Abstract
Background
Sepsis is one of the most common diseases that seriously threaten human health. Although a large number of markers related to sepsis have been reported in the last two decades, the diagnostic accuracy of these biomarkers remains unclear due to the lack of similar baselines among studies. Therefore, we conducted a large systematic review and meta-analysis to evaluate the diagnostic value of biomarkers from studies that included non-infectious systemic inflammatory response syndrome patients as a control group.
Methods
We searched Medline, Embase and the reference lists of identified studies beginning in April 2014. The last retrieval was updated in September 2016.
Results
Ultimately, 86 articles fulfilled the inclusion criteria. Sixty biomarkers and 10,438 subjects entered the final analysis. The areas under the receiver operating characteristic curves for the 7 most common biomarkers, including procalcitonin, C-reactive protein, interleukin 6, soluble triggering receptor expressed on myeloid cells-1, presepsin, lipopolysaccharide binding protein and CD64, were 0.85, 0.77, 0.79, 0.85, 0.88, 0.71 and 0.96, respectively. The remaining 53 biomarkers exhibited obvious variances in diagnostic value and methodological quality.
Conclusions
Although some biomarkers displayed moderate or above moderate diagnostic value for sepsis, the limitations of the methodological quality and sample size may weaken these findings. Currently, we still lack an ideal biomarker to aid in the diagnosis of sepsis. In the future, biomarkers with better diagnostic value as well as a combined diagnosis using multiple biomarkers are expected to solve the challenge of the diagnosis of sepsis.
Electronic supplementary material
The online version of this article (doi:10.1186/s40064-016-3591-5) contains supplementary material, which is available to authorized users.
Keywords: Biomarkers, Sepsis, Systemic inflammatory response syndrome, Diagnosis, Meta-analysis
Background
Epidemiological surveys indicate that sepsis is the leading cause of non-cardiac death in intensive care units and causes at least 30% of the deaths in patients who are septic (Levy et al. 2010). Along with the aging of the population, the incidence of sepsis shows an obvious increase in countries around the world (Wafaisade et al. 2011; Martin et al. 2003; Angus et al. 2001). An important aspect of improving survival rates in septic patients is early diagnosis, which is helpful to ensure timely treatment and to avoid deterioration of organ function. The classical method of diagnosis is based on signs of an inflammatory response and microbial cultures. However, doctors must wait for several days before getting culture results, and what is worse, negative culture results account for 30–40%. Because microbial cultures have the features of being time-consuming and having a low positive rate as well as being non-specific for systemic inflammatory response syndrome (SIRS), many patients may lose the opportunity of timely and effective treatment. Unlike microbial culture, biomarkers, primarily from the blood, increase in the early stage of the inflammatory response and show different expression between non-infectious inflammation and sepsis. Over the last 20 years, many researchers have been dedicated to finding blood biomarkers for the early diagnosis of infection or sepsis, and they have obtained a substantial number of research results. However, due to the large amounts of experimental data and the inconsistency of the baselines among these studies, it is difficult for medical researchers and workers to make comparisons across various biomarkers or to identify biomarkers with potential diagnostic value. Therefore, we performed a large-scale meta-analysis to summarize potential biomarkers for the differential diagnosis between non-infectious SIRS and sepsis.
Methods
Literature search
We conducted the first systematic retrieval from PubMed and Embase in April 2014. The basic retrieval scheme included the following three search keywords: ‘sepsis’, ‘systemic inflammatory response syndrome’ and ‘diagnosis’. Then, we excluded ‘review’, ‘erratum’, ‘editorial’ and ‘letter’ from the retrieval results. In addition, the reference lists of the included original studies and relevant meta-analysis articles were examined for any eligible documents that were missed. The last retrieval was updated in September 2016. The study protocol was approved by the ethics committee affiliated with Daping Hospital and did not require written informed consent from the patients.
Selection criteria
Articles were included if they evaluated the diagnostic accuracy of biomarkers for distinguishing patients with sepsis from those with non-infectious SIRS. Sepsis was defined as the coexistence of SIRS with infection, according to the diagnostic criteria proposed by the American College of Chest Physicians and the Society of Critical Care Medicine (Bone et al. 1992). We excluded articles that lacked non-infectious SIRS patients as a control group. We also eliminated studies with immunocompromised patients, hematologic patients or pediatric patients. Moreover, articles that could not provide sufficient data to build a 2 × 2 contingency table were likewise excluded.
Data collection and quality assessment
The data were extracted independently by two reviewers (YL and WX) using a pre-designed Microsoft Excel spreadsheet table that included the categories of methodological quality, methods of biomarker detection, features of the participants and results of diagnostic accuracy. If needed, the authors were contacted for any missing information. We evaluated the quality of the included studies according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS). Because the analysis of the test results of the biomarkers did not involve clinical data, we omitted item 12 of QUADAS in the quality assessment. Discrepancies between the two reviewers were resolved by discussion with the third author (SHW).
Data synthesis and statistical analysis
The scheme of the systematic review and meta-analysis was implemented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al. 2010). Stata 13.0 software was used to perform the statistical analysis of the pooled data. We used an exact binomial rendition of the bivariate mixed-effects regression model for the synthesis of diagnostic test data (Reitsma et al. 2005). I2 statistics were used to reflect the percentage of total variation across articles that were attributable to heterogeneity rather than chance. I2 values of 25, 50, and 75% describe the heterogeneity as low, moderate, and high, respectively (Higgins et al. 2003). If heterogeneity existed, and the number of studies was larger than 10, the potential reasons for heterogeneity were identified by meta-regression. Publication bias was evaluated by employing a scatter plot with the inverse of the square root of the effective sample size versus the log diagnostic odds ratio, with a symmetrical funnel shape indicating less publication bias (Deeks et al. 2005).
Results
We retrieved articles from the PubMed and EMBASE databases. A total of 31,874 articles remained after duplicates were removed. Three hundred and thirty-two articles were preserved after examining the titles and abstracts. We further excluded 267 articles after reviewing the full content. Sixty-five studies were included in the quantitative synthesis after the first retrieval. Finally, 86 studies were included after two updated searches in February 2015 and September 2016 (Fig. 1) (Abidi et al. 2008; Ahmadinejad et al. 2009; Al-Nawas et al. 1996; Anand et al. 2015; Balc et al. 2003; Barati et al. 2010; Battista et al. 2016; Bell et al. 2003; Beqja-Lika et al. 2013; Carpio et al. 2015; Castelli et al. 2004; Clec’h et al. 2006; de Pablo et al. 2013; Dorizzi et al. 2006; Du et al. 2003; Endo et al. 2012; Farag et al. 2013; Feng et al. 2012; Gaini et al. 2006; Garnacho-Montero et al. 2014; Gerrits et al. 2013; Giamarellos-Bourboulis et al. 2008; Gibot et al. 2004; Godnic et al. 2015; Guven et al. 2002; Han et al. 2016; Harbarth et al. 2001; Hoenigl et al. 2013; Hou et al. 2012, 2016; Hsu et al. 2011; Ishikura et al. 2014; Ivancevic et al. 2008; Jekarl et al. 2013, 2014; Jiang et al. 2015; Kim and Zhang 2012; Kofoed et al. 2007; Latour-Perez et al. 2010; Lewis et al. 2015; Li et al. 2013a; Lin et al. 2015; Matera et al. 2013; Mat-Nor et al. 2016; Mearelli et al. 2014; Meynaar et al. 2011; Miglietta et al. 2015; Miller et al. 1999; Muthiah et al. 2007, Naeini and Montazerolghaem 2006; Oshita et al. 2010; Papadimitriou-Olivgeris et al. 2015; Ratzinger et al. 2013; Reichsoellner et al. 2014; Righi et al. 2014; Rivera-Chavez and Minei 2009; Rogina et al. 2014; Romualdo et al. 2014; Ruiz-Alvarez et al. 2009; Sakr et al. 2008; Scherpereel et al. 2006; Schulte et al. 2011; Selberg et al. 2000; Seok et al. 2012; Shozushima et al. 2011; Sierra et al. 2004; Su et al. 2012, 2013; Sungurtekin et al. 2006; Suprin et al. 2000; Takahashi et al. 2014; Talebi-Taher et al. 2014; Tan et al. 2016; Tian et al. 2014; Tromp et al. 2012; Tsalik et al. 2012; Tsangaris et al. 2009; Tugrul et al. 2002; Ulla et al. 2013; Vaschetto et al. 2008; Vodnik et al. 2013; Wang et al. 2012, 2013; Wanner et al. 2000; Xiao et al. 2015; Yousef et al. 2010). The study by Clec’h et al. reported results separately for medical and surgical patients, and the study by Anand et al. reported results for positive and negative cultures. Furthermore, the study by Lin et al. was divided into a training group and validation group. The results of these three studies were divided into six parts (Anand et al. 2015; Clec’h et al. 2006; Lin et al. 2015).
