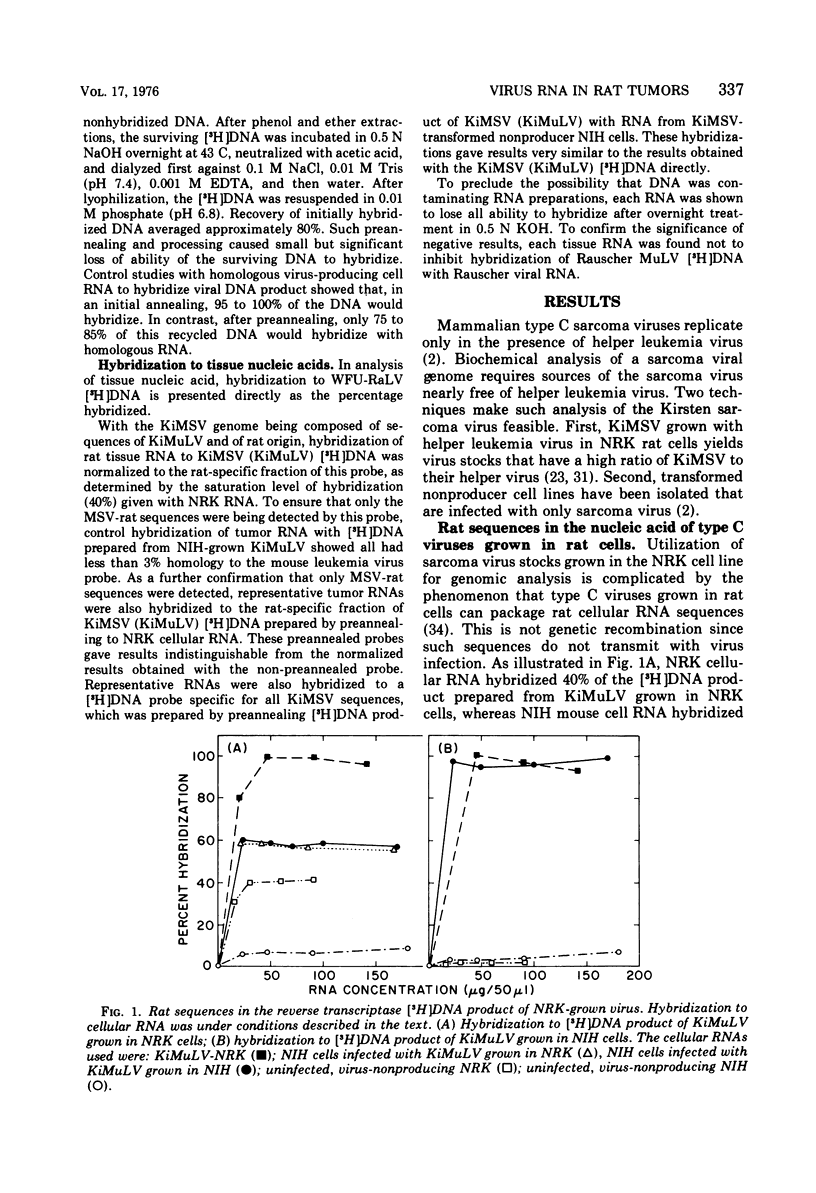
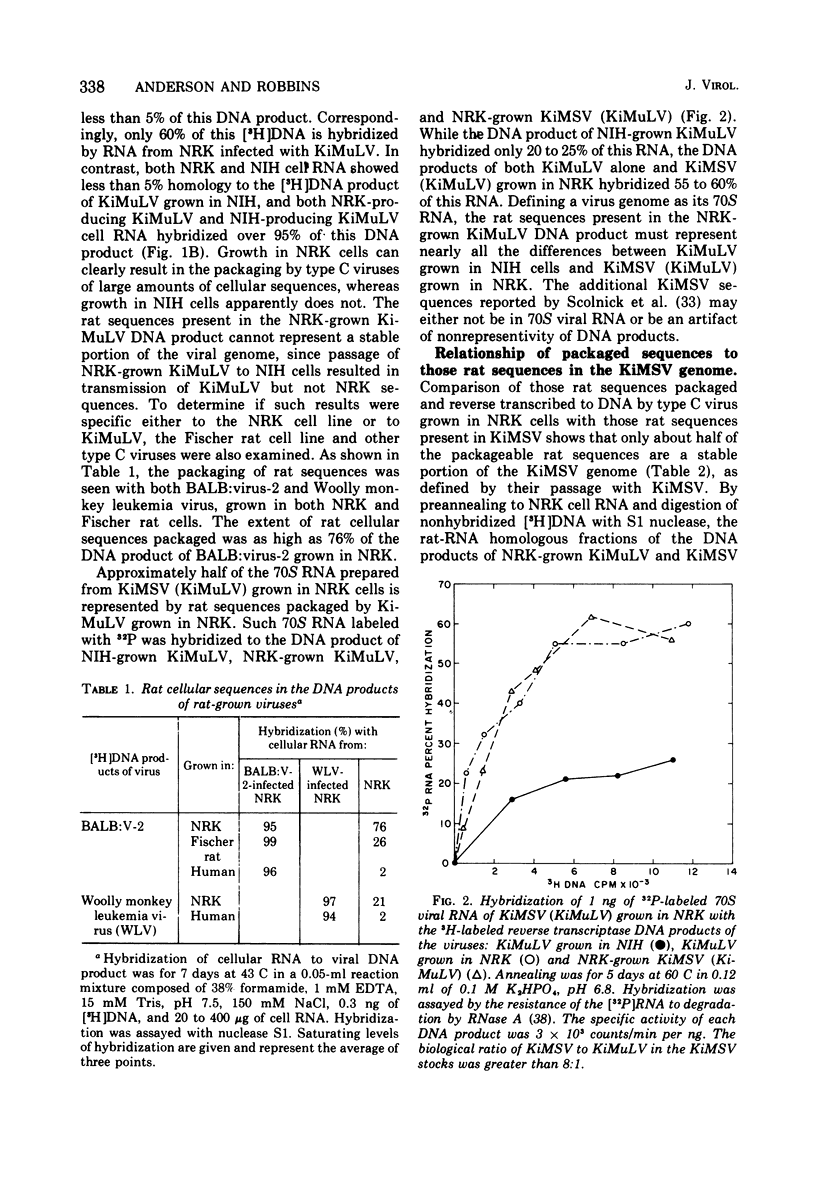
