Abstract
Background
The aim of this study was to investigate the effect of marine n-3 polyunsaturated fatty acids (PUFA) on cardiac autonomic function and vascular function in patients with psoriatic arthritis.
Methods
The study was conducted as a randomized, double-blind, placebo-controlled trial, where 145 patients with psoriatic arthritis were supplemented with 3 g of n-3 PUFA or olive oil (control) daily for 24 weeks. Blood pressure, heart rate, heart rate variability (HRV), central blood pressure, pulse wave velocity (PWV) and fatty acid composition of granulocytes, were determined at baseline and after supplementation.
Results
At baseline we found a significant difference in the mean of all normal RR intervals (inverse of heart rate, vary from beat to beat) when comparing subjects with the highest vs the lowest fish intake (p = 0.03). After supplementation for 24 weeks there was a trend towards an increase in RR (p = 0.13) and decrease in heart rate (p = 0.12) comparing the n-3 PUFA group with the control group. However, per-protocol analysis showed significantly increased RR (p = 0.01) and lowered heart rate (p = 0.01) in the n-3 PUFA supplemented patients compared with controls. Blood pressure, PWV and Central blood pressure did not change after supplementation with n-3 PUFA. Adjustment for disease activity and conventional cardiovascular risk factors did not change the results.
Conclusions
Marine n-3 PUFA increased RR intervals in patients with psoriatic arthritis which may suggest a protective effect of n-3 PUFA against cardiovascular disease in this population.
Trial registration
Clinicaltrials.gov Identifier: NCT01818804
Keywords: Psoriatic arthritis, n-3 PUFA, Heart rate variability, Cardiac autonomic function, Pulse wave velocity, Arterial stiffness
Background
Psoriatic arthritis (PsA) is a chronic inflammatory joint disease occurring in 6–39% of patients with psoriasis and with a prevalence in the general population of approximately 0.2% [1–3].
Patients with inflammatory diseases such as rheumatoid arthritis and systemic lupus erythematosus are at an increased risk of cardiovascular disease (CVD) [4] and recent observational studies indicate that PsA patients also have an increased cardiovascular mortality and morbidity [5, 6]. Accelerated atherosclerosis due to inflammation, and autonomic dysfunction can both play a role in the pathogenesis of CVD in patients with PsA in addition to conventional risk factors for CVD such as smoking, hypertension, hypercholesterolemia and diabetes mellitus [7].
Several studies have indicated that chronic inflammation may impair autonomic cardiac regulation leading to a decrease in heart rate variability (HRV) [8, 9]. A low HRV has been identified as an independent predictor of coronary heart disease [10], as well as malignant ventricular arrhythmias and sudden cardiac death [11–15]. Also, arterial stiffness has been recognised as an independent predictor of CVD [16, 17]. Studies using non-invasive methods such as pulse wave velocity (PWV) for evaluation of CVD risk have revealed increased arterial stiffness in patients with PsA [18–20].
A beneficial effect of marine n-3 polyunsaturated fatty acids (PUFA) on CVD has been suggested from several epidemiological studies, experimental data and clinical trials [21]. Interestingly, the major marine n-3 PUFA, eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), have been shown to have anti-inflammatory effects [22] and beneficial effects on blood pressure (BP) and cardiac autonomic function [23, 24].
The present study aimed to examine whether supplementation with a moderate to high (3 g) daily dose of marine n-3 PUFA for 24 weeks had a beneficial effect on cardiac autonomic and hemodynamic function represented by BP, heart rate (HR), HRV, PWV and central BP in patients with PsA.
Methods
Study design
The study was designed as a randomized, double-blind, placebo-controlled trial. The patients were randomly assigned in blocks of five by a computer-generated block sequence. For 24 weeks patients were assigned to daily intake of six capsules containing either 3 g of n-3 PUFA (50% EPA and 50% DHA) or 3 g of olive oil (approximately 80% of oleic acid and 20% linoleic acid). Investigators, patients and research staff were blinded to the supplementation codes. Patients were asked to maintain their usual diet during the entire study. The study was conducted in a 2 year period between 2013 and 2015. Clinical assessment, blood samples, HRV and PWV were carried out at baseline and after 24 weeks. The study was conducted in accordance with the Declaration of Helsinki and registered at ClinicalTrials.gov (NCT01818804). Furthermore the study was monitored by Good Clinical Practice (GCP) inspectors and the GCP ethical and scientific quality requirements were followed.
Subjects
Patients with PsA defined by Classification criteria for psoriatic arthritis (CASPAR) [25] were enrolled from the Department of Rheumatology, Aalborg University Hospital and Department of Rheumatology, Vendsyssel Hospital, in Denmark. The inclusion criteria were PsA in adults above 18 years of age with any disease activity while exclusion criteria were documented known cardiac arrhythmias, treatment with biological drugs, or treatment with oral corticosteroids. Patients using fish oil supplements (n = 2) underwent a wash-out period of at least 8 weeks before inclusion.
Compliance was assessed by counting capsules during the last visit. Patients were defined as non-compliant if missing >15% of capsules and these patients were not included in the per-protocol analysis.
All participants gave their written informed consent and the regional ethics committee of Northern Region DK, approved the study (reference number N20120076).
Clinical assessment
At baseline, duration of PsA, medical history, smoking habits and diets were obtained. Medical history of diabetes mellitus, hypertension and dyslipidemia was assessed and was defined as present if the patient received dietary or medical therapy for the condition. A food questionnaire was used to assess patients’ fish consumption at lunch and dinner. A score for fish intake was given according to the following: never eating fish = 1; eating fish once a month =2; eating fish two to three times a month = 3; eating fish once a week = 4; eating fish two to three times a week = 5; and eating fish at least once daily = 6.
At both visits conventional cardiovascular risk factors such as smoking habits, BP, body mass index (BMI) and waist to hip ratio (WHR) were assessed. Additionally, a clinical evaluation was performed, consisting of 68 tender joint count, 66 swollen joint count, disease activity score (DAS66/68) and psoriatic skin area involvement (PASI).