Fig. 1.
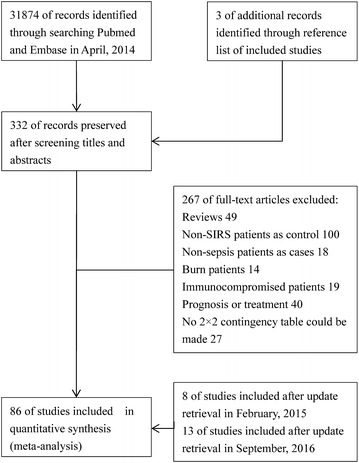
Flow diagram of the study selection
The main characteristics of the studies are shown in Additional file 1: S1. Altogether, 10,438 patients with non-infectious SIRS or sepsis (including 30,043 test instances) and 60 biomarkers were included in the analysis, of which 18,542 instances (61.72%) indicated sepsis, and 11,501 (38.28%) indicated a SIRS of non-infectious origin. The proportion of sepsis among the studies ranged between 16 and 93% (median 61%).
The methodological quality of the included studies was evaluated according to QUADAS. None of the studies fulfilled all of the items. The included studies fulfilled 766 (69%) of the total 1118 items. The quality was poor for item 10 (index test results blinded), item 11 (reference standard results blinded) and item 13 (uninterpretable results) (Additional file 2: S2). Three biomarkers with more than 10 references, including procalcitonin (PCT), C-reactive protein (CRP) and interleukin 6 (IL-6), were evaluated for publication bias by using Deeks’ regression test of asymmetry (Fig. 2). There was significant publication bias for PCT (P = 0.02) but not for CRP (P = 0.62) and IL-6 (P = 0.70).
Fig. 2.
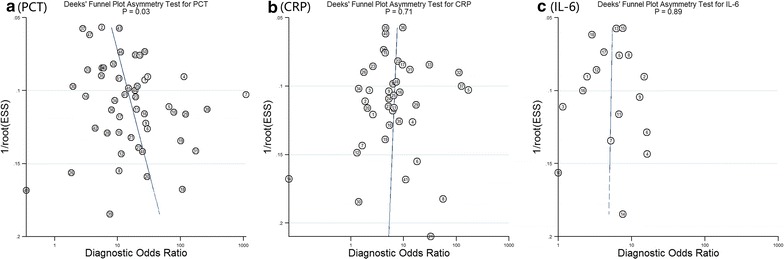
Funnel plots for detection of publication bias of PCT (a), CRP (b) and IL-6 (c)
Because of there being fewer than 4 references for each, the diagnostic accuracy data of 53 biomarkers could not be pooled by Stata 13.0 software. Thus, we pooled the sensitivity and specificity of 7 biomarkers, including PCT, CRP, IL-6, soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), presepsin (sCD14-ST), lipopolysaccharide binding protein (LBP) and CD64, with 7376, 5654, 3450, 831, 1510, 1136 and 558 participants, respectively, and with the area under the receiver operating characteristic curve (AUC) being 0.85, 0.77, 0.79, 0.85, 0.88, 0.71 and 0.96, respectively (Table 1). The forest plots for the biomarkers are shown in the Additional file 3: S3.
Table 1.
Research results of biomarkers with at least 4 references
| Test | Studies | Cut-off | n | TP | FP | FN | TN | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| PCT | 59 | 0.96 (0.5, 1.7) ng/mla | 7376 | 3173 | 847 | 1060 | 2296 | 0.85 [0.82, 0.88] | 0.79 [0.75, 0.83] | 0.78 [0.74, 0.81] |
| CRP | 45 | 84 (38, 140) mg/l | 5654 | 2356 | 719 | 1014 | 1565 | 0.77 [0.73, 0.81] | 0.75 [0.69, 0.79] | 0.67 [0.58, 0.74] |
| IL-6 | 22 | 138 (75, 220) pg/ml | 3450 | 1376 | 403 | 625 | 1046 | 0.79 [0.75, 0.82] | 0.72 [0.63, 0.80] | 0.73 [0.67, 0.79] |
| sTREM-1 | 8 | 123 (635, 594) pg/ml | 831 | 406 | 82 | 126 | 217 | 0.85 [0.82, 0.88] | 0.78 [0.66, 0.87] | 0.78 [0.65, 0.87] |
| Presepsin | 9 | 600 (415, 647) pg/ml | 1510 | 777 | 155 | 168 | 410 | 0.88 [0.85, 0.90] | 0.84 [0.79, 0.88] | 0.77 [0.68, 0.84] |
| LBP | 5 | 30 (24.35, 32) μg/ml | 1136 | 305 | 208 | 191 | 432 | 0.71 [0.67, 0.75] | 0.62 [0.53, 0.71] | 0.70 [0.59, 0.79] |
| CD64 | 4 | – | 558 | 300 | 13 | 76 | 169 | 0.96 [0.94, 0.97] | 0.87 [0.75, 0.94] | 0.93 [0.87, 0.96] |
TP true positive, FP false positive, FN false negative, TN true negative
aMedian (25% percentiles, 75% percentiles)
The biomarkers with less than 4 references are displayed in another table (Table 2). Several biomarkers presented high diagnostic values, with AUCs equal to or greater than 0.9 but fewer than 100 participants, including decoy receptor 3 (DcR3), endocan, soluble intercellular adhesion molecule-1 (sICAM-1) and complement 3a (C3a) (with AUCs of 0.96, 0.92, 0.9 and 0.9, respectively).
Table 2.
