Abstract
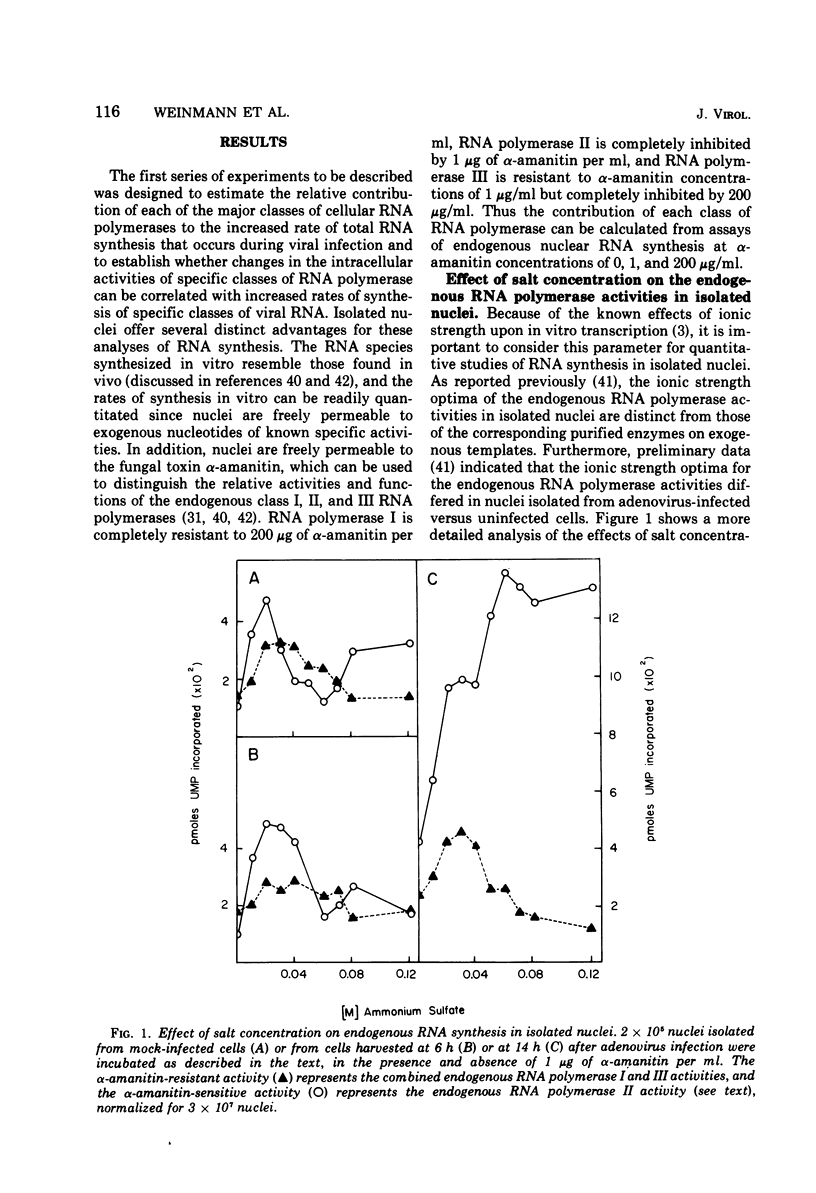
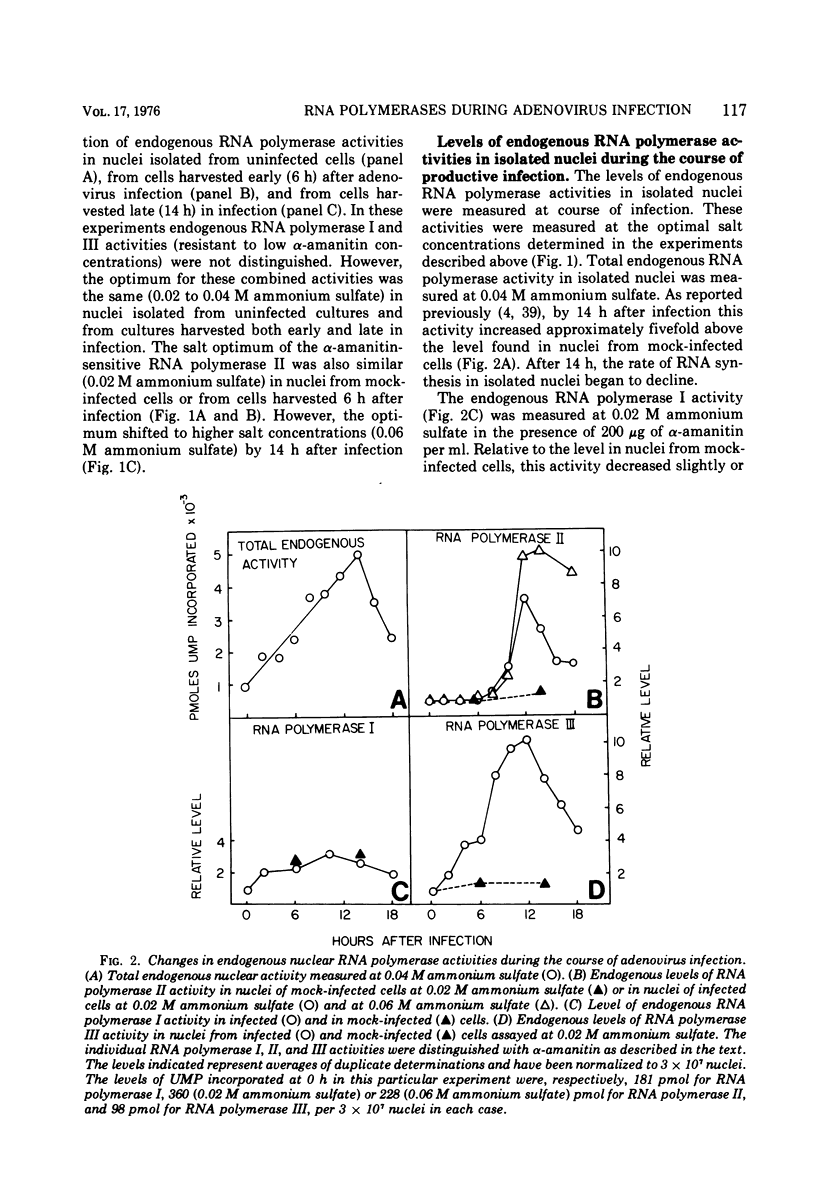
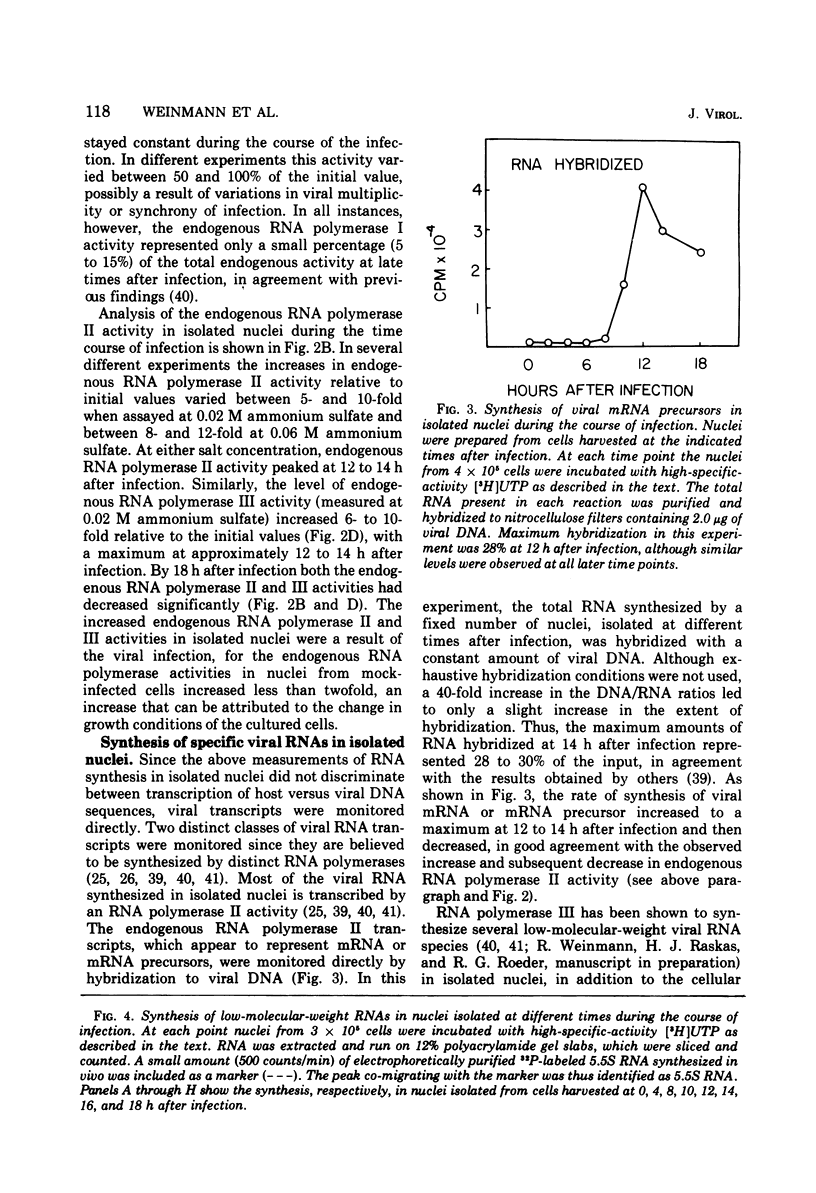
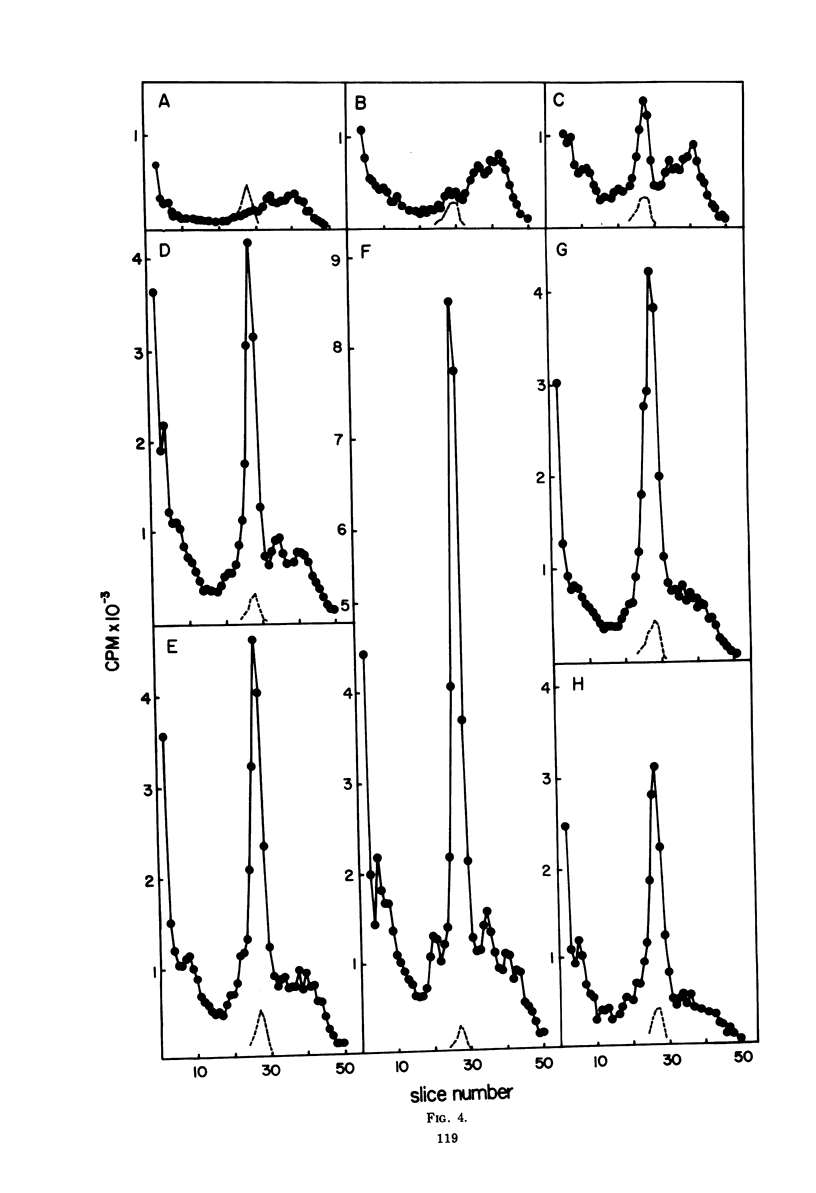
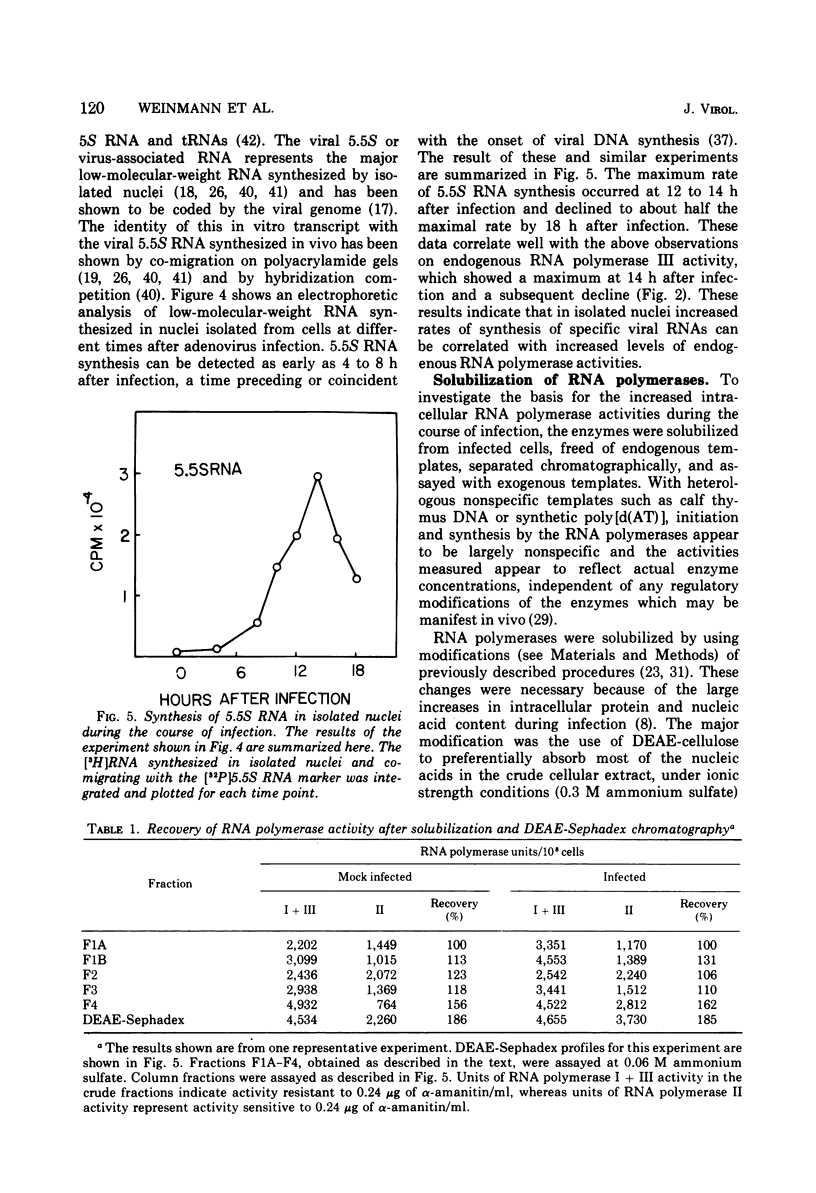
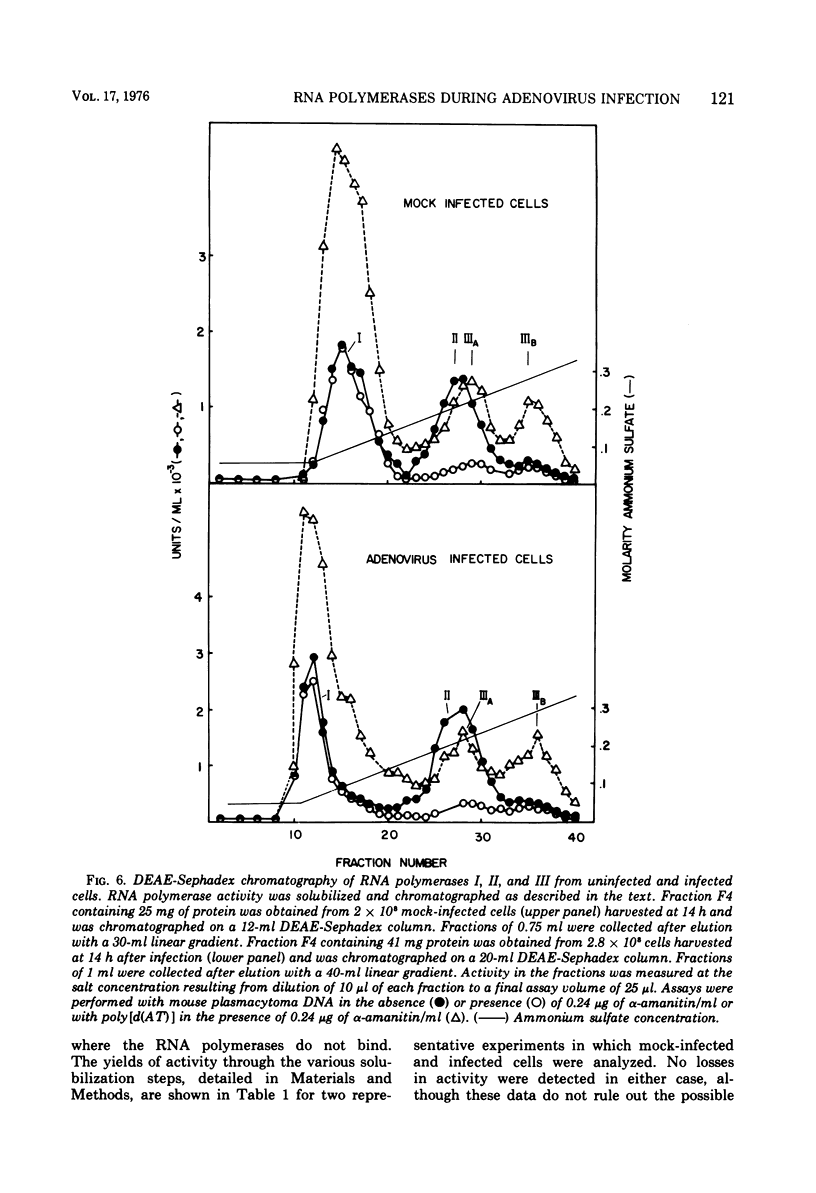
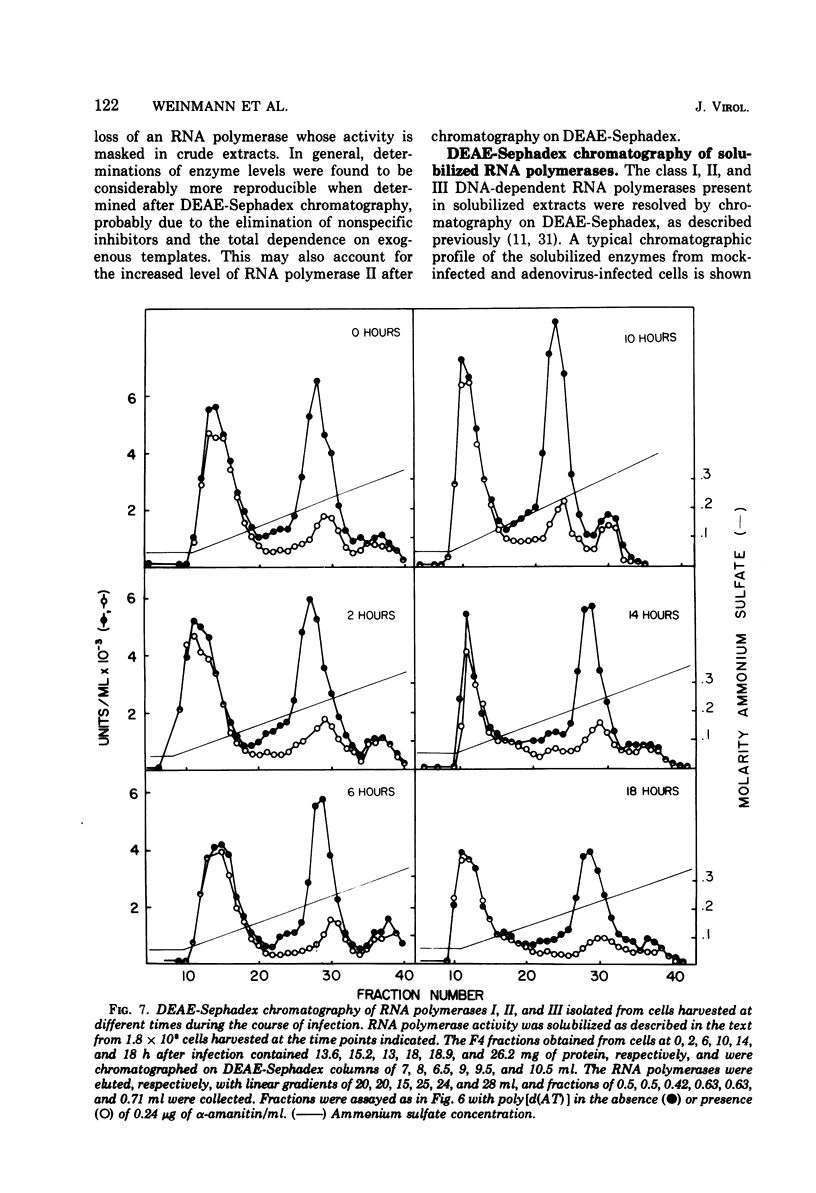
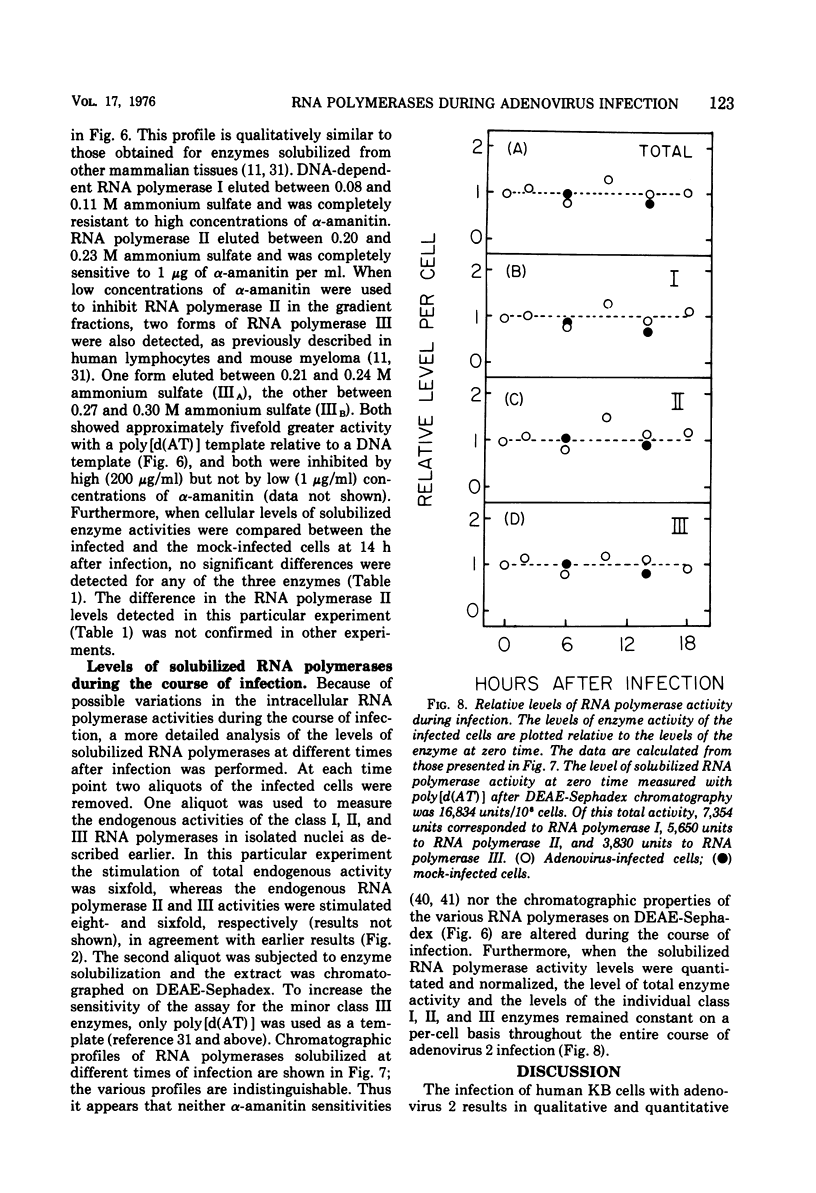
The rates of RNA synthesis in cultured human KB cells infected by adenovirus 2 were estimated by measuring the endogenous RNA polymerase activities in isolated nuclei. The fungal toxin α-amanitin was used to determine the relative and absolute levels of RNA synthesis by RNA polymerases I, II, and III in nuclei isolated during the course of infection. Whereas the level of endogenous RNA polymerase I activity in nuclei from infected cells remained constant relative to the level in nuclei from mock-infected cells, the endogenous RNA polymerase II and III activities each increased about 10-fold. These increases in endogenous RNA polymerase activities were accompanied by concomitant increases in the rates of synthesis in isolated nuclei of viral mRNA precursor, which was monitored by hybridization to viral DNA, and of viral 5.5S RNA, which was quantitated by