEK-GC cells expressing WT (filled columns) or mutant PDE5 (S102A) (open columns) were stimulated with GSNO (100 μM; 60 s) and lysed. PDE5 activity was determined in the supernatant fractions.
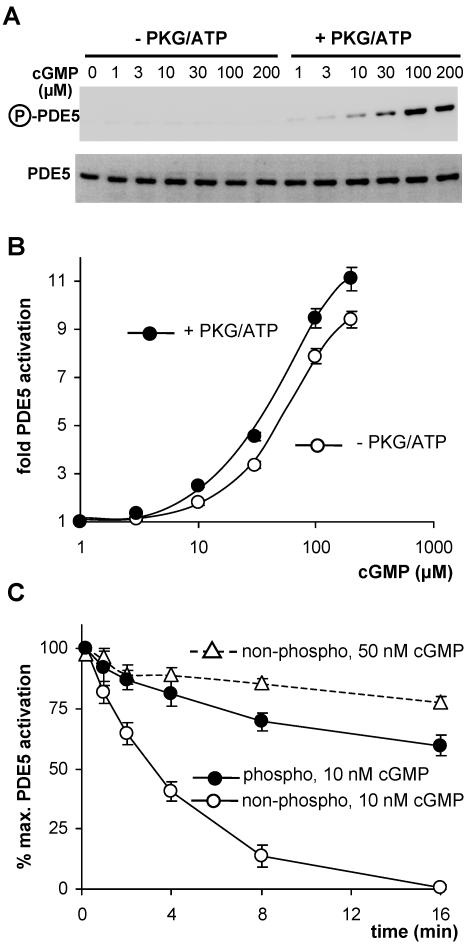
To strengthen the hypothesis that PDE5 phosphorylation assists activation, we studied the cGMP-induced activation of recombinant PDE5 in cytosolic preparations of HEK cells. Cytosols were incubated with increasing concentrations of cGMP under phosphorylating and nonphosphorylating conditions, i.e., in the presence or absence of purified PKG and ATP. The respective PDE5 phosphorylation was controlled in Western blots (Figure 7A). The concentration-response for cGMP activation of phosphorylated PDE5 was slightly but significantly shifted to the left, activation of phosphorylated PDE5 was stronger at every cGMP concentration tested (Figure 7B). These results are compatible with the concept of an enhanced affinity of phosphorylated PDE5 for cGMP. We performed deactivation experiments to confirm the effect of phosphorylation on the affinity for cGMP. PDE5 in cytosolic preparations was activated with 30 μM cGMP (5 min) under phosphorylating and nonphosphorylating conditions, i.e., in the presence or absence of purified PKG and ATP. After activation, cytosols were diluted 1000-fold to lower the cGMP concentration and initiate deactivation; the residual cGMP concentration was determined to be below 10 nM. The time course of deactivation was monitored by measuring PDE5 activity at the indicated time points. As can be seen in Figure 7C, phosphorylation of PDE5 had a profound impact on the rate of deactivation at 10 nM cGMP. Whereas activation of nonphospho-PDE5 was completely reversed after 16 min, phospho-PDE5 was still substantially activated (∼60%). Under these experimental conditions, the estimated half-lives for activated nonphospho- and phospho-PDE5 were 3 and 20 min, respectively. Clearly, phosphorylation decelerated deactivation of PDE5 that could be explained by a reduced cGMP dissociation rate. If cGMP affinity was increased by phosphorylation, a higher ambient cGMP concentration during deactivation should compensate for the effect of phosphorylation. Therefore, we measured deactivation of nonphosphorylated PDE5 at a higher ambient cGMP concentration (50 nM). Figure 7C (dashed line) shows that at 50 nM cGMP deactivation of nonphospho-PDE5 was markedly decelerated and resembled deactivation of phospho-PDE5 seen at 10 nM cGMP.
Figure 7.
