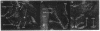Abstract
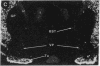
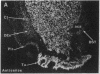
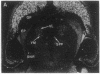
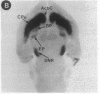
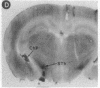
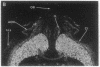
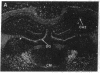
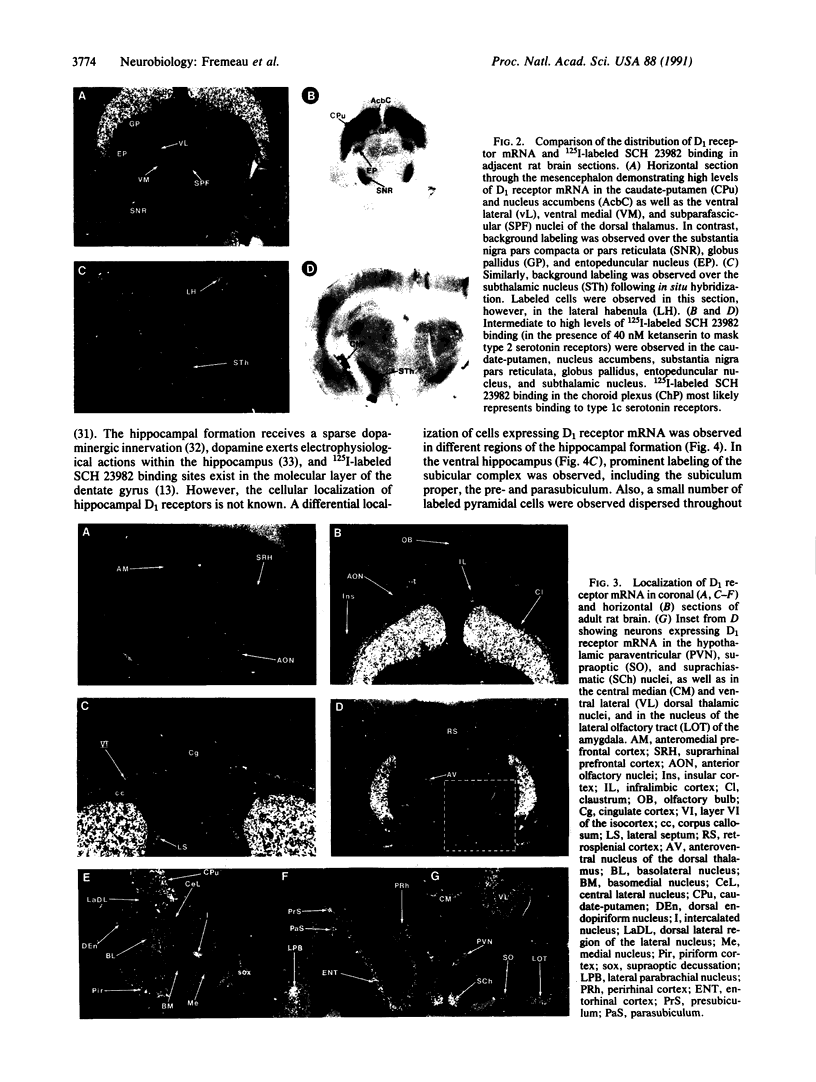
Expression of a D1 dopamine receptor was examined in the rat brain by using a combination of in situ hybridization and in vitro receptor autoradiography. Cells expressing D1 receptor mRNA were localized to many, but not all, brain regions receiving dopaminergic innervation. The highest levels of hybridization were detected in the caudate-putamen, nucleus accumbens, and olfactory tubercle. Cells expressing D1 receptor mRNA were also detected throughout the cerebral cortex, limbic system, hypothalamus, and thalamus. D1 receptor mRNA was differentially expressed in distinct regions of the hippocampal formation. Dentate granule cells were labeled in dorsal but not ventral regions, whereas the subicular complex was prominently labeled in ventral but not dorsal regions. Intermediate to high levels of D1 binding sites, but no hybridizing D1 receptor mRNA, were detected in the substantia nigra pars reticulata, globus pallidus, entopeduncular nucleus, and subthalamic nucleus. In these brain regions, which are involved in the efferent flow of information from the basal ganglia, D1 receptors may be localized on afferent nerve terminals originating in other brain regions. These results indicate that in addition to a role in control of motor function, the D1 receptor may also participate in the cognitive, affective, and neuroendocrine effects of dopaminergic neurotransmission.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benardo L. S., Prince D. A. Dopamine action on hippocampal pyramidal cells. J Neurosci. 1982 Apr;2(4):415–423. doi: 10.1523/JNEUROSCI.02-04-00415.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkirane S., Arbilla S., Langer S. Z. A functional response to D1 dopamine receptor stimulation in the central nervous system: inhibition of the release of [3H]-serotonin from the rat substantia nigra. Naunyn Schmiedebergs Arch Pharmacol. 1987 May;335(5):502–507. doi: 10.1007/BF00169115. [DOI] [PubMed] [Google Scholar]
- Bischoff S., Scatton B., Korf J. Biochemical evidence for a transmitter role of dopamine in the rat hippocampus. Brain Res. 1979 Apr 6;165(1):161–165. doi: 10.1016/0006-8993(79)90056-8. [DOI] [PubMed] [Google Scholar]
- Boyson S. J., McGonigle P., Molinoff P. B. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986 Nov;6(11):3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D., White F. J. D1 dopamine receptor--the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1(4):347–388. doi: 10.1002/syn.890010408. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Barone P., Sidhu A., Wamsley J. K., Chase T. N. The D1 dopamine receptor in the rat brain: quantitative autoradiographic localization using an iodinated ligand. Neuroscience. 1988 Jul;26(1):83–100. doi: 10.1016/0306-4522(88)90129-7. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Gehlert D. R., McCabe R. T., Barnett A., Wamsley J. K. D-1 dopamine receptors in the rat brain: a quantitative autoradiographic analysis. J Neurosci. 1986 Aug;6(8):2352–2365. doi: 10.1523/JNEUROSCI.06-08-02352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearry A., Gingrich J. A., Falardeau P., Fremeau R. T., Jr, Bates M. D., Caron M. G. Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature. 1990 Sep 6;347(6288):72–76. doi: 10.1038/347072a0. [DOI] [PubMed] [Google Scholar]