Blood samples
Blood samples were taken non-fasting for assessment of fatty acid composition of granulocytes and routine laboratory evaluation including plasma levels of C-reactive protein (CRP). Granulocytes were isolated from whole blood and their fatty acid composition were determined by gas chromatography with a Chrompack CP-9002 gas chromatograph (Varian, Middelberg, The Netherlands) and expressed as weight percent (wt %) of total fatty acids. The following fatty acids were evaluated in the further analyses: eicosapentaenoic acid (EPA; 20:5n-3), docosahexaenoic acid (DHA; 22:6n-3), docosapentaenoic acid (DPA; 22:5n-3), arachidonic acid (AA; 20:4n-6), oleic acid (18:1n-9), linoleic acid (LA; 18:2n-6), palmitic acid (16:0) and stearic acid (18:0).
HRV
Five min. HRV recordings were obtained with SphygmoCor Technology (SphygmoCor, Software version 8.2; AtCor Medical, Sydney, NSW, Australia) in each patient. HRV was recorded according to current recommendations [26] with measurements obtained in the morning hours after resting for 15 min in a room with a constant temperature of 20 °C. Patients were instructed not to smoke and avoid alcohol and caffeine-containing beverages within 12 h prior to investigation. A trained technician blinded to the type of supplement performed these analyses. The patients were in a supine position (resting) for 10 min, breathing spontaneously without talking. HRV were analysed in the time-domain and the following variables were obtained:
HR: heart rate
RR: mean of all normal RR intervals during the 5 min recording (inverse of heart rate, vary from beat to beat)
SDNN: standard deviation of all normal RR intervals in the 5 min recording
SDNNindex: mean of the standard deviation of all the normal RR intervals
pNN50: percentage of successive RR-interval differences > 50 ms
RMSSD: square root of the mean of the sum of the squares of differences between adjacent intervals
PWV
PWV and pulse wave analysis were performed non-invasively with the Sphygmocor system (AtCor Medical, Sydney, NSW, Australia), as described previously [27] and according to international recommendations [28]. All measurements were made in duplicate by a single trained operator and the mean of the two values was used in the analysis. Carotid-radial and carotid-femoral PVW were measured using arterial tonometry. The measurements were obtained under the same conditions as described under HRV. The patients were in a supine position and all measurements were performed on the right side extremities. The surface distance was measured with a tape measure as a straight line from the suprasternal notch to the carotid location (proximal pulse) and subtracted the distance from the suprasternal notch to the radial or femoral location (distal pulse) [29, 30]. The pressure wave transit time was determined as the time between the R-wave of the ECG and the proximal pulse subtracted from the time between the R-wave of the ECG and the distal pulse. PWV was subsequently calculated by dividing the surface distance by the pressure wave transit time.
The central BP was estimated using the SphygmoCor® device. After 10 min of rest in the supine position, brachial BP was measured three times at 2-min intervals on the left arm with an automatic Microlife® device, and the last measurement was taken as representative of brachial artery BP. Hereafter, radial artery pressure waveforms of the right arm were sampled. Using the validated generalized transfer function, central BP was estimated using brachial systolic and diastolic BP [31, 32]. Aortic augmentation index (AIx) was standardized to a HR of 75 beat per minute to minimize the effect of HR.
Statistical Analysis
All statistical analyses were performed using Stata: Release 13 (StataCorp LP, TX, US). HRV measurements were primary outcome and PWV measurements secondary outcome.
We hypothesized that intervention with n-3 PUFA would increase RR. Based on previous literature [33, 34] and to achieve α = 0.05 and 1-β = 0.80 we needed a sample size with 63 subjects in each group.
The score for fish intake at baseline was grouped according to tertiles. The content of DHA and EPA in granulocytes were also grouped according to tertiles.
The difference in the continuous outcomes between baseline and 24 weeks after randomization was compared between the two treatment groups in a one-way analysis of variance (ANOVA). Equality of variances between the treatment groups was assessed using Bartlett’s test. Due to the potential for confounding after the random treatment assignment an analysis of covariance (ANCOVA) was performed. Prior to this analysis a check for collinearity between the covariates was performed and the model was modified accordingly. In this model equality of variances was assessed using Levene’s test. All analyses were performed both as intention to treat and per-protocol (patients who completed the entire clinical trial according to the protocol and consumed > 85% of the assigned supplement) analyses. The ANCOVA analyses were controlled for age, sex, smoking status, presence or absence of diabetes mellitus, hypertension and hypercholesterolemia, use of nonsteroidal anti-inflammatory drugs (NSAID) and DAS66/68.
Differences were considered significant with a p-value of <0.05 (two-tailed).
Results
A total of 145 patients were enrolled and 133 patients (92%) completed the study. Seven patients in the control arm and five patients in the n-3 PUFA arm withdrew from the study before last visit and five patients were excluded because of insufficient HRV recordings at baseline. Ten patients in the n-3 PUFA group and four in the control group were defined as noncompliant according to the number of capsules returned and were not included in the per-protocol analysis. Figure 1 shows the study flow diagram.
Fig. 1.
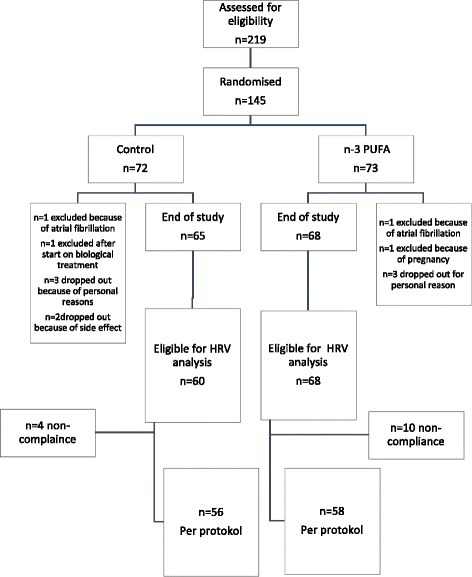
Flow diagram of the study participants
The n-3 PUFA and the control groups were comparable regarding baseline characteristics, apart from number of current smokers and number of patients with known hypercholesterolemia (Table 1). Thus, adjustments were made for these to parameters in the final analysis. Patients excluded from final analysis did not differ from patients eligible for final analysis in regard to baseline characteristics (data not shown). Nine participants in the n-3 PUFA supplemented group and six participants in the control group reported mild gastrointestinal adverse effect. In 13 of these participants the side effects was avoided by changing the intake of capsules from six capsules once daily to two capsules three times daily. The remaining two participants were in the placebo group and dropped out because of nausea and mild diarrhea.