The research results for the biomarkers with less than 4 references
| Test | References | Cutoff value | N | TP | FP | FN | TN | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|---|---|
| Acute phase protein | ||||||||||
| AGP | Xiao et al. (2015) | 1462 mg/l | 277 | 150 | 8 | 42 | 77 | 0.869 | 0.782 | 0.902 |
| MBL | Ruiz-Alvarez et al. (2009) | – | 104 | 49 | 13 | 29 | 13 | 0.6 | 0.63 | 0.5 |
| SAA | Reichsoellner et al. (2014) | 289.4 μg/ml | 159 | 80 | 32 | 30 | 17 | 0.519 | 0.73 | 0.35 |
| sPLA2-IIA | Tan et al. (2016) | 2.13 μg/l | 51 | 38 | 2 | 4 | 7 | – | 0.91 | 0.78 |
| Biomarkers related to vaosdilation | ||||||||||
| Substance P | Reichsoellner et al. (2014) | 0.3 ng/ml | 159 | 62 | 23 | 48 | 26 | 0.524 | 0.56 | 0.53 |
| Cell marker biomarkers | ||||||||||
| CD64/CD16 | Hsu et al. (2011) | – | 66 | 47 | 2 | 8 | 9 | 0.883 | 0.855 | 0.818 |
| CD11C | Lewis et al. (2015) | 48.50% | 103 | 67 | 4 | 16 | 16 | 0.89 | 0.807 | 0.8 |
| sCD22 | Jiang et al. (2015) | 2.3 ng/ml | 64 | 31 | 6 | 7 | 20 | – | 0.8158 | 0.7692 |
| sCD163 | Feng et al. (2012) | 1.49 μg/ml | 132 | 75 | 2 | 27 | 28 | 0.856 | 0.74 | 0.9333 |
| sCD25 | Matera et al. (2013) | – | 53 | 25 | 6 | 4 | 18 | 0.812 | 0.875 | 0.75 |
| Coagulation biomarkers | ||||||||||
| protein C activity | Ishikura et al. (2014) | 47% | 82 | 33 | 7 | 10 | 32 | – | 0.775 | 0.811 |
| Thrombomodulin | Reichsoellner et al. (2014) | 0 ng/ml | 159 | 30 | 9 | 80 | 40 | 0.543 | 0.27 | 0.81 |
| Cytokine/chemokine biomarkers | ||||||||||
| IFN-r | Jekarl et al. (2014) | 45 pg/ml | 127 | 68 | 16 | 29 | 14 | 0.573 | 0.702 | 0.464 |
| IFN-r | Matera et al. (2013) | 9 pg/ml | 52 | 13 | 7 | 15 | 17 | 0.486 | 0.4545 | 0.7 |
| IL-1 | Jekarl et al. (2014) | 30 pg/ml | 128 | 38 | 8 | 59 | 23 | 0.554 | 0.394 | 0.75 |
| IL-10 | Jekarl et al. (2014) | 40 pg/ml | 127 | 32 | 1 | 65 | 29 | 0.661 | 0.329 | 0.964 |
| IL-10 | Matera et al. (2013) | 3.05 pg/ml | 52 | 22 | 5 | 6 | 19 | 0.767 | 0.7826 | 0.8 |
| IL-10 | Reichsoellner et al. (2014) | 1.9 ng/ml | 159 | 25 | 16 | 85 | 33 | 0.508 | 0.23 | 0.67 |
| IL-12 | Jekarl et al. (2014) | 2 pg/ml | 127 | 18 | 9 | 79 | 21 | 0.504 | 0.181 | 0.714 |
| IL-13 | Jekarl et al. (2014) | 40 pg/ml | 128 | 85 | 23 | 12 | 8 | 0.508 | 0.872 | 0.25 |
| IL-17 | Jekarl et al. (2014) | 1.5 pg/ml | 127 | 41 | 9 | 56 | 21 | 0.586 | 0.426 | 0.714 |
| IL-2 | Jekarl et al. (2014) | 35 pg/ml | 127 | 87 | 24 | 10 | 6 | 0.534 | 0.894 | 0.214 |
| IL-2 | Balc et al. (2003) | 1288.5 pg/ml | 83 | 22 | 22 | 13 | 26 | 0.641 | 0.63 | 0.55 |
| IL22 | Jekarl et al. (2014) | 300 pg/ml | 127 | 75 | 18 | 22 | 12 | 0.542 | 0.776 | 0.393 |
| IL-4 | Jekarl et al. (2014) | 25 pg/ml | 127 | 85 | 24 | 12 | 6 | 0.516 | 0.872 | 0.214 |
| IL-5 | Jekarl et al. (2014) | 5 pg/ml | 127 | 69 | 9 | 28 | 21 | 0.714 | 0.713 | 0.714 |
| IL-8 | Balc et al. (2003) | 31.5 pg/ml | 83 | 24 | 21 | 11 | 27 | 0.663 | 0.68 | 0.57 |
| IL-8 | Harbarth et al. (2001) | 30 ng/ml | 78 | 38 | 4 | 22 | 14 | 0.71 | 0.63 | 0.78 |
| IL-8 | Reichsoellner et al. (2014) | 507.2 pg/ml | 160 | 50 | 11 | 61 | 38 | 0.625 | 0.45 | 0.77 |
| IL-9 | Jekarl et al. (2014) | 5 pg/ml | 128 | 83 | 23 | 14 | 8 | 0.532 | 0.851 | 0.25 |
| MIF | Kofoed et al. (2007) | 0.81 ng/ml | 151 | 77 | 29 | 19 | 26 | 0.63 | 0.8 | 0.47 |
| Osteopontin | Vaschetto et al. (2008) | 1.7 ng/ml | 56 | 19 | 6 | 8 | 23 | 0.796 | 0.7 | 0.79 |
| TNF-α | Balc et al. (2003) | 11.5 pg/ml | 83 | 19 | 16 | 16 | 32 | 0.607 | 0.55 | 0.66 |
| TNF-α | Jekarl et al. (2014) | 15 pg/ml | 128 | 47 | 8 | 50 | 23 | 0.598 | 0.489 | 0.75 |
| TNF-α | Li et al. (2013a, b) | 9.75 pg/ml | 52 | 26 | 4 | 12 | 10 | 0.796 | 0.68 | 0.71 |
| Receptor biomarkers | ||||||||||
| DcR3 | Hou et al. (2012) | 2.85 ng/ml | 67 | 23 | 14 | 1 | 29 | 0.896 | 0.958 | 0.674 |
| DcR3 | Kim et al. (2012) | 3.24 ng/ml | 48 | 24 | 4 | 1 | 19 | 0.958 | 0.96 | 0.826 |
| PLA2-II | Mearelli et al. (2014) | 6 ng/ml | 80 | 58 | 8 | 2 | 12 | 0.851 | 0.97 | 0.6 |
| suPAR | Hoenigl et al. (2013) | 7.9 ng/ml | 132 | 34 | 18 | 21 | 59 | 0.726 | 0.62 | 0.77 |
| suPAR | Kofoed et al. (2007) | 2.7 ng/ml | 151 | 34 | 18 | 62 | 37 | 0.5 | 0.35 | 0.67 |
| suPAR | Reichsoellner et al. (2014) | 7.6 ng/ml | 160 | 61 | 7 | 50 | 42 | 0.66 | 0.55 | 0.86 |
| Vascular endothelial biomarkers | ||||||||||
| Endocan | Scherpereel et al. (2006) | 1.2 ng/ml | 70 | 52 | 0 | 11 | 7 | 0.923 | 0.825 | 1 |
| sICAM-1 | de Pablo et al. (2013) | 904 ng/ml | 92 | 39 | 2 | 13 | 38 | 0.9 | 0.743 | 0.941 |
| Other biomarkers | ||||||||||
| Ang 2 | Mearelli et al. (2014) | 3.2 ng/ml | 80 | 49 | 12 | 11 | 8 | 0.581 | 0.82 | 0.4 |
| Biotin | Reichsoellner et al. (2014) | 70.4 pg/ml | 159 | 55 | 9 | 55 | 40 | 0.646 | 0.5 | 0.81 |
| C2 | Ruiz-Alvarez et al. (2009) | – | 104 | 6 | 3 | 72 | 23 | 0.5 | 0.08 | 0.9 |
| C3 | Sungurtekin et al. (2006) | 54 mg/dL | 99 | 25 | 22 | 16 | 36 | 0.566 | 0.61 | 0.625 |
| C3a | Selberg et al. (2000) | 540 ng/ml | 33 | 19 | 2 | 3 | 9 | 0.9 | 0.86 | 0.8 |
| C4 | Sungurtekin et al. (2006) | 28 mg/dL | 99 | 32 | 36 | 9 | 22 | 0.544 | 0.78 | 0.382 |
| cf-DNA | Garnacho-Montero et al. (2014) | 2850GE/ml | 81 | 41 | 20 | 11 | 9 | 0.51 | 0.7931 | 0.3023 |
| cf-DNA | Hou et al. (2016) | 493 pg/ml | 67 | 23 | 13 | 1 | 30 | 0.856 | 0.9412 | 0.7059 |
| Copeptin | Battista et al. (2016) | 23.2 pmol/l | 90 | 47 | 3 | 17 | 23 | – | 0.74 | 0.87 |
| Cystatin C | Reichsoellner et al. (2014) | 2.1 μg/ml | 159 | 55 | 14 | 55 | 35 | 0.578 | 0.5 | 0.71 |
| Delta neutrophil index | Seok et al. (2012) | 0.03% | 174 | 93 | 1 | 34 | 46 | 0.88 | – | – |
| Elastase | Selberg et al. (2000) | 91 μg/ml | 33 | 19 | 10 | 3 | 1 | 0.57 | 0.86 | 0.09 |