- Dubois A., Savasta M., Curet O., Scatton B. Autoradiographic distribution of the D1 agonist [3H]SKF 38393, in the rat brain and spinal cord. Comparison with the distribution of D2 dopamine receptors. Neuroscience. 1986 Sep;19(1):125–137. doi: 10.1016/0306-4522(86)90010-2. [DOI] [PubMed] [Google Scholar]
- Fremeau R. T., Jr, Popko B. In situ analysis of myelin basic protein gene expression in myelin-deficient oligodendrocytes: antisense hnRNA and readthrough transcription. EMBO J. 1990 Nov;9(11):3533–3538. doi: 10.1002/j.1460-2075.1990.tb07562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault J. A., Spampinato U., Savaki H. E., Glowinski J., Besson M. J. In vivo release of [3H]gamma-aminobutyric acid in the rat neostriatum--I. Characterization and topographical heterogeneity of the effects of dopaminergic and cholinergic agents. Neuroscience. 1986 Dec;19(4):1101–1108. doi: 10.1016/0306-4522(86)90126-0. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Moratalla R., Robertson H. A. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A., Fuxe K., Johansson O. Dopamine nerve terminals in the rat limbic cortex: aspects of the dopamine hypothesis of schizophrenia. Science. 1974 Apr 12;184(4133):177–179. doi: 10.1126/science.184.4133.177. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Lankford K. L., DeMello F. G., Klein W. L. D1-type dopamine receptors inhibit growth cone motility in cultured retina neurons: evidence that neurotransmitters act as morphogenic growth regulators in the developing central nervous system. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4567–4571. doi: 10.1073/pnas.85.12.4567-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A., Meador-Woodruff J. H., Bunzow J. R., Civelli O., Akil H., Watson S. J. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci. 1990 Aug;10(8):2587–2600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff J. H., Mansour A., Bunzow J. R., Van Tol H. H., Watson S. J., Jr, Civelli O. Distribution of D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7625–7628. doi: 10.1073/pnas.86.19.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer J. H., Rietveld W. J. Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiol Rev. 1989 Jul;69(3):671–707. doi: 10.1152/physrev.1989.69.3.671. [DOI] [PubMed] [Google Scholar]
- Mengod G., Martinez-Mir M. I., Vilaró M. T., Palacios J. M. Localization of the mRNA for the dopamine D2 receptor in the rat brain by in situ hybridization histochemistry. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8560–8564. doi: 10.1073/pnas.86.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma F. J., Jr, Mahan L. C., McVittie L. D., Gerfen C. R., Sibley D. R. Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6723–6727. doi: 10.1073/pnas.87.17.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet C. C., Miller P. E., Hemmings H. C., Jr, Walaas S. I., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984 Jan;4(1):111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi J. C., Iversen L. L., Jessell T. M. Dopamine selectively increases 3H-GABA release from slices of rat substantia nigra in vitro. Nature. 1977 Aug 18;268(5621):652–654. doi: 10.1038/268652a0. [DOI] [PubMed] [Google Scholar]
- Roberts G. W. Schizophrenia: the cellular biology of a functional psychosis. Trends Neurosci. 1990 Jun;13(6):207–211. doi: 10.1016/0166-2236(90)90161-3. [DOI] [PubMed] [Google Scholar]