Table 1.
Baseline demographics, biochemical and hemodynamic characteristics of the 143 patients with psoriatic arthritis
| n-3 PUFA (N = 72) | Control (N = 71) | Total (N = 143) | ||
|---|---|---|---|---|
| Covariates | Age | |||
| Mean age | 53.2 (11.4) | 50.7 (11.5) | 52.0 (11.5) | |
| Sex | ||||
| - Female | 40 (55.6) | 43 (60.6) | 83 (58.0) | |
| - Male | 32 (44.4) | 28 (39.4) | 60 (42.0) | |
| Smoking | ||||
| - Non-smoker | 36 (50.0) | 41 (57.7) | 77 (53.8) | |
| - Former smoker | 27 (37.5) | 14 (19.7) | 41 (28.7) | |
| - Current smoker | 9 (12.5) | 16 (22.5) | 25 (17.5) | |
| Documented coronary heart disease | 4 (5.6) | 4 (5.6) | 8 (5.6) | |
| Hypertension | 21 (29.2) | 20 (28.2) | 41 (28.7) | |
| Hypercholesterolemia | 18 (25.0) | 11 (15.5) | 29 (20.3) | |
| NSAID use | 40 (55.6) | 32 (45.1) | 72 (50.3) | |
| Outcomes | Systolic BP, mmHg | 138 (18) | 134 (19) | 136 (19) |
| Diastolic BP, mmHg | 83 (11) | 82 (12) | 82 (12) | |
| Hip and waste ratio | 1.1 (0.1) | 1.2 (0.1) | 1.1 (0.1) | |
| Body Mass Index | 28.6 (5.7) | 28.0 (5.0) | 28.3 (5.4) | |
| Disease Activity Score | 2.5 (0.9) | 2.7 (0.9) | 2.6 (0.9) | |
| CRP mg/l | 4.6 (4.2) | 6.1 (7.7) | 5.3 (6.2) | |
| PASI | 2.2 (3.0) | 2.3 (4.0) | 2.3 (3.5) | |
| Total cholesterol, mmol/l | 5.0 (1.0) | 4.8 (0.8) | 4.9 (0.9) |
Data are given as mean (sd) or n (%) as appropriate
N non-missing values, BP Blood pressure
Baseline analyses
Patients with the highest fish intake had a significantly higher RR than patients with the lowest intake (p = 0.03). Also, patients in the tertile with the highest content of DHA in granulocytes had the highest RR (p = 0.04) whereas the content of EPA in granulocytes was not associated with RR. The associations between dietary fish intake and RR seemed to be dose dependent (Figs. 2 and 3). There were no association between DAS66/68 and RR at baseline.
Fig. 2.
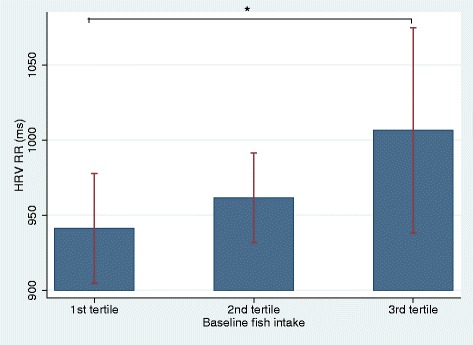
The relation between baseline RR (ms) and fish intake presented in tertiles. Footnote: RR: Mean of all normal RR-intervals in HRV recording; *: Significant difference in RR between the lower and the upper tertile
Fig. 3.
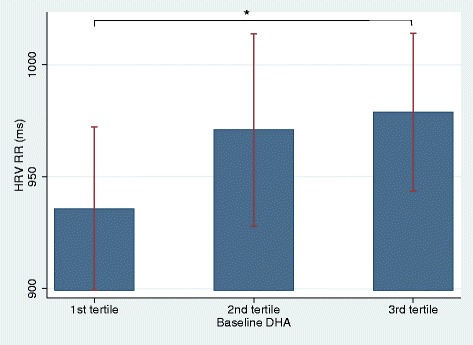
The relation between baseline RR (ms) and content of DHA in granulocytes presented in tertiles. Footnote: HRV: Heart Rate Variability; RR: Mean of all normal RR-intervals in HRV recording; DHA: docosahexaenoic acid; *: Significant difference in RR between the lower and the upper tertile
Intervention analyses
The group randomized to n-3 PUFA had a significant increase in total content of n-3 PUFA (DHA + EPA + DPA), DHA and EPA in granulocytes from baseline to study end compared to the control group. There were also a significant decrease in the granulocyte content of AA and LA in the n-3 PUFA supplemented group compared to control group (Table 2).
Table 2.
Content of fatty acids in granulocytes presented as means with 95% confidence intervals at baseline and after 24 weeks of supplementation for both groups
| n-3 PUFA (n = 68) | Control (n = 65) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 24 | Difference (CI) | Baseline | Week 24 | Difference (CI) | P | |
| Palmitic acid (16:0) wt.% | 12.32 | 12.40 | 0.08 (−0.04 - 0.21) | 12.38 | 12.37 | −0.00 (−0.12 - 0.12) | 0.33 |
| Stearic acid (18:0) wt % | 17.36 | 17.35 | −0.01 (−0.12 - 0.11) | 17.41 | 17.30 | −0.11 (−0.24 - 0.01) | 0.20 |
| Oleic acid (18:1n-9) wt.% | 32.90 | 33.21 | 0.30 (0.03; 0.57) | 32.97 | 33.44 | 0.47 (0.24; 0.70) | 0.35 |
| Linoleic acid (18:2n-6) wt. % | 9.12 | 8.62 | −0.50 (−0.80; −0.21) | 9.32 | 9.21 | −0.11 (−0.26; 0.05) | 0.02 |
| AA (20:4n-6) wt % | 13.05 | 11.44 | −1.61 (−1.84; −1.38) | 13.01 | 12.80 | −0.20 (−0.37; −0.04) | <0.01 |
| Total n-6 wt % | 22.17 | 20.05 | −2.12 (−2.47 - -1.76) | 22.32 | 22.01 | −0.31 (−0.51 - -0.11) | <0.01 |
| EPA wt % | 0.60 | 1.99 | 1.40 (1.24; 1.56) | 0.53 | 0.50 | −0.03 (−0.08; 0.02) | <0.01 |
| DPA wt % | 1.58 | 2.61 | 1.03 (0.86; 1.21) | 1.46 | 1.42 | −0.04 (−0.10; 0.02) | <0.01 |
| DHA wt % | 1.21 | 2.10 | 0.89 (0.77; 1.00) | 1.14 | 1.11 | −0.02 (−0.07; 0.02) | <0.01 |
| Total n-3 PUFA (DHA + EPA + DPA) wt % | 3.39 | 6.71 | 3.32 (2.92; 3.71) | 3.13 | 3.03 | −0.10 (−0.22; 0.03) | <0.01 |
AA arachidonic acid, DHA docosahexaenoic acid, EPA eicosapentaenoic acid, DPA docosapentaenoic acid, wt. % weight percent, P P for the difference between the two groups of supplement
Intention to treat analysis
After supplementation for 24 weeks there was a trend towards an increase in RR (p = 0.06) and a decrease in HR (p = 0.12) comparing the n-3 PUFA group with the control group (Table 3).