| eosinophil | Abidi et al. (2008) | – | 140 | 96 | 4 | 24 | 16 | 0.84 | 0.8 | 0.8 |
| Fibronectin | Reichsoellner et al. (2014) | 377.4 μg/ml | 159 | 59 | 15 | 51 | 34 | 0.384 | 0.54 | 0.69 |
| Interferon-induced protein 10 | Mearelli et al. (2014) | 19.5 ng/ml | 80 | 16 | 0 | 44 | 20 | 0.666 | 0.27 | 1 |
| leptin | Farag et al. (2013) | 38.05 ng/ml | 30 | 14 | 0 | 1 | 15 | – | ||
| Leptin | Yousef et al. (2010) | 38 ng/ml | 74 | 36 | 5 | 4 | 29 | – | 0.912 | 0.85 |
| miR-143 | Han et al. (2016) | 15.9 ng/ml | 198 | 81 | 8 | 22 | 87 | – | 0.786 | 0.916 |
| miR-146a | Wang et al. (2013) | – | 18 | 6 | 1 | 4 | 7 | 0.813 | 0.6 | 0.875 |
| miR-15a | Wang et al. (2012) | – | 198 | 113 | 2 | 53 | 30 | 0.858 | 0.683 | 0.944 |
| NGAL | Reichsoellner et al. (2014) | 82 ng/ml | 159 | 29 | 2 | 81 | 47 | 0.599 | 0.26 | 0.96 |
| Peroxiredoxin4 | Schulte et al. (2011) | 4.5 U/l | 79 | 32 | 7 | 11 | 29 | 0.824 | – | – |
| Thrombocytes | Sungurtekin et al. (2006) | – | 99 | 27 | 17 | 14 | 41 | 0.656 | 0.659 | 0.707 |
Except for CD64, the remaining pooled data of 6 biomarkers showed significant heterogeneity. We conducted a meta-regression analysis for 3 biomarkers (PCT, CRP and IL-6) for which the number of studies was larger than 10. Six factors were analyzed as potential sources of heterogeneity, including sample size, publication year, patient age, patient sex, proportion of patients with sepsis and methodological quality. Although the results of the meta-regression analysis showed that the race that was divided into Caucasian and Asian may be the heterogeneity source for PCT and CRP, the heterogeneity did not disappear in subgroup analysis by race. Therefore, there was no one factor that could satisfactorily explain the heterogeneity source of the three biomarkers.
Discussion
A total of 60 types of markers were included in our research. Most of the biomarkers had a small number of references. Six biomarkers with the largest number of participants or studies presented a moderate degree of diagnostic value, including PCT, CRP, IL-6, presepsin, LBP and sTREM-1, with AUC values of 0.85, 0.77, 0.79, 0.88, 0.71 and 0.85, respectively. Presepsin and sTREM-1, two popular research biomarkers over the last several years, presented diagnostic values similar to PCT. Several biomarkers with AUCs greater than or equal to 0.9 may be potential biomarkers for sepsis, including CD64, DcR3, endocan, sICAM-1 and C3a. However, the biomarkers with the highest AUCs were described in studies with limited sample sizes and inadequate methodological quality.
Although the reference standard for SIRS and sepsis of the included studies was in accordance with the American College of Chest Physicians and the Society of Critical Care Medicine Consensus Conference, most studies did not provide details that described how the patients were diagnosed with SIRS or sepsis. In some studies, only patients with positive cultures were diagnosed with sepsis, while in other studies, all patients with positive cultures or clinically suspected infections were diagnosed with sepsis. We believe that the cohort being investigated should include different types of patients, such as those with positive cultures and those with clinically confirmed infections. Only in this way can the results of the studies be more representative and have more clinical application value. In addition, we believe studies should exclude the patients whose infection status cannot be confirmed, as these patients may lead to selective bias.
We evaluated the publication bias for three biomarkers, PCT, CRP and IL-6. Among them, the funnel plot of PCT presented publication bias. The PCT funnel plot showed a negative correlation between diagnostic value and sample size. In other words, large sample sizes tended to have a relatively small diagnostic value. Although our meta-analysis only searched two databases, PubMed and Embase, our included references and the results of merged data for PCT were similar to the study by Wacker et al. (2013) who searched 7 databases (pooled sensitivity: 0.79 vs. 0.77; pooled specificity: 0.78 vs. 0.79). Therefore, we believe that one of the major reasons for publication bias in our meta-analysis was more likely the publication of studies with positive or expected results rather than negative results.
Except for CD64, the remaining six biomarkers presented significant heterogeneity. Because the cutoff value for the same biomarker often varied among different studies, the diverse cutoff values often led to the threshold effect as a source of heterogeneity. We used meta-regression analysis to explore the sources of heterogeneity, but no single factor could satisfactorily explain the origins of the heterogeneity, including sample size, publication year, patient age, patient sex, the proportion of patients with sepsis and the methodological quality. Although the heterogeneity among studies was significant, we had stable results for sensitivity analysis. Moreover, the pooled diagnostic test results are consistent with the other meta-analysis results (Wacker et al. 2013; Wu et al. 2012; Li et al. 2013a, b).
Research quality could be an important factor that affected the results. For example, because of limited sample sizes and narrow disease spectra, some studies could not represent the overall state of the patients. In addition, most of the studies did not use blinded methods, which may have resulted in the judgment of sepsis to be affected by the biomarker determination results. These deficiencies may affect the authenticity of results and also lead to heterogeneity among the studies.