electrophoretic analysis on polyacrylamide gels. The cellular RNA polymerase levels were measured with exogenous templates after solubilization and chromatographic resolution of the enzymes on DEAE-Sephadex, using procedures in which no losses of activity were apparent. In contrast to the endogenous RNA polymerase activities in isolated nuclei, the cellular levels of the solubilized class I, II, and III RNA polymerases remained constant throughout the course of the infection. Furthermore, no differences were detected in the chromatographic properties of the RNA polymerases obtained from infected or control mock-infected cells. These observations suggest that the increases in endogenous RNA polymerase activities in isolated nuclei are not due to variations in the cellular concentrations of the enzymes. Instead, it is likely that the increased endogenous enzyme activities result from either the large amounts of viral DNA template available as a consequence of viral replication or from functional modifications of the RNA polymerases or from a combination of these effects.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin G. E., Bello L. J., Furth J. J. DNA dependent RNA polymerase of KB cells. I. Isolation of the enzymes and transcription of viral DNA, mammalian DNA and chromatin. Biochim Biophys Acta. 1973 Nov 14;324(4):488–500. doi: 10.1016/0005-2787(73)90208-6. [DOI] [PubMed] [Google Scholar]
- Axel R., Cedar H., Felsenfield G. The structure of the globin genes in chromatin. Biochemistry. 1975 Jun 3;14(11):2489–2495. doi: 10.1021/bi00682a031. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Gazzolo L., Pogo B. G. Effect of -amanitin on adenovirus 5 multiplication. Virology. 1972 Apr;48(1):300–304. doi: 10.1016/0042-6822(72)90144-4. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Two classes of cytoplasmic viral RNA synthesized early in productive infection with adenovirus 2. J Virol. 1974 Oct;14(4):751–757. doi: 10.1128/jvi.14.4.751-757.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., DAESCH G. E. Biochemical studies on adenovirus multiplication. II. Kinetics of nucleic acid and protein synthesis in suspension cultures. Virology. 1961 Feb;13:169–176. doi: 10.1016/0042-6822(61)90051-4. [DOI] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Jaehning J. A., Stewart C. C., Roeder R. G. DNA-dependent RNA polymerase levels during the response of human peripheral lymphocytes to phytohemagglutinin. Cell. 1975 Jan;4(1):51–57. doi: 10.1016/0092-8674(75)90133-6. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Ledinko N. Nucleolar ribosomal precursor RNA and protein metabolism in human embryo kidney cultures infected with adenovirus 12. Virology. 1972 Jul;49(1):79–89. doi: 10.1016/s0042-6822(72)80008-4. [DOI] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Transcription and transport of virus-specific ribonucleic acids in African green monkey kidney cells abortively infected with type 2 adenovirus. J Virol. 1972 Dec;10(6):1109–1117. doi: 10.1128/jvi.10.6.1109-1117.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohe K. Virus-coded origin of a low molecular weight RNA from KB cells infected with adenovirus 2. Virology. 1972 Mar;47(3):726–733. doi: 10.1016/0042-6822(72)90562-4. [DOI] [PubMed] [Google Scholar]
- Ohe K., Weissman S. M., Cooke N. R. Studies on the origin of a low molecular weight ribonucleic acid from human cells infected with adenoviruses. J Biol Chem. 1969 Oct 10;244(19):5320–5332. [PubMed] [Google Scholar]
- Ohe K., Weissman S. M. The nucleotide sequence of a low molecular weight ribonucleic acid from cells infected with adenovirus 2. J Biol Chem. 1971 Nov 25;246(22):6991–7009. [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Gardner J., Green M. Biochemical studies on adenovirus multiplication, XIX. Resolution of late viral RNA species in the nucleus and cytoplasm. Proc Natl Acad Sci U S A. 1971 Mar;68(3):557–560. doi: 10.1073/pnas.68.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Philipson L. Synthesis of complementary RNA sequences during productive adenovirus infection. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4887–4891. doi: 10.1073/pnas.71.12.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. XIV. Macromolecule and enzyme synthesis in cells replicating oncogenic and nononcogenic human adenovirus. Virology. 1969 Aug;38(4):573–586. doi: 10.1016/0042-6822(69)90178-0. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. A distinct RNA polymerase activity, synthesizing 5-5 s, 5 s and 4 s RNA in nuclei from adenovirus 2-infected HeLa cells. J Mol Biol. 1972 Oct 14;70(3):435–450. doi: 10.1016/0022-2836(72)90551-7. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. Transcription of the adenovirus genome by an -amanitine-sensitive ribonucleic acid polymerase in HeLa cells. J Virol. 