Table 3.
Intention to treat data with no adjustments
| n-3 PUFA (n = 68) | Control (n = 60) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 24 | Difference (CI) | Baseline | Week 24 | Difference (CI) | P | |
| Heart rate, min−1 | 63.83 | 63.29 | −0.61 (−1.92; 0.70) | 63.39 | 64.38 | 0.96 (−0.55; 2.47) | 0.12 |
| RR, ms | 956.55 | 969.94 | 13.38 (−5.06; 31.83) | 964.02 | 950.53 | −13.48 (−35; − 8.69) | 0.06 |
| PNN50 % | 10.68 | 11.29 | 0.62 (−3.25; 4.48) | 15.83 | 14.37 | −1.46 (−4.76; 1.84) | 0.42 |
| SDNN ms | 49.37 | 48.12 | −1.24 (−8.46; 5.97) | 49.71 | 47.41 | −2.30 (−6.79; 2.20) | 0.81 |
| RMSSD ms | 36.59 | 37.58 | 0.99 (−7.13; 9.12) | 39.84 | 39.15 | −0.69 (−6.23; 4.85) | 0.73 |
| Peripheral systolic BP mmHg | 138.20 | 134.53 | −3.67 (−6.69; −0.65) | 134.41 | 133.18 | −1.23 (−4.59; 2.14) | 0.28 |
| Peripheral diastolic BP mmHg | 82.61 | 81.82 | −0.79 (−2.35; 0.77) | 82.36 | 80.92 | −1.44 (−3.26; 0.39) | 0.59 |
| PWV m/s | 7.80 | 7.81 | 0.01 (−0.44; 0.46) | 7.40 | 7.48 | 0.08 (−0.33; 0.49) | 0.82 |
| Central systolic BP mmHg | 114.82 | 112.24 | −2.58 (−4.84; −0.32) | 113.29 | 111.38 | −1.91 (−4.77; 0.95) | 0.71 |
| Central diastolic BP mmHg |
96.04 | 93.76 | −2.28 (−4.10; −0.47) | 95.45 | 94.17 | −1.29 (−3.51; 0.94) | 0.49 |
| PWA AIx | 26.42 | 27.54 | 1.12 (−0.56; 2.80) | 26.97 | 25.82 | −1.15 (−2.78; 0.47) | 0.05 |
Outcomes presented as means with 95% confidence intervals at baseline and after 24 weeks of supplement for both groups
CI Confidence Interval, P P for difference between the two groups of supplement, HRV Heart rate variability, PWV Pulse wave velocity, BP blood pressure, AIx central Augmentation Index
There were no significant change in BP, PWV or central BP in the n-3 PUFA supplemented group or between the n-3 PUFA and control group. Adjustment for conventional CVD risk factors and DAS66/68 did not affect the results.
DAS66/68 and CRP were not associated with HRV or PWV.
Per-protocol analysis
Analyses of outcomes after 24 weeks revealed a significant increase in RR and decrease in HR in the fish oil supplemented group and there was a significant difference in changes in RR (p = 0.03) and HR (0.02) between the n-3 PUFA and control group (Table 4). In contrast, there were no significant change in BP, PWV or central BPs in the n-3 PUFA supplemented group or between the n-3 PUFA and control group. Adjustment for conventional CVD risk factors and DAS66/68 did not change the results (Table 5).
Table 4.
Per-protocol data with no adjustments
| n-3 PUFA (n = 58) | Control (n = 56) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 24 | Difference (CI) | Baseline | Week 24 | Difference (CI) | P | |
| Heart rate | 63.24 | 61.73 | −1.51 (−2.89; −0.13) | 63.39 | 64.38 | 0.98 (−0.54; 2.50) | 0.02 |
| RR ms | 964.14 | 990.39 | 26.25 (6.21; 46.30) | 964.02 | 950.53 | −13.48 (−35.66; 8.69) | 0.01 |
| PNN50 % | 11.39 | 11.65 | 0.26 (−4.06; 4.58) | 15.83 | 14.37 | −1.46 (−4.76; 1.84) | 0.52 |
| SDNN ms | 51.56 | 49.36 | −2.20 (−9.96; 5.56) | 49.71 | 47.41 | −2.30 (−6.79; 2.20) | 0.98 |
| RMSSD ms | 39.10 | 37.85 | −1.25 (−9.41; 6.91) | 39.84 | 39.15 | −0.69 (−6.23; 4.85) | 0.91 |
| Peripheral BP Systolic mmHg | 137.67 | 134.29 | −3.38 (−7.03; 0.26) | 134.41 | 133.18 | −1.23 (−4.59; 2.14) | 0.39 |
| Peripheral BP Diastolic mmHg | 81.96 | 80.88 | −1.08 (−2.89; 0.74) | 82.36 | 80.92 | −1.44 (−3.26; 0.39) | 0.78 |
| PWV m/s | 7.66 | 7.61 | −0.04 (−0.51;- 0.43) | 7.40 | 7.48 | 0.08 (−0.33; 0.49) | 0.70 |
| Central BP systolic mmHg | 114.62 | 112.17 | −2.44 (−5.17; 0.28) | 113.29 | 111.38 | −1.91 (−4.77; 0.95) | 0.79 |
| Central BP diastolic mmHg | 95.50 | 93.13 | −2.37 (−4.49; −0.24) | 95.45 | 94.17 | −1.29 (−3.51; 0.94) | 0.49 |
| PWA AIx | 26.42 | 27.51 | 1.12 (−0.56; 2.80) | 27.02 | 25.84 | −1.15 (−2.78; 0.47) | 0.06 |
Outcomes presented as means with 95% confidence intervals at baseline and after 24 weeks of supplement for both groups
CI Confidence Interval, P P for difference between the two groups of supplement, PWV Pulse wave velocity, BP blood pressure, AIx central Augmentation Index
Table 5.