In the review by Pierrakos and Vincent (2010), the researchers retrieved a large number of biomarkers related to sepsis and made a detailed classification of them. However, they did not collect all articles in accordance with the inclusion criteria, nor did they quantitatively evaluate the diagnostic value of biomarkers. Two systematic reviews by Wacker et al. (2013) and Wu et al. (2012) evaluated the diagnostic accuracy of two popular biomarkers to differentiate sepsis from SIRS—PCT and sTREM-1, respectively—but they did not include any other biomarkers. In addition, two other systematic reviews by Simon et al. (2004) and Li et al. (2013b) assessed whether biomarkers could diagnose bacterial infection rather than sepsis. Our review included almost all diagnostic trials for the differential diagnosis of septic patients from those with a SIRS of non-infectious origin published before September 2016. Inconsistent control groups from different studies that may result in heterogeneity were ruled out in our analysis, such as those including healthy individuals, infected patients without SIRS, febrile patients without SIRS and immunocompromised patients. We believed the inconsistency of baselines among the control groups would lead to incorrect assessments of the diagnostic value of biomarkers.
The biomarker CD64, a cell surface marker, showed a high value for the differential diagnosis of sepsis and SIRS. However, this test requires flow cytometry and trained technical personnel, which limits its feasibility in clinical applications. Obviously, its high cost means that the promotion of one biomarker will be limited in developing or poor countries. Therefore, we believe that a desirable biomarker for diagnosing sepsis should have the following features: high sensitivity and specificity, elevation in the early phase of the infection, low cost and rapid results.
Sepsis is defined as life-threatening organ dysfunction caused by a serious infection, according to a new international expert consensus (Singer et al. 2016). Therefore, SIRS is not a part of the diagnostic criteria of sepsis according to the new guideline. However, previous studies on sepsis markers still have a certain reference value, such as for PCT, which is still widely used in the clinical setting. Summarizing previous research can provide information for new research and guide the development of new studies.
For the objective assessment of the diagnostic value of septic biomarkers, future trials should compare new putative markers with classical biomarkers such as PCT and CRP in the same trial and follow the Standards for Reporting of Diagnostic Accuracy (Bossuyt et al. 2003). Moreover, medical journals should consider accepting more studies with negative or unintended results to avoid publication bias.
The present meta-analysis shows that plasma PCT, sTREM-1 and presepsin have moderate diagnostic utility in differentiating sepsis from SIRS. Several biomarkers with high AUC values, including CD64, DcR3, endocan, sICAM-1 and C3a, need more studies with larger sample sizes and rigorous methodological designs to confirm the results. Not surprisingly, because sepsis is a non-specific clinical syndrome related to serious microorganism infection and uncontrolled immune responses, it is less likely that one biomarker could satisfactorily differentiate sepsis from SIRS patients. In clinical practice, in addition to the dynamic changes of one septic biomarker, doctors should incorporate biomarkers with medical history, clinical symptoms, physical signs and other tests related to infection when diagnosing sepsis. In the future, biomarkers with better diagnostic value and combined diagnosis with multiple biomarkers are expected to solve the challenge of the diagnosis of sepsis.
Authors’ contributions
QL and JH conceived and designed this study. JW, S-NW and YL were responsible for searching the articles in database. YL and KC were jointly involving in extracting data and writing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank all the people and patients who participated in this study.
Competing interests
The authors declare that they have no competing interests.
Funding
Funding was provided by Youth Fund of National Natural Science Foundation of China (Grant No. 81401412).
Additional files
Additional file 1. Characteristics of the included studies.
Additional file 2. The quality assessment of the included studies by QUADAS (Diagnostic Accuracy included in Systematic Reviews).
Additional file 3. Forest plots of biomarkers for the diagnosis of sepsis.
Footnotes
Yong Liu and Jun-huan Hou contributed equally to this work
Contributor Information
Yong Liu, Email: 616756156@qq.com.
Jun-huan Hou, Email: 470820531@qq.com.
Qing Li, Email: likunze88824@163.com.
Kui-jun Chen, Email: chen_kuijun@163.com.
Shu-Nan Wang, Email: wangshunan2001@hotmail.com.
Jian-min Wang, Phone: +86 023 68757461, Email: jmwang@tmmu.edu.cn.
References
- Abidi K, Khoudri I, Belayachi J, Madani N, Zekraoui A, Zeggwagh A, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care. 2008;12:1. doi: 10.1186/cc6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadinejad Z, Dadsetan B, Jalili M, Soudbakhsh A, Rasolinejad M. Evaluation of serum procalcitonin in patients with systemic inflammatory response syndrome with and without infection. Acta Med Iran. 2009;47:383–388. [Google Scholar]
- Al-Nawas B, Krammer I, Shah PM. Procalcitonin in diagnosis of severe infections. Eur J Med Res. 1996;1:331–333. [PubMed] [Google Scholar]
- Anand D, Das S, Bhargava S, Srivastava LM, Garg A, Tyagi N, et al. Procalcitonin as a rapid diagnostic biomarker to differentiate between culture-negative bacterial sepsis and systemic inflammatory response syndrome: a prospective, observational, cohort study. J Crit Care. 2015;30:218.e7–218.e12. doi: 10.1016/j.jcrc.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Balc IC, Sungurtekin H, Gurses E, Sungurtekin U, Kaptanoglu B. Usefulness of procalcitonin for diagnosis of sepsis in the intensive care unit. Crit Care. 2003;7:85–90. doi: 10.1186/cc1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barati M, Bashar FR, Shahrami R, Zadeh MH, Taher MT, Nojomi M. Soluble triggering receptor expressed on myeloid cells 1 and the diagnosis of sepsis. J Crit Care. 2010;25:362.e1–362.e6. doi: 10.1016/j.jcrc.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Battista S, Audisio U, Galluzzo C, Maggiorotto M, Masoero M, Forno D, et al. Assessment of diagnostic and prognostic role of copeptin in the clinical setting of sepsis. Biomed Res Int. 2016;2016:3624730. doi: 10.1155/2016/3624730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell K, Wattie M, Byth K, Silvestrini R, Clark R, Stachowski E, et al. Procalcitonin: a marker of bacteraemia is SIRS. Anaesth Intensive Care. 2003;31:629–636. doi: 10.1177/0310057X0303100603. [DOI] [PubMed] [Google Scholar]