Effect of phosphorylation on activation and deactivation of PDE5. (A) Western blot detection of phosphorylated PDE5 (top) after incubation of cytosols with increasing cGMP concentrations in the absence of presence of PKG/ATP; bottom shows loading of PDE5. (B) Activation of PDE5 by preincubation of cytosols with increasing cGMP concentrations. (C) Deactivation of PDE5. PDE5 was activated by preincubation with 30 μM cGMP in the presence or absence of PKG/ATP to obtain phospho- or a nonphospho-PDE5. Then samples were diluted to yield a cGMP concentration of 10 nM (solid lines) and further incubated. At the indicated time points, PDE5 activity was determined. The dashed line represents deactivation of nonphospho-PDE5 at a cGMP concentration of 50 nM.
DISCUSSION
In human platelets, NO elicits a transient spike-like cGMP response with a rapid increase in cGMP peaking within 3-5 s and a subsequent decrease to almost resting levels in <1 min. Aortic smooth muscle displays a somewhat different response with a delayed maximum at 20-30 s followed by a slower and less pronounced decline of intracellular cGMP (Mullershausen et al., 2001). Homologous NO-induced desensitization of the cGMP response is another feature of the signaling cascade in both cell types. Through analysis of cGMP formation and degradation, the activation of PDE5 was shown to be responsible for the termination of the cGMP response in platelets (Mullershausen et al., 2001). The activation of PDE5 was sustained (at least 60 min) and therefore sufficient to cause long-term desensitization (Mullershausen et al., 2003). Here, we reconstituted the cGMP response in HEK cells by coexpressing NO-sensitive GC, PDE5, and PKG to examine whether these participants are sufficient to define the special features of the signaling cascade observed in platelets and vascular smooth muscle.
HEK cells expressing both subunits of NO-sensitive GC showed a pronounced NO-induced increase in cGMP (∼200-fold). After a fast initial rise, cGMP levels reached a plateau, indicating that the rate of cGMP formation declined with time. Desensitization of NO-sensitive GC in the course of NO stimulation has been suggested and would explain the reduced rate of cGMP formation (Bellamy et al., 2000; Wykes et al., 2002; Gibb et al., 2003). However, NO-induced cGMP elevations in the HEK cells were calculated to reach concentrations up to 1 mM, which is in the range of the reported cellular GTP concentration. Therefore, we hypothesized depletion of the substrate GTP as the reason for the decreased rate of cGMP formation. And indeed, 80% of the GTP was shown to be consumed after NO stimulation of HEK cells expressing high levels of NO-sensitive GC. Similar results were obtained in NO-stimulated platelets when cGMP degradation was inhibited with sildenafil (Mullershausen et al., 2001). We assume that in cells with high GC and low PDE activity, cGMP formation is limited by the GTP content because PDE activity is required for the recycling process of cGMP to GTP.
The NO-induced cGMP response in HEK cells coexpressing NO-sensitive GC and PDE5 displayed the characteristic spike-like elevation of cGMP. In the first phase of the response, NO causes the immediate stimulation of NO-sensitive GC. The rising cGMP levels lead to substrate-linked and allosteric activation of PDE5 (Figure 5B) that eventually exceeds GC activity, causing cGMP levels to fall. The allosteric PDE5 activation is shown to be sustained and unaffected by the decline of intracellular cGMP. However, because catalytic rate also depends on the substrate concentration a decrease of intracellular cGMP is expected to lead to a reduced PDE5 activity. In the cGMP plateau the rate of cGMP formation and degradation is equal. The plateau observed in the recombinant HEK-GC/PDE5 cells is considerably higher than that in platelets where cGMP returns to almost basal levels (Figure 3B). Because platelets contain an approximately twofold higher relative amount of PDE5 versus GC activity, it seems that a higher relative degrading capacity is required to return cGMP to resting levels. Thus, the ratio of NO-sensitive GC and PDE5 activity not only determines the amplitude of the cGMP response but also the level of the cGMP plateau. The cGMP response in aortic smooth muscle was similar to that in HEK-GC/PDE5 cells (Figure 3B), emphasizing the physiological relevance of the cell model. Moreover, as in platelets, long-term desensitization of NO/cGMP signaling was observed in GC/PDE5-expressing HEK cells, which was paralleled by sustained allosteric activation of PDE5 (at least 60 min). Apparently, all features of the NO-induced cGMP response observed in platelets and smooth muscle can be reconstituted by coexpression of both enzymes.