Per-protocol analysis
| Estimated effect (95% CI) | F statistic | P | N | |
|---|---|---|---|---|
| RR ms | 35.26 (3.00 to 67.53) | 4.70 | 0.03 | 114 |
| PNN50 % | 1.81 (−3.99 to 7.61) | 0.38 | 0.54 | 114 |
| SDNN ms | −0.05 (−9.49 to 9.39) | 0.00 | 0.99 | 114 |
| RMSSD ms | 1.35 (−9.06 to 11.76) | 0.07 | 0.80 | 114 |
| Peripheral systolic BP, mmHg | −1.48 (−6.25 to 3.29) | 0.38 | 0.54 | 117 |
| Peripheral diastolic BP, mmHg | 0.26 (−2.45 to 2.96) | 0.03 | 0.85 | 117 |
| Pulse Wave Velocity, m/s | −0.15 (−0.83 to 0.54) | 0.18 | 0.67 | 111 |
| Central systolic BP mmHg | −0.90 (−5.23 to 3.44) | 0.17 | 0.68 | 117 |
| Central diastolic BP mmHg | −1.57 (−4.94 to 1.79) | 0.86 | 0.36 | 117 |
| PWA AIx | 2.91 (0.12 to 5.71) | 4.26 | 0.04 | 117 |
ANCOVA analysis of differences in outcomes from baseline to 24 weeks comparing n-3 PUFA to control. Analysis are controlled for age, sex, smoking, diabetes mellitus, hypertension, blood pressure, hypercholesterolemia, NSAID treatment and Disease activity scores. N are numbers of patients
CI Confidence Interval, HRV Heart rate variability, BP Blood pressure, PWV Pulse wave velocity, AIx central Augmentation Index
Discussion
With 145 participants and only 8% not completing, this study is the largest investigation of the effect of marine n-3 PUFA on cardiovascular function in patients with PsA. We found that supplementation with 3 g n-3 PUFA daily for 24 weeks had a beneficial effect on autonomic control of the heart in PsA patients by decreasing heart rate and increasing RR interval.
At baseline we found a significantly higher RR in the patients with the highest fish intake. Baseline analysis also showed that the content of DHA in granulocytes was positively associated with RR. These associations seemed to be dose dependent and the results suggest beneficial effect of dietary fish consumption on the cardiac autonomic tone in patients with PsA.
After intervention for 24 weeks there was a significant shift in the PUFA composition in the active group with an increase in the granulocyte content of n-3 PUFA and a decrease in content of AA and LA. In the intention to treat analysis there was a trend towards increased RR and thereby a reciprocal lowering of HR. However, the per-protocol analysis revealed a significant increase in RR and decrease in HR in the n-3 PUFA supplemented group.
HRV is considered a useful and reliable measurement of cardiac autonomic tone [35]. A depressed HRV indicates an increased cardiovascular risk in the general population [11] and in patients with known CVD [36]. Evidence also suggests that HRV improves with fish consumption and intervention with n-3 PUFA [37]. The positive association between fish intake and RR at baseline and the increase in RR after intervention with n-3 PUFA found in this study of patients with PsA is in line with previous studies of other high-risk patients and healthy subjects [38]. The effect of n-3 PUFA on HR in our study is also consistent with previous data showing that n-3 PUFA reduces resting HR [39, 40], an important risk marker for cardiovascular disease [41]. As a surrogate marker for cardiac autonomic tone, HRV can indicate changes mediated by n-3 PUFA at the level of cardiac efferent stimuli [42, 43]. Interestingly, in our study baseline content of DHA but not EPA in granulocytes was positively associated with RR, which is consistent with findings of previous studies [44, 45]. DHA is most abundant in the heart, brain and nervous system membrane lipids [46] and therefore might be more influential regarding effects on cardiac autonomic function.
Interactions between the autonomic nervous system and the immune system have been reviewed recently and direct autonomic innervation and non-synaptic communication with lymphoid organs has been shown [47]. Abnormalities in the autonomic nervous system as well as cardiovascular autonomic dysfunction has been reported in patients with inflammatory rheumatic diseases [48]. A few studies have investigated HRV in PsA and demonstrated attenuated HRV. In a study with 38 patients with PsA and 25 healthy controls using 5-min HRV Gaydukova et al. [49] found that SDNN was 65.1 ms and pNN50 12.9% in the PsA patients whereas in healthy controls SDNN and pNN50 were higher (83.2 ms and 20.6%, respectively). Proietti et al. [50] also used short-term HRV in 26 patients with psoriasis and 27 healthy controls and found a significant difference in RMSDD between patients with psoriasis (39.4 ms) and the controls (53.2 ms). Our results revealed a baseline SDNN of 50 ms, pNN50 of 13%, and RMSSD of 38 ms, supporting an attenuation of HRV in patients with PsA. A likely explanation for a low HRV in PsA is the presence of systemic inflammation leading to a decreased parasympathetic regulation of cardiac autonomic tone [51, 52]. In this study, DAS66/68 and CRP were not associated with HRV or PWV. However, DAS and CRP may be a more important marker of disease activity in patients with RA [53] than in patients with PsA [47, 54, 55].
In a small study with 20 patients with PsA, Syngle et al. observed improvement in autonomic dysfunction after treatment with different synthetic DMARDs over 12 weeks [33]. However, no treatment strategy for cardiac autonomic dysfunction in PsA has yet been demonstrated. Thus, the possible beneficial effect of n-3 PUFA found in our study may be of importance in the approach towards an improved cardiac autonomic function in PsA.
n-3 PUFA is known to have a mildly antihypertensive effect [56]. Furthermore, studies and a meta-analysis of randomized and controlled human clinical trials examining the effect of n-3 PUFA on arterial stiffness has shown a reduction in arterial stiffness after treatment with less than 4 g/d of n-3 PUFA in populations with hypertension, diabetes mellitus, dyslipidemia, metabolic syndrome and obesity [57, 58].