- Beqja-Lika A, Bulo-Kasneci A, Refatllari E, Heta-Alliu N, Rucaj-Barbullushi A, Mone I, et al. Serum procalcitonine levels as an early diagnostic indicator of sepsis. Mater Sociomed. 2013;25:23–25. doi: 10.5455/msm.2013.25.23-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpio R, Zapata J, Spanuth E, Hess G. Utility of presepsin (sCD14-ST) as a diagnostic and prognostic marker of sepsis in the emergency department. Clin Chim Acta. 2015;450:169–175. doi: 10.1016/j.cca.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Castelli GP, Pognani C, Meisner M, Stuani A, Bellomi D, Sgarbi L. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit Care. 2004;8:R234–R242. doi: 10.1186/cc2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clec’h C, Fosse JP, Karoubi P, Vincent F, Chouahi I, Hamza L, et al. Differential diagnostic value of procalcitonin in surgical and medical patients with septic shock. Crit Care Med. 2006;34:102–107. doi: 10.1097/01.CCM.0000195012.54682.F3. [DOI] [PubMed] [Google Scholar]
- de Pablo R, Monserrat J, Reyes E, Diaz D, Rodriguez-Zapata M, de la Hera A, et al. Circulating sICAM-1 and sE-Selectin as biomarker of infection and prognosis in patients with systemic inflammatory response syndrome. Eur J Intern Med. 2013;24:132–138. doi: 10.1016/j.ejim.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Dorizzi RM, Polati E, Sette P, Ferrari A, Rizzotti P, Luzzani A. Procalcitonin in the diagnosis of inflammation in intensive care units. Clin Biochem. 2006;39:1138–1143. doi: 10.1016/j.clinbiochem.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Du B, Pan J, Cheng D, Li Y. Serum procalcitonin and interleukin-6 levels may help to differentiate systemic inflammatory response of infectious and non-infectious origin. Chin Med J (Engl) 2003;116:538–542. [PubMed] [Google Scholar]
- Endo S, Suzuki Y, Takahashi G, Shozushima T, Ishikura H, Murai A, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother. 2012;18:891–897. doi: 10.1007/s10156-012-0435-2. [DOI] [PubMed] [Google Scholar]
- Farag NA, Taema KM, Abdel-Latiff E, Hamed G. Differentiating sepsis from non-infective systemic inflammatory response syndrome: comparison between C-reactive protein and Leptin. Egypt J Crit Care Med. 2013;1:111–118. doi: 10.1016/j.ejccm.2013.11.003. [DOI] [Google Scholar]
- Feng L, Zhou X, Su LX, Feng D, Jia YH, Xie LX. Clinical significance of soluble hemoglobin scavenger receptor CD163 (sCD163) in sepsis, a prospective study. PLoS ONE. 2012;7:e38400. doi: 10.1371/journal.pone.0038400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaini S, Koldkjaer OG, Pedersen C, Pedersen SS. Procalcitonin, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in community-acquired infections and sepsis: a prospective study. Crit Care. 2006;10:R53. doi: 10.1186/cc4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnacho-Montero J, Huici-Moreno MJ, Gutierrez-Pizarraya A, Lopez I, Marquez-Vacaro JA, Macher H, et al. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin, and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Crit Care. 2014;18:R116. doi: 10.1186/cc13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits JH, McLaughlin PMJ, Nienhuis BN, Smit JW, Loef B. Polymorphic mononuclear neutrophils CD64 index for diagnosis of sepsis in postoperative surgical patients and critically ill patients. Clin Chem Lab Med. 2013;51:897–905. doi: 10.1515/cclm-2012-0279. [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis EJ, Mouktaroudi M, Tsaganos T, Koutoukas P, Spyridaki E, Pelekanou A, et al. Evidence for the participation of soluble triggering receptor expressed on myeloid cells-1 in the systemic inflammatory response syndrome after multiple trauma. J Trauma. 2008;65:1385–1390. doi: 10.1097/TA.0b013e31814699cc. [DOI] [PubMed] [Google Scholar]
- Gibot S, Kolopp-Sarda MN, Bene MC, Cravoisy A, Levy B, Faure GC, et al. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med. 2004;141:9–15. doi: 10.7326/0003-4819-141-1-200407060-00009. [DOI] [PubMed] [Google Scholar]
- Godnic M, Stubljar D, Skvarc M, Jukic T. Diagnostic and prognostic value of sCD14-ST–presepsin for patients admitted to hospital intensive care unit (ICU) Wien Klin Wochenschr. 2015;127(13):521–527. doi: 10.1007/s00508-015-0719-5. [DOI] [PubMed] [Google Scholar]
- Guven H, Altintop L, Baydin A, Esen S, Aygun D, Hokelek M, et al. Diagnostic value of procalcitonin levels as an early indicator of sepsis. Am J Emerg Med. 2002;20:202–206. doi: 10.1053/ajem.2002.33005. [DOI] [PubMed] [Google Scholar]
- Han Y, Dai QC, Shen HL, Zhang XW, et al. Diagnostic value of elevated serum miRNA-143 levels in sepsis. J Int Med Res. 2016;44(4):875–881. doi: 10.1177/0300060516645003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164:396–402. doi: 10.1164/ajrccm.164.3.2009052. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenigl M, Raggam RB, Wagner J, Valentin T, Leitner E, Seeber K, et al. Diagnostic accuracy of soluble urokinase plasminogen activator receptor (suPAR) for prediction of bacteremia in patients with systemic inflammatory response syndrome. Clin Biochem. 2013;46:225–229. doi: 10.1016/j.clinbiochem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Hou YQ, Xu P, Zhang M, Han D, Peng L, Liang DY, et al. Serum decoy receptor 3, a potential new biomarker for sepsis. Clin Chim Acta. 2012;413:744–748. doi: 10.1016/j.cca.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Hou YQ, Liang DY, Lou XL, Zhang M, Zhang ZH, Zhang LR. Branched DNA-based Alu quantitative assay for cell-free plasma DNA levels in patients with sepsis or systemic inflammatory response syndrome. J Crit Care. 2016;31(1):90–95. doi: 10.1016/j.jcrc.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Hsu KH, Chan MC, Wang JM, Lin LY, Wu CL. Comparison of Fcgamma receptor expression on neutrophils with procalcitonin for the diagnosis of sepsis in critically ill patients. Respirology. 2011;16:152–160. doi: 10.1111/j.1440-1843.2010.01876.x. [DOI] [PubMed] [Google Scholar]
- Ishikura H, Nishida T, Murai A, Nakamura Y, Irie Y, Tanaka J, et al. New diagnostic strategy for sepsis-induced disseminated intravascular coagulation: a prospective single-center observational study. Crit Care. 2014;18:R19. doi: 10.1186/cc13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivancevic N, Radenkovic D, Bumbasirevic V, Karamarkovic A, Jeremic V, Kalezic N, et al. Procalcitonin in preoperative diagnosis of abdominal sepsis. Langenbecks Arch Surg. 2008;393:397–403. doi: 10.1007/s00423-007-0239-5. [DOI] [PubMed] [Google Scholar]
- Jekarl DW, Lee SY, Lee J, Park YJ, Kim Y, Park JH, et al. Procalcitonin as a diagnostic marker and IL-6 as a prognostic marker for sepsis. Diagn Microbiol Infect Dis. 2013;75:342–347. doi: 10.1016/j.diagmicrobio.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Jekarl DW, Kim JY, Lee S, Kim M, Kim Y, Han K, et al. Diagnosis and evaluation of severity of sepsis via the use of biomarkers and profiles of 13 cytokines: a multiplex analysis. Clin Chem Lab Med. 2014;53:575–581. doi: 10.1515/cclm-2014-0607. [DOI] [PubMed] [Google Scholar]
- Jiang YN, Cai X, Zhou HM, Jin WD, Zhang M, Zhang Y, et al. Diagnostic and prognostic roles of soluble CD22 in patients with Gram-negative bacterial sepsis. Hepatobiliary Pancreat Dis Int. 2015;14(5):523–529. doi: 10.1016/S1499-3872(15)60394-0. [DOI] [PubMed] [Google Scholar]