Phosphorylation of PDE5 by PKG (in vivo) and PKA (in vitro) has been reported before and has been shown to parallel PDE5 activation in platelets and smooth muscle cells (Wyatt et al., 1998; Corbin et al., 2000; Mullershausen et al., 2001; Rybalkin et al., 2002). Although it is established that PDE5 can be directly activated by cGMP independently of phosphorylation, the functional role of the phosphorylation has not been completely elucidated yet. A minor PDE5 phosphorylation occurred in NO-stimulated GC/PDE5-containing cells without heterologous expression of PKG. To assess the impact of phosphorylation we used the phosphorylation site mutant PDE5(S102A) and were able to rule out a pronounced effect of phosphorylation on the spike-like shape of the cGMP response.
On the other hand, a strong increase in PDE5 phosphorylation by the coexpression of PKG in HEK cells did have an effect. Here, a considerable degree of PDE5 phosphorylation was already observed under resting conditions, i.e., in the absence of NO. On NO stimulation, the spike-like shape of the cGMP response was completely blunted. However, the level of the resulting cGMP plateau was similar in PKG-transfected and nontransfected HEK-GC/PDE5 cells. These data suggest that PDE5 phosphorylation promotes activation, allowing PDE5-catalyzed degradation to outcompete cGMP formation before peak cGMP levels are reached; the observed plateau results from the balance of NO-stimulated GC and maximally activated PDE5. The PDE5 catalytic rate in the plateau seems to be almost unaffected by phosphorylation.
Activation of PDE5 has been demonstrated to be induced by binding of cGMP to the GAF-A domain located in the N-terminal regulatory domain. With cGMP bound, PDE5 can be readily phosphorylated by PKG or PKA at Ser-102, which has been shown to increase cGMP-binding affinity of the holoenzyme and the isolated regulatory domain (Corbin et al., 2000; Francis et al., 2002) and enhance cGMP-induced activation in lysed platelets (Mullershausen et al., 2003). Moreover, the biphasic dissociation pattern of cGMP from the isolated regulatory domain was converted into a single high-affinity component by phosphorylation, greatly slowing dissociation (Francis et al., 2002).
In line with these data, phosphorylation of PDE5 caused a leftward shift of the concentration-response curve for cGMP activation. The effect was discrete, and it should be noted that in these experiments, PDE5 cannot be phosphorylated before cGMP activation because cGMP binding is a prerequisite for phosphorylation (Turko et al., 1998). Therefore, the effect of phosphorylation on cGMP affinity was studied by monitoring deactivation of phospho- versus nonphospho-PDE5, which was initiated by lowering the cGMP concentration to 10 nM. Phosphorylation had a crucial effect on the rate of deactivation because the phospho-PDE5 remained substantially activated, whereas the deactivation of nonphospho-PDE5 was completely reversed within 16 min. The data suggest that phosphorylation maintains PDE5 in the activated cGMP-bound conformation and that an increase in cGMP affinity accounts for the reduced rate of deactivation of phosphorylated PDE5. This notion is supported by the finding that at higher cGMP concentrations, the deactivation profile of nonphospho-PDE5 resembled that of the phosphorylated enzyme. Remarkably, even the nonphospho-PDE5 displayed a very high affinity for cGMP because a concentration of 50 nM was already sufficient to drastically decelerate deactivation. On the other hand, relatively high cGMP concentrations were required to induce PDE5 activation, indicating a low-affinity state of the GAF-A domain for cGMP before activation. Therefore, binding of cGMP seems to cause a conformational change in the regulatory domain that leads to a dramatic increase in cGMP affinity. In fact, structural data on the analogous cGMP-binding GAF-B domain of PDE2 revealed that cGMP is deeply buried in the protein explaining the high affinity with a low nanomolar kD value (Martinez et al., 2002).
In sum, our experimental data obtained using intact HEK cells expressing NO-sensitive GC and PDE5 suggest that the important characteristics of NO/cGMP signaling largely depend on the presence of these two enzymes. The cGMP formed by NO-stimulated GC binds and activates PDE5, causing a negative feedback similar to the mechanism found in platelets. Concomitant PKG-mediated phosphorylation of PDE5 further stabilizes the activated conformation of PDE5 and leads to a long-term desensitization of the cascade toward NO. Therefore, the NO-induced desensitization is inherent to the signaling cascade and very likely represents a feature of any GC/PDE5/PKG-expressing cell throughout the body. Within this process, phosphorylation of PDE5 seems to act as a memory switch for NO-induced bursts in cGMP and subsequently maintains the signaling cascade in a desensitized state.
Acknowledgments
We thank Erika Mannheim, Ursula Krabbel, Katja Rezny, and Gabriele Scheibel for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-12-0890. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-12-0890.
Abbreviations used: GC, guanylyl cyclase; GSNO, S-nitrosoglutathion; PDE, phosphodiesterase; PKG, cGMP-dependent protein kinase.
References
- Bellamy, T.C., Wood, J., Goodwin, D.A., and Garthwaite, J. (2000). Rapid desensitization of the nitric oxide receptor, soluble guanylyl cyclase, underlies diversity of cellular cGMP responses. Proc. Natl. Acad. Sci. USA 97, 2928-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin, J.D., Blount, M.A., Weeks, J.L., 2nd, Beasley, A., Kuhn, K.P., Ho, Y.S., Saidi, L.F., Hurley, J.H., Kotera, J., and Francis, S.H. (2003). [3H]sildenafil binding to phosphodiesterase-5 is specific, kinetically heterogeneous, and stimulated by cGMP. Mol. Pharmacol. 63, 1364-1372. [DOI] [PubMed] [Google Scholar]
- Corbin, J.D., Turko, I.V., Beasley, A., and Francis, S.H. (2000). Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur. J. Biochem. 267, 2760-2767. [DOI] [PubMed] [Google Scholar]
- Francis, S.H., Bessay, E.P., Kotera, J., Grimes, K.A., Liu, L., Thompson, W.J., and Corbin, J.D. (2002). Phosphorylation of isolated human phosphodiesterase-5 regulatory domain induces an apparent conformational change and increases cGMP binding affinity. J. Biol. Chem. 277, 47581-47587. [DOI] [PubMed] [Google Scholar]
- Francis, S.H., Turko, I.V., and Corbin, J.D. (2000). Cyclic nucleotide phosphodiesterases: relating structure and function. Prog. Nucleic. Acid. Res. Mol. Biol. 65, 1-52. [DOI] [PubMed] [Google Scholar]
- Friebe, A., and Koesling, D. (2003). Regulation of nitric oxide-sensitive guanylyl cyclase. Circ. Res. 93, 96-105. [DOI] [PubMed] [Google Scholar]
- Friebe, A., Mullershausen, F., Smolenski, A., Walter, U., Schultz, G., and Koesling, D. (1998). YC-1 potentiates nitric oxide- and carbon monoxide-induced cyclic GMP effects in human platelets. Mol. Pharmacol. 54, 962-967. [DOI] [PubMed] [Google Scholar]
- Gibb, B.J., Wykes, V., and Garthwaite, J. (2003). Properties of NO-activated guanylyl cyclases expressed in cells. Br. J. Pharmacol. 139, 1032-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juilfs, D.M., Soderling, S., Burns, F., and Beavo, J.A. (1999). Cyclic GMP as substrate and regulator of cyclic nucleotide phosphodiesterases (PDEs). Rev. Physiol. Biochem. Pharmacol. 135, 67-104. [DOI] [PubMed] [Google Scholar]