In our study we were unable to demonstrate any changes in BP, PWV, central BP and AIx measurements. However, in most of the previous studies arterial stiffness was not assessed with carotid-femoral PWV (regarded as “golden standard”) as in our study. Supplementation with n-3-PUFA for 24 weeks might be too short a period to observe changes and modulation in the vessels measured by PWV. Patients in this study had a low mean disease activity score of 2.6 (sd = 0.9) at baseline and 75% of the patients received disease-modifying antirheumatic drugs. Thus, we investigated a patient group in remission and results may not apply to patients with a more severely disease activity. In other chronic inflammatory diseases, such as RA and systemic vasculitis, PWV and AIx were increased compared to controls, but only in patients with active disease [59, 60].
Limitation of the study
Five minutes HRV was used to assess the function of the autonomic nervous system. This method limits the measurement of vagal predominance during nighttime. However, other studies assessing patients with PsA have also obtained 5 min HRV with results comparable to our findings.
The difference in HRV in the intention to treat and per-protocol results might be explained by non-compliance. Compliance and adherence to the study protocol might have been improved with administration of more concentrated capsules of n-3 PUFA resulting in fewer capsules per day for the patients.
Olive oil has often been used as control oil in studies investigating the effects of fish oil in patients with arthritis but olive oil itself may have some effect on inflammation [61]. However, marine n-3 PUFA have been shown to be superior to olive oil in several studies of CVD [37] and olive oil had no anti-arrhythmic effect in previous studies [62, 63]. The mean intake of monounsaturated fat in Denmark is 36 g/d [64] and therefore, adding 3 g/d of olive oil would not be expected to have a substantial effect on the results. Also, we found no significant changes in the content in granulocytes of oleic acid and linoleic acid (main components of olive oil) in the control group after 24 weeks of supplementation (Table 2).
Conclusion
In conclusion, our study demonstrated a beneficial effect of n-3 PUFA on RR and HR in a well-characterized group of patients with PsA. The results may indicate a beneficial effect of n-3 PUFA on cardiac autonomic tone in patients with PsA and further large-scale studies are needed to demonstrate whether this translates into a reduction of CVD in these patients.
Acknowledgements
We express our gratitude to the patients for participating in this study. We thank C. M. Skov, K. Holdensen, V. Mellergaard, R. B. Eschen, A. Andreasen and B. Thomsen for invaluable laboratory assistance and excellent work participating in the examinations.
Funding
The study was supported by Aalborg University Hospital Research Foundation, The Medical Research Foundation of the Northern Region, Denmark, The Danish Rheumatism Association, The Danish Psoriasis Foundation, The Aage Bang Foundation, Abbvie Foundation, Heinrich Kopps Foundation and Jacob Madsen and wife Olga Madsen Foundation.
The capsules of marine n-3 PUFA and olive oil were kindly delivered by Marine Pharma, Norway.
Availability of data and materials
The datasets generated during the current study are available in the Clinical Trials, www.clinicaltrials.gov Identifier: NCT01818804.
Authors’ contributions
SK designed and coordinated the study, collected data, performed statistical analysis and drafted and revised the manuscript. JHC, EBS, and AS participated in the study design and data interpretation and were involved in critically revising the manuscript for important intellectual content. CR participated in the study design and coordination and helped to revise the manuscript. EL helped collecting data and revising the manuscript. MBJ contributed to the study design and helped with the statistical analysis. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All participants gave their written informed consent and the regional ethics committee of Northern Region DK, approved the study (reference number N20120076).
Abbreviations
- AA
Arachidonic acid
- AI
Augmentation index
- AIx
Aortic augmentation index
- ANCOVA
Analysis of covariance
- ANOVA
One-way analysis of variance
- BMI
Body mass index
- BP
Blood pressure
- CASPAR
Classification criteria for psoriatic arthritis
- CI
Confidence intervals
- CRP
C-reactive protein
- CVD
Cardiovascular disease
- DAS
Disease activity score
- DHA
Docosahexaenoic acid
- DMARD
Disease-modifying antirheumatic drugs
- DPA
Docosapentaenoic acid
- EPA
Eicosapentaenoic acid
- GCP
Good clinical practice
- HR
Heart rate
- HRV
Heart rate variability
- LA
Linoleic acid
- n-3 PUFA
n-3 polyunsaturated fatty acids
- NSAID
Nonsteroidal anti-inflammatory drugs
- PASI
Psoriatic skin area involvement
- pNN50
Percentage of successive RR-interval differences > 50 ms
- PsA
Psoriatic arthritis
- PWV
Pulse wave velocity
- RMSSD
Square root of the mean of the sum of the squares of differences between adjacent intervals
- RR
Mean of all normal RR intervals during the 5 min recording
- SD
Standard deviation
- SDNN
Standard deviation of all normal RR intervals in the 5 min recording
- SDNNindex
Mean of the standard deviation of all the normal RR intervals
Contributor Information
Salome Kristensen, Phone: +4597664015, Email: sakr@rn.dk.
Erik Berg Schmidt, Email: ebs@rn.dk.
Annette Schlemmer, Email: a.schlemmer@rn.dk.
Claus Rasmussen, Email: clara@rn.dk.
Esther Lindgreen, Email: esli@rn.dk.
Martin Berg Johansen, Email: martin.johansen@rn.dk.
Jeppe Hagstrup Christensen, Email: jeppe.hagstrup.christensen@rn.dk.