- Kim S, Mi L, Zhang L. Specific elevation of DcR3 in sera of sepsis patients and its potential role as a clinically important biomarker of sepsis. Diagn Microbiol Infect Dis. 2012;73:312–317. doi: 10.1016/j.diagmicrobio.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofoed K, Andersen O, Kronborg G, Tvede M, Petersen J, Eugen-Olsen J, et al. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care. 2007;11:R38. doi: 10.1186/cc5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour-Perez J, Alcala-Lopez A, Garcia-Garcia MA, Sanchez-Hernandez JF, Abad-Terrado C, Viedma-Contreras JA, et al. Diagnostic accuracy of sTREM-1 to identify infection in critically ill patients with systemic inflammatory response syndrome. Clin Biochem. 2010;43:720–724. doi: 10.1016/j.clinbiochem.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36:222–231. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SM, Treacher DF, Edgeworth J, Mahalingam G, Brown CS, Mare TA, Stacey M. Expression of CD11c and EMR2 on neutrophils: potential diagnostic biomarkers for sepsis and systemic inflammation. Clin Exp Immunol. 2015;182(2):184–194. doi: 10.1111/cei.12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhu Z, Chen J, Ouyang B, Chen M, Guan X. Diagnostic value of soluble triggering receptor expressed on myeloid cells-1 in critically-ill, postoperative patients with suspected sepsis. Am J Med Sci. 2013;345:178–184. doi: 10.1097/MAJ.0b013e318253a1a6. [DOI] [PubMed] [Google Scholar]
- Li S, Huang X, Chen Z, Zhong H, Peng Q, Deng Y, et al. Neutrophil CD64 expression as a biomarker in the early diagnosis of bacterial infection: a meta-analysis. Int J Infect Dis. 2013;17:e12–e23. doi: 10.1016/j.ijid.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Lin S, Huang Z, Wang M, Weng Z, Zeng D, Zhang Y, et al. Interleukin-6 as an early diagnostic marker for bacterial sepsis in patients with liver cirrhosis. J Crit Care. 2015;30(4):732–738. doi: 10.1016/j.jcrc.2015.03.031. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Matera G, Puccio R, Giancotti A, Quirino A, Pulicari MC, Zicca E, et al. Impact of interleukin-10, soluble CD25 and interferon-gamma on the prognosis and early diagnosis of bacteremic systemic inflammatory response syndrome: a prospective observational study. Crit Care. 2013;17:R64. doi: 10.1186/cc12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mat-Nor MB, Md-Ralib A, Abdulah NZ, Pickering JW. The diagnostic ability of procalcitonin and interleukin-6 to differentiate infectious from noninfectious systemic inflammatory response syndrome and to predict mortality. J Crit Care. 2016;33:245–251. doi: 10.1016/j.jcrc.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Mearelli F, Fiotti N, Altamura N, Zanetti M, Fernandes G, Burekovic I, et al. Heterogeneous models for an early discrimination between sepsis and non-infective SIRS in medical ward patients: a pilot study. Intern Emerg Med. 2014;9:749–757. doi: 10.1007/s11739-013-1031-x. [DOI] [PubMed] [Google Scholar]
- Meynaar IA, Droog W, Batstra M, Vreede R, Herbrink P. In Critically Ill Patients, Serum Procalcitonin Is More Useful in Differentiating between Sepsis and SIRS than CRP, Il-6, or LBP. Crit Care Res Pract. 2011;2011:594645. doi: 10.1155/2011/594645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglietta F, Faneschi ML, Lobreglio G, Palumbo C, Rizzo A, Cucurachi M, et al. Procalcitonin, C-reactive protein and serum lactate dehydrogenase in the diagnosis of bacterial sepsis. SIRS and systemic candidiasis. Infez Med. 2015;23(3):230–237. [PubMed] [Google Scholar]
- Miller PR, Munn DD, Meredith JW, Chang MC. Systemic inflammatory response syndrome in the trauma intensive care unit: who is infected? J Trauma. 1999;47:1004–1008. doi: 10.1097/00005373-199912000-00003. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Muthiah KA, Rachakonda KS, Davis MJ, Simmons EG, Schier G, Gil FS. Prospective evaluation of procalcitonin in sepsis in the Illawarra area of Australia: PEPSIA study. Crit Care Resusc. 2007;9:137–142. [PubMed] [Google Scholar]
- Naeini AE, Montazerolghaem S. Procalcitonin marker for sepsis diagnosis. Evaluating a rapid immunochromatografic test. Saudi Med J. 2006;27:422–424. [PubMed] [Google Scholar]
- Oshita H, Sakurai J, Kamitsuna M. Semi-quantitative procalcitonin test for the diagnosis of bacterial infection: clinical use and experience in Japan. J Microbiol Immunol Infect. 2010;43:222–227. doi: 10.1016/S1684-1182(10)60035-7. [DOI] [PubMed] [Google Scholar]
- Papadimitriou-Olivgeris M, Lekka K, Zisimopoulos K, Spiliopoulou I, Logothetis D, Theodorou G, et al. Role of CD64 expression on neutrophils in the diagnosis of sepsis and the prediction of mortality in adult critically ill patients. Diagn Microbiol Infect Dis. 2015;82(3):234–239. doi: 10.1016/j.diagmicrobio.2015.03.022. [DOI] [PubMed] [Google Scholar]
- Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzinger F, Schuardt M, Eichbichler K, Tsirkinidou I, Bauer M, Haslacher H, et al. Utility of sepsis biomarkers and the infection probability score to discriminate sepsis and systemic inflammatory response syndrome in standard care patients. PLoS ONE. 2013;8:e82946. doi: 10.1371/journal.pone.0082946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichsoellner M, Raggam RB, Wagner J, Krause R, Hoenigl M. Clinical evaluation of multiple inflammation biomarkers for diagnosis and prognosis for patients with systemic inflammatory response syndrome. J Clin Microbiol. 2014;52:4063–4066. doi: 10.1128/JCM.01954-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Righi S, Santambrogio L, Monsagrati A, Saliu M, Locati L, Radrizzani D. Clinical evaluation of neutrophil CD64 as a diagnostic marker of infection in a polyvalent intensive care unit. Infect Dis Clin Pract. 2014;22:32–37. doi: 10.1097/IPC.0b013e31828f4b6a. [DOI] [Google Scholar]
- Rivera-Chavez FA, Minei JP. Soluble triggering receptor expressed on myeloid cells-1 Is an early marker of infection in the surgical intensive care unit. Surg Infect (Larchmt) 2009;10:435–439. doi: 10.1089/sur.2009.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina P, Stubljar D, T LZ, Osredkar J, Skvarc M (2014) Expression of CD64 on neutrophils (CD64 index): diagnostic accuracy of CD64 index to predict sepsis in critically ill patients is better than of procalcitonin C-reactive protein, research note. Clin Chem Lab Med [DOI] [PubMed]
- Romualdo LGDG, Torrella PE, Gonzalez MV, Sanchez RJ, Holgado AH, Freire AO, et al. Diagnostic accuracy of presepsin (soluble CD14 subtype) for prediction of bacteremia in patients with systemic inflammatory response syndrome in the Emergency Department. Clin Biochem. 2014;47:505–508. doi: 10.1016/j.clinbiochem.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Ruiz-Alvarez MJ, Garcia-Valdecasas S, De Pablo R, Sanchez Garcia M, Coca C, Groeneveld TW, et al. Diagnostic efficacy and prognostic value of serum procalcitonin concentration in patients with suspected sepsis. J Intensive Care Med. 2009;24:63–71. doi: 10.1177/0885066608327095. [DOI] [PubMed] [Google Scholar]
- Sakr Y, Burgett U, Nacul FE, Reinhart K, Brunkhorst F. Lipopolysaccharide binding protein in a surgical intensive care unit: a marker of sepsis? Crit Care Med. 2008;36:2014–2022. doi: 10.1097/CCM.0b013e31817b86e3. [DOI] [PubMed] [Google Scholar]
- Scherpereel A, Depontieu F, Grigoriu B, Cavestri B, Tsicopoulos A, Gentina T, et al. Endocan, a new endothelial marker in human sepsis. Crit Care Med. 2006;34:532–537. doi: 10.1097/01.CCM.0000198525.82124.74. [DOI] [PubMed] [Google Scholar]