1972 Apr;9(4):621–626. doi: 10.1128/jvi.9.4.621-626.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskas H. J., Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XVII. Ribosome synthesis in uninfected and infected KB cells. Virology. 1970 Apr;40(4):893–902. doi: 10.1016/0042-6822(70)90135-2. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Lawrence C., Thach R. E., Roeder R. G. Encephalomyocarditis virus infection of mouse plasmacytoma cells. II. Effect on host RNA synthesis and RNA polymerases. J Virol. 1974 Sep;14(3):611–619. doi: 10.1128/jvi.14.3.611-619.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. B., Roeder R. G. Purification and subunit structure of deoxyribonucleic acid-dependent ribonucleic acid polymerase II from the mouse plasmacytoma, MOPC 315. J Biol Chem. 1975 May 10;250(9):3221–3228. [PubMed] [Google Scholar]
- Schwartz L. B., Sklar V. E., Jaehning J. A., Weinmann R., Roeder R. G. Isolation and partial characterization of the multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in the mouse myeloma, MOPC 315. J Biol Chem. 1974 Sep 25;249(18):5889–5897. [PubMed] [Google Scholar]
- Shmookler R. J., Buss J., Green M. H. Properties of the polyoma virus transcription complex obtained from mouse nuclei. Virology. 1974 Jan;57(1):122–127. doi: 10.1016/0042-6822(74)90113-5. [DOI] [PubMed] [Google Scholar]
- Sklar V. E., Schwartz L. B., Roeder R. G. Distinct molecular structures of nuclear class I, II, and III DNA-dependent RNA polymerases. Proc Natl Acad Sci U S A. 1975 Jan;72(1):348–352. doi: 10.1073/pnas.72.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Keller W. Mammalian deoxyribonucleic acid-dependent ribonucleic acid polymerases. I. Purification and properties of an -amanitin-sensitive ribonucleic acid polymerase and stimulatory factors from HeLa and KB cells. J Biol Chem. 1973 Jun 10;248(11):3777–3788. [PubMed] [Google Scholar]
- Tal J., Craig E. A., Zimmer S., Raskas H. J. Localization of adenovirus 2 messenger RNA's to segments of the viral genome defined by endonuclease R-R1. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4057–4061. doi: 10.1073/pnas.71.10.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XV. Transcription of the adenovirus type II genome during productive infection. Virology. 1969 Oct;39(2):205–210. doi: 10.1016/0042-6822(69)90040-3. [DOI] [PubMed] [Google Scholar]
- Wall R., Philipson L., Darnell J. E. Processing of adenovirus specific nuclear RNA during virus replication. Virology. 1972 Oct;50(1):27–34. doi: 10.1016/0042-6822(72)90342-x. [DOI] [PubMed] [Google Scholar]
- Wallace R. D., Kates J. State of adenovirus 2 deoxyribonucleic acid in the nucleus and its mode of transcription: studies with isolated viral deoxyribonucleic acid-protein complexes and isolated nuclei. J Virol. 1972 Apr;9(4):627–635. doi: 10.1128/jvi.9.4.627-635.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Raskas H. J., Roeder R. G. Role of DNA-dependent RNA polymerases II and III in transcription of the adenovirus genome late in productive infection. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3426–3439. doi: 10.1073/pnas.71.9.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Roeder R. G. Role of DNA-dependent RNA polymerase 3 in the transcription of the tRNA and 5S RNA genes. Proc Natl Acad Sci U S A. 1974 May;71(5):1790–1794. doi: 10.1073/pnas.71.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. Products of RNA polymerases in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2861–2865. doi: 10.1073/pnas.68.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]