- Savasta M., Dubois A., Scatton B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res. 1986 Jun 11;375(2):291–301. doi: 10.1016/0006-8993(86)90749-3. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T., Goldman-Rakic P. S. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991 Feb 22;251(4996):947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Schachter M., Bédard P., Debono A. G., Jenner P., Marsden C. D., Price P., Parkes J. D., Keenan J., Smith B., Rosenthaler J. The role of D-1 and D-2 receptors. Nature. 1980 Jul 10;286(5769):157–159. doi: 10.1038/286157a0. [DOI] [PubMed] [Google Scholar]
- Schalling M., Dagerlind A., Goldstein M., Ehrlich M., Greengard P., Hökfelt T. Comparison of gene expression of the dopamine D-2 receptor and DARPP-32 in rat brain, pituitary and adrenal gland. Eur J Pharmacol. 1990 Apr 25;188(4-5):277–281. doi: 10.1016/0922-4106(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Schalling M., Djurfeldt M., Hökfelt T., Ehrlich M., Kurihara T., Greengard P. Distribution and cellular localization of DARPP-32 mRNA in rat brain. Brain Res Mol Brain Res. 1990 Feb;7(2):139–149. doi: 10.1016/0169-328x(90)90091-q. [DOI] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980 Sep;32(3):229–313. [PubMed] [Google Scholar]
- Sokoloff P., Giros B., Martres M. P., Bouthenet M. L., Schwartz J. C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990 Sep 13;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sunahara R. K., Niznik H. B., Weiner D. M., Stormann T. M., Brann M. R., Kennedy J. L., Gelernter J. E., Rozmahel R., Yang Y. L., Israel Y. Human dopamine D1 receptor encoded by an intronless gene on chromosome 5. Nature. 1990 Sep 6;347(6288):80–83. doi: 10.1038/347080a0. [DOI] [PubMed] [Google Scholar]
- Tassin J. P., Simon H., Hervé D., Blanc G., Le Moal M., Glowinski J., Bockaert J. Non-dopaminergic fibres may regulate dopamine-sensitive adenylate cyclase in the prefrontal cortex and nucleus accumbens. Nature. 1982 Feb 25;295(5851):696–698. doi: 10.1038/295696a0. [DOI] [PubMed] [Google Scholar]
- Trugman J. M., Wooten G. F. Selective D1 and D2 dopamine agonists differentially alter basal ganglia glucose utilization in rats with unilateral 6-hydroxydopamine substantia nigra lesions. J Neurosci. 1987 Sep;7(9):2927–2935. doi: 10.1523/JNEUROSCI.07-09-02927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar L., Meldolesi J. Mechanisms of signal transduction at the dopamine D2 receptor. Trends Pharmacol Sci. 1989 Feb;10(2):74–77. doi: 10.1016/0165-6147(89)90082-5. [DOI] [PubMed] [Google Scholar]
- Verney C., Baulac M., Berger B., Alvarez C., Vigny A., Helle K. B. Morphological evidence for a dopaminergic terminal field in the hippocampal formation of young and adult rat. Neuroscience. 1985 Apr;14(4):1039–1052. doi: 10.1016/0306-4522(85)90275-1. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Aswad D. W., Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983 Jan 6;301(5895):69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Sakai M., Kani K., Nagatsu I., Tanaka M. The dopaminergic innervation as observed by immunohistochemistry using anti-dopamine serum in the rat cerebral cortex. Experientia. 1988 Aug 15;44(8):700–702. doi: 10.1007/BF01941033. [DOI] [PubMed] [Google Scholar]
- Zhou Q. Y., Grandy D. K., Thambi L., Kushner J. A., Van Tol H. H., Cone R., Pribnow D., Salon J., Bunzow J. R., Civelli O. Cloning and expression of human and rat D1 dopamine receptors. Nature. 1990 Sep 6;347(6288):76–80. doi: 10.1038/347076a0. [DOI] [PubMed] [Google Scholar]