References
- 1.Gelfand JM, Gladman DD, Mease PJ, Smith N, Margolis DJ, Nijsten T, Stern RS, Feldman SR, Rolstad T. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53(4):573–7. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 2.Shbeeb M, Uramoto KM, Gibson LE, O’Fallon WM, Gabriel SE. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982–1991. J Rheumatol. 2000;27(5):1247–50. [PubMed] [Google Scholar]
- 3.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61(2):233–9. doi: 10.1002/art.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33(11):2167–72. [PubMed] [Google Scholar]
- 5.Ahlehoff O, Gislason GH, Charlot M, Jorgensen CH, Lindhardsen J, Olesen JB, Abildstrom SZ, Skov L, Torp-Pedersen C, Hansen PR. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270(2):147–57. doi: 10.1111/j.1365-2796.2010.02310.x. [DOI] [PubMed] [Google Scholar]
- 6.Gladman DD, Ang M, Su L, Tom BD, Schentag CT, Farewell VT. Cardiovascular morbidity in psoriatic arthritis. Ann Rheum Dis. 2009;68(7):1131–5. doi: 10.1136/ard.2008.094839. [DOI] [PubMed] [Google Scholar]
- 7.Kimhi O, Caspi D, Bornstein NM, Maharshak N, Gur A, Arbel Y, Comaneshter D, Paran D, Wigler I, Levartovsky D, Berliner S, Elkayam O. Prevalence and risk factors of atherosclerosis in patients with psoriatic arthritis. Semin Arthritis Rheum. 2007;36(4):203–9. doi: 10.1016/j.semarthrit.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Bruchfeld A, Goldstein RS, Chavan S, Patel NB, Rosas-Ballina M, Kohn N, Qureshi AR, Tracey KJ. Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis. J Intern Med. 2010;268(1):94–101. doi: 10.1111/j.1365-2796.2010.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011;269(1):45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annu Rev Med. 1999;50:249–61. doi: 10.1146/annurev.med.50.1.249. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–5. doi: 10.1161/01.CIR.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 12.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI. Lancet. 1998;351(9101):478–84. doi: 10.1016/S0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz PJ. The autonomic nervous system and sudden death. Eur Hear J. 1998;19(Suppl F):F72–80. [PubMed] [Google Scholar]
- 14.Barron HV, Lesh MD. Autonomic nervous system and sudden cardiac death. J Am Coll Cardiol. 1996;27(5):1053–60. doi: 10.1016/0735-1097(95)00615-X. [DOI] [PubMed] [Google Scholar]
- 15.Airaksinen KEJ. Autonomic mechanisms and sudden death after abrupt coronary occlusion. Ann Med. 1999;31(4):240–5. doi: 10.3109/07853899908995886. [DOI] [PubMed] [Google Scholar]
- 16.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–41. doi: 10.1161/01.HYP.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 17.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113(5):664–70. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 18.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 19.Gisondi P, Fantin F, Del Giglio M, Valbusa F, Marino F, Zamboni M, Girolomoni G. Chronic plaque psoriasis is associated with increased arterial stiffness. Dermatology. 2009;218(2):110–3. doi: 10.1159/000182256. [DOI] [PubMed] [Google Scholar]
- 20.Costa L, Caso F, D’Elia L, Atteno M, Peluso R, Del Puente A, Strazzullo P, Scarpa R. Psoriatic arthritis is associated with increased arterial stiffness in the absence of known cardiovascular risk factors: A case control study. Clin Rheumatol. 2012;31(4):711–5. doi: 10.1007/s10067-011-1892-1. [DOI] [PubMed] [Google Scholar]
- 21.De Caterina R. N-3 Fatty Acids in Cardiovascular Disease. N Engl J Med. 2011;364(25):2439–50. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 22.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2014;1851(4):469–84. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Christensen JH, Gustenhoff P, Korup E, Aarøe J, Toft E, Møller J, Rasmussen K, Dyerberg J, Schmidt EB. Effect of fish oil on heart rate variability in survivors of myocardial infarction: a double blind randomised controlled trial. BMJ. 1996;312(7032):677–8. doi: 10.1136/bmj.312.7032.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376(9740):540–50. doi: 10.1016/S0140-6736(10)60445-X. [DOI] [PubMed] [Google Scholar]
- 25.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 26.Maksymowych WP, Mallon C, Morrow S, Shojania K, Olszynski WP, Wong RL, Sampalis J, Conner-Spady B. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index. Ann Rheum Dis. 2009;68(6):948–53. doi: 10.1136/ard.2007.084244. [DOI] [PubMed] [Google Scholar]
- 27.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy B. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26(3):485–90. doi: 10.1161/01.HYP.26.3.485. [DOI] [PubMed] [Google Scholar]
- 28.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, A. European Network for Non-invasive Investigation of Large Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Hear J. 2006;27(21):2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 29.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FUS, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–8. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 30.Dzeko M, Peters CD, Kjaergaard KD, Jensen JD, Jespersen B. Aortic pulse wave velocity results depend on which carotid artery is used for the measurements. J Hypertens. 2013;31(1):117–22. doi: 10.1097/HJH.0b013e32835b051b. [DOI] [PubMed] [Google Scholar]
- 31.Chen C-H, Nevo E, Fetics B, Pak PH, Yin FCP, Maughan WL, Kass DA. Estimation of Central Aortic Pressure Waveform by Mathematical Transformation of Radial Tonometry Pressure : Validation of Generalized Transfer Function. Circulation. 1997;95(7):1827–36. doi: 10.1161/01.CIR.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 32.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38(4):932–7. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 33.Syngle A, Verma I, Krishan P, Garg N, Syngle V. Disease-modifying anti-rheumatic drugs improve autonomic neuropathy in arthritis: DIANA study. Clin Rheumatol. 2014;34(7):1233–41. [DOI] [PubMed]
- 34.Janse van Rensburg DC, Ker JA, Grant CC, Fletcher L. Effect of exercise on cardiac autonomic function in females with rheumatoid arthritis. Clin Rheumatol. 2012;31(8):1155–62. doi: 10.1007/s10067-012-1985-5. [DOI] [PubMed] [Google Scholar]