- Martinez, S.E., Wu, A.Y., Glavas, N.A., Tang, X.B., Turley, S., Hol, W.G., and Beavo, J.A. (2002). The two GAF domains in phosphodiesterase 2A have distinct roles in dimerization and in cGMP binding. Proc. Natl. Acad. Sci. USA 99, 13260-13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister-Lucas, L.M., et al. (1993). The structure of a bovine lung cGMP-binding, cGMP-specific phosphodiesterase deduced from a cDNA clone. J. Biol. Chem. 268, 22863-22873. [PubMed] [Google Scholar]
- Mergia, E., Zoidl, G., Russwurm, M., and Koesling, D. (2003). Major occurrence of the new alpha2beta1 isoform of NO-sensitive guanylyl cyclase in brain. Cell Signal 15, 189-195. [DOI] [PubMed] [Google Scholar]
- Mullershausen, F., Friebe, A., Feil, R., Thompson, W.J., Hofmann, F., and Koesling, D. (2003). Direct activation of PDE5 by cGMP: long-term effects within NO/cGMP signaling. J. Cell Biol. 160, 719-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullershausen, F., Russwurm, M. Thompson, W.J., Liu, L. Koesling, D., and Friebe, A. (2001). Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme. J. Cell Biol. 155, 271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, D., and Asakawa, S. (2002). Allosteric activation of cGMP-specific, cGMP-binding phosphodiesterase (PDE5) by cGMP. Biochemistry 41, 9672-9679. [DOI] [PubMed] [Google Scholar]
- Russwurm, M., Wittau, N., and Koesling, D. (2001). Guanylyl cyclase/PSD-95 interaction: targeting of the nitric oxide-sensitive alpha2beta1 guanylyl cyclase to synaptic membranes. J. Biol. Chem. 276, 44647-44652. [DOI] [PubMed] [Google Scholar]
- Rybalkin, S.D., Rybalkina, I.G., Feil, R., Hofmann, F., and Beavo, J.A. (2002). Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J. Biol. Chem. 277, 3310-3317. [DOI] [PubMed] [Google Scholar]
- Rybalkin, S.D., Rybalkina, I.G., Shimizu-Albergine, M., Tang, X.B., and Beavo, J.A. (2003). PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J. 22, 469-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Albergine, M., Rybalkin, S.D., Rybalkina, I.G., Feil, R., Wolfsgruber, W., Hofmann, F., and Beavo, J.A. (2003). Individual cerebellar Purkinje cells express different cGMP phosphodiesterases (PDEs): in vivo phosphorylation of cGMP-specific PDE (PDE5) as an indicator of cGMP-dependent protein kinase (PKG) activation. J. Neurosci. 23, 6452-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turko, I.V., Francis, S.H., and Corbin, J.D. (1998). Binding of cGMP to both allosteric sites of cGMP-binding cGMP-specific phosphodiesterase (PDE5) is required for its phosphorylation. Biochem. J. 329, 505-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt, T.A., Naftilan, A.J., Francis, S.H., and Corbin, J.D. (1998). ANF elicits phosphorylation of the cGMP phosphodiesterase in vascular smooth muscle cells. Am. J. Physiol. 274, 448-455. [DOI] [PubMed] [Google Scholar]
- Wykes, V., Bellamy, T.C., and Garthwaite, J. (2002). Kinetics of nitric oxide-cyclic GMP signalling in CNS cells and its possible regulation by cyclic GMP. J. Neurochem. 83, 37-47. [DOI] [PubMed] [Google Scholar]