- Schulte J, Struck J, Kohrle J, Muller B. Circulating levels of peroxiredoxin 4 as a novel biomarker of oxidative stress in patients with sepsis. Shock. 2011;35:460–465. doi: 10.1097/SHK.0b013e3182115f40. [DOI] [PubMed] [Google Scholar]
- Selberg O, Hecker H, Martin M, Klos A, Bautsch W, Kohl J. Discrimination of sepsis and systemic inflammatory response syndrome by determination of circulating plasma concentrations of procalcitonin, protein complement 3a, and interleukin-6. Crit Care Med. 2000;28:2793–2798. doi: 10.1097/00003246-200008000-00019. [DOI] [PubMed] [Google Scholar]
- Seok Y, Choi JR, Kim J, Kim YK, Lee J, Song J, et al. Delta neutrophil index: a promising diagnostic and prognostic marker for sepsis. Shock. 2012;37:242–246. doi: 10.1097/SHK.0b013e3182454acf. [DOI] [PubMed] [Google Scholar]
- Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother. 2011;17:764–769. doi: 10.1007/s10156-011-0254-x. [DOI] [PubMed] [Google Scholar]
- Sierra R, Rello J, Bailen MA, Benitez E, Gordillo A, Leon C, et al. C-reactive protein used as an early indicator of infection in patients with systemic inflammatory response syndrome. Intensive Care Med. 2004;30:2038–2045. doi: 10.1007/s00134-004-2434-y. [DOI] [PubMed] [Google Scholar]
- Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–217. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Han B, Liu C, Liang L, Jiang Z, Deng J, et al. Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: a prospective cohort study. BMC Infect Dis. 2012;12:157. doi: 10.1186/1471-2334-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Feng L, Song Q, Kang H, Zhang X, Liang Z, et al. Diagnostic value of dynamics serum sCD163, sTREM-1, PCT, and CRP in differentiating sepsis, severity assessment, and prognostic prediction. Mediators Inflamm. 2013;2013:969875. doi: 10.1155/2013/969875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungurtekin H, Sungurtekin U, Balci C. Circulating complement (C3 and C4) for differentiation of SIRS from sepsis. Adv Ther. 2006;23:893–901. doi: 10.1007/BF02850211. [DOI] [PubMed] [Google Scholar]
- Suprin E, Camus C, Gacouin A, Le Tulzo Y, Lavoue S, Feuillu A, et al. Procalcitonin: a valuable indicator of infection in a medical ICU? Intensive Care Med. 2000;26:1232–1238. doi: 10.1007/s001340000580. [DOI] [PubMed] [Google Scholar]
- Takahashi G, Shibata S, Ishikura H, Miura M, Fukui Y, Inoue Y et al (2014) Presepsin in the prognosis of infectious diseases and diagnosis of infectious disseminated intravascular coagulation: a prospective, multicentre, observational study. Eur J Anaesthesiol [DOI] [PubMed]
- Talebi-Taher M, Babazadeh S, Barati M, Latifnia M. Serum inflammatory markers in the elderly: are they useful in differentiating sepsis from SIRS? Acta Med Iran. 2014;52:438–442. [PubMed] [Google Scholar]
- Tan TL, Ahmad NS, Nasuruddin DN, Ithnin A, Tajul Arifin K, Zaini IZ, et al. CD64 and Group II secretory phospholipase A2 (sPLA2-IIA) as biomarkers for distinguishing adult sepsis and bacterial infections in the emergency department. PLoS ONE. 2016;11(3):e0152065. doi: 10.1371/journal.pone.0152065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Pan S, Ma G, Liao W, Su Q, Gu B, et al. Serum levels of procalcitonin as a biomarker for differentiating between sepsis and systemic inflammatory response syndrome in the neurological intensive care unit. J Clin Neurosci. 2014;21:1153–1158. doi: 10.1016/j.jocn.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Tromp M, Lansdorp B, Bleeker-Rovers CP, Gunnewiek JM, Kullberg BJ, Pickkers P. Serial and panel analyses of biomarkers do not improve the prediction of bacteremia compared to one procalcitonin measurement. J Infect. 2012;65:292–301. doi: 10.1016/j.jinf.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Tsalik EL, Jaggers LB, Glickman SW, Langley RJ, van Velkinburgh JC, Park LP, et al. Discriminative value of inflammatory biomarkers for suspected sepsis. J Emerg Med. 2012;43:97–106. doi: 10.1016/j.jemermed.2011.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsangaris I, Plachouras D, Kavatha D, Gourgoulis GM, Tsantes A, Kopterides P, et al. Diagnostic and prognostic value of procalcitonin among febrile critically ill patients with prolonged ICU stay. BMC Infect Dis. 2009;9:213. doi: 10.1186/1471-2334-9-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugrul S, Esen F, Celebi S, Ozcan PE, Akinci O, Cakar N, et al. Reliability of procalcitonin as a severity marker in critically ill patients with inflammatory response. Anaesth Intensive Care. 2002;30:747–754. doi: 10.1177/0310057X0203000605. [DOI] [PubMed] [Google Scholar]
- Ulla M, Pizzolato E, Lucchiari M, Loiacono M, Soardo F, Forno D, et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care. 2013;17:R168. doi: 10.1186/cc12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschetto R, Nicola S, Olivieri C, Boggio E, Piccolella F, Mesturini R, et al. Serum levels of osteopontin are increased in SIRS and sepsis. Intensive Care Med. 2008;34:2176–2184. doi: 10.1007/s00134-008-1268-4. [DOI] [PubMed] [Google Scholar]
- Vodnik T, Kaljevic G, Tadic T, Majkic-Singh N. Presepsin (sCD14-ST) in preoperative diagnosis of abdominal sepsis. Clin Chem Lab Med. 2013;51:2053–2062. doi: 10.1515/cclm-2013-0061. [DOI] [PubMed] [Google Scholar]
- Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:426–435. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- Wafaisade A, Lefering R, Bouillon B, Sakka SG, Thamm OC, Paffrath T, et al. Epidemiology and risk factors of sepsis after multiple trauma: an analysis of 29,829 patients from the Trauma Registry of the German Society for Trauma Surgery. Crit Care Med. 2011;39:621–628. doi: 10.1097/CCM.0b013e318206d3df. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie LX. Evidence for serum miR-15a and miR-16 levels as biomarkers that distinguish sepsis from systemic inflammatory response syndrome in human subjects. Clin Chem Lab Med. 2012;50:1423–1428. doi: 10.1515/cclm-2011-0826. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang HC, Chen C, Zeng J, Wang Q, Zheng L, et al. Differential expression of plasma miR-146a in sepsis patients compared with non-sepsis-SIRS patients. Exp Ther Med. 2013;5:1101–1104. doi: 10.3892/etm.2013.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner GA, Keel M, Steckholzer U, Beier W, Stocker R, Ertel W. Relationship between procalcitonin plasma levels and severity of injury, sepsis, organ failure, and mortality in injured patients. Crit Care Med. 2000;28:950–957. doi: 10.1097/00003246-200004000-00007. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang F, Fan X, Bao R, Bo L, Li J, et al. Accuracy of plasma sTREM-1 for sepsis diagnosis in systemic inflammatory patients: a systematic review and meta-analysis. Crit Care. 2012;16:R229. doi: 10.1186/cc11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Su L, Yan P, Han B, Li J, Wang H, et al. alpha-1-Acid glycoprotein as a biomarker for the early diagnosis and monitoring the prognosis of sepsis. J Crit Care. 2015;30(4):744–751. doi: 10.1016/j.jcrc.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Yousef AA, Amr YM, Suliman GA. The diagnostic value of serum leptin monitoring and its correlation with tumor necrosis factor-alpha in critically ill patients: a prospective observational study. Crit Care. 2010;14:R33. doi: 10.1186/cc8911. [DOI] [PMC free article] [PubMed] [Google Scholar]