- 35.Task Force of the European Society of Cardiology; t. N. A. S. Electrophysiology Heart Rate Variability : Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation. 1996;93(5):1043–65. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 36.Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59(4):256–62. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 37.La Rovere MT, Christensen JH. The autonomic nervous system and cardiovascular disease: role of n-3 PUFAs. Vascul Pharmacol. 2015;71:1–10. doi: 10.1016/j.vph.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Christensen JH. Omega-3 polyunsaturated Fatty acids and heart rate variability. Front Physiol. 2011;2:84. doi: 10.3389/fphys.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112(13):1945–52. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 40.Dallongeville J, Yarnell J, Ducimetière P, Arveiler D, Ferfières J, Montaye M, Luc G, Evans A, Bingham A, Hass B, Ruidavets JB, Amouyel P. Fish consumption is associated with lower heart rates. Circulation. 2003;108(7):820–5. doi: 10.1161/01.CIR.0000084542.64687.97. [DOI] [PubMed] [Google Scholar]
- 41.Palatini P, Julius S. Elevated Heart Rate: A Major Risk Factor for Cardiovascular Disease. Clin Exp Hypertens. 2004;26:637–44. doi: 10.1081/CEH-200031959. [DOI] [PubMed] [Google Scholar]
- 42.La Rovere MT, Staszewsky L, Barlera S, Maestri R, Mezzani A, Midi P, Marchioli R, Maggioni AP, Tognoni G, Tavazzi L, Latini R. n-3PUFA and Holter-derived autonomic variables in patients with heart failure: data from the Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca (GISSI-HF) Holter substudy. Heart Rhythm. 2013;10(2):226–32. doi: 10.1016/j.hrthm.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 43.Billman GE. Heart Rate Variability ? A Historical Perspective. Front Physiol. 2011;2(November):1–13. doi: 10.3389/fphys.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christensen JH, Schmidt EB. Autonomic nervous system, heart rate variability and n-3 fatty acids. J Cardiovasc Med. 2007;8 Suppl 1:S19–22. doi: 10.2459/01.JCM.0000289276.10675.a1. [DOI] [PubMed] [Google Scholar]
- 45.Grimsgaard S, Bønaa KH, Hansen JB, Myhre ES. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. Am J Clin Nutr. 1998;68(1):52–9. doi: 10.1093/ajcn/68.1.52. [DOI] [PubMed] [Google Scholar]
- 46.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137(4):855–9. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- 47.Helliwell PS. Assessment of disease activity in psoriatic arthritis. 2015;33(5 Suppl 93):S44–7. [PubMed]
- 48.Stojanovich L. Autonomic dysfunction in autoimmune rheumatic disease. Autoimmun Rev. 2009;8(7):569–72. doi: 10.1016/j.autrev.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 49.Gaydukova I, Rebrov A, Nikitina N, Poddubnyy D. Decreased heart rate variability in patients with psoriatic arthritis. Clin Rheumatol. 2012;31(9):1377–81. [DOI] [PubMed]
- 50.Proietti I, Raimondi G, Skroza N, Pampena R, Bernardini N, La Viola G, Nicolucci F, Tolino E, Zuber S, Scordamaglia B, Balduzzi V, Soccodato V, Potenza C. Cardiovascular Risk in Psoriatic Patients Detected by Heart Rate Variability (HRV) Analysis. Drug Dev Res. 2014;75:S81–4. doi: 10.1002/ddr.21204. [DOI] [PubMed] [Google Scholar]
- 51.Madsen T, Christensen JH, Toft E, Schmidt EB. C-reactive protein is associated with heart rate variability. Ann Noninvasive Electrocardiol. 2007;12(3):216–22. doi: 10.1111/j.1542-474X.2007.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sloan RP, Mccreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR Interval Variability Is Inversely Related to Inflammatory Markers : The CARDIA Study. Mol Med. 2007;13(3–4):178–84. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spiegel TM, King W, Weiner SR, Paulus HE. Measuring disease activity: comparison of joint tenderness, swelling, and ultrasonography in rheumatoid arthritis. Arthritis Rheum. 1987;30:1283–8. doi: 10.1002/art.1780301111. [DOI] [PubMed] [Google Scholar]
- 54.Daunt AO, Cox NL, Robertson JC, Cawley MI. Indices of disease activity in psoriatic arthritis. J R Soc Med. 1987;80:556–8. doi: 10.1177/014107688708000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coates L. Outcome Measures in Psoriatic Arthritis. Rheum Dis Clin North Am. 2015;41(4):699–710. doi: 10.1016/j.rdc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88(2):523–33. doi: 10.1161/01.CIR.88.2.523. [DOI] [PubMed] [Google Scholar]
- 57.Pase MP, Grima NA, Sarris J. Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials. Br J Nutr. 2011;106(7):974–80. doi: 10.1017/S0007114511002819. [DOI] [PubMed] [Google Scholar]
- 58.Monahan KD, Feehan RP, Blaha C, McLaughlin DJ. Effect of omega‐3 polyunsaturated fatty acid supplementation on central arterial stiffness and arterial wave reflections in young and older healthy adults. Physiol Rep. 2015;3(6):e12438. doi: 10.14814/phy2.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Booth AD, Wallace S, McEniery CM, Brown J, Jayne DR, Wilkinson IB. Inflammation and arterial stiffness in systemic vasculitis: a model of vascular inflammation. Arthritis Rheum. 2004;50(2):581–8. doi: 10.1002/art.20002. [DOI] [PubMed] [Google Scholar]
- 60.Provan SA, Semb AG, Hisdal J, Stranden E, Agewall S, Dagfinrud H, Angel K, Atar D, Kvien TK. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: a cross-sectional comparative study. Ann Rheum Dis. 2011;70(5):812–7. doi: 10.1136/ard.2010.141523. [DOI] [PubMed] [Google Scholar]
- 61.Kremer JM, Lawrence DA, Jubiz W, DiGiacomo R, Rynes R, Bartholomew LE, Sherman M. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33(6):810–20. doi: 10.1002/art.1780330607. [DOI] [PubMed] [Google Scholar]
- 62.Kirkegaard E, Svensson M, Strandhave C, Schmidt EB, Jorgensen KA, Christensen JH. Marine n-3 fatty acids, atrial fibrillation and QT interval in haemodialysis patients. Br J Nutr. 2012;107:903–9. doi: 10.1017/S0007114511003771. [DOI] [PubMed] [Google Scholar]
- 63.McLennan PL. Relative effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on cardiac arrhythmias in rats. Am J Clin Nutr. 1993;57(2):207–12. doi: 10.1093/ajcn/57.2.207. [DOI] [PubMed] [Google Scholar]
- 64.Knudsen VK, Matthiessen J, Biltoft-Jensen A, Sorensen MR, Groth MV, Trolle E, Christensen T, Fagt S. Identifying dietary patterns and associated health-related lifestyle factors in the adult Danish population. Eur J Clin Nutr. 2014;68(6):736–40. doi: 10.1038/ejcn.2014.38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available in the Clinical Trials, www.clinicaltrials.gov Identifier: NCT01818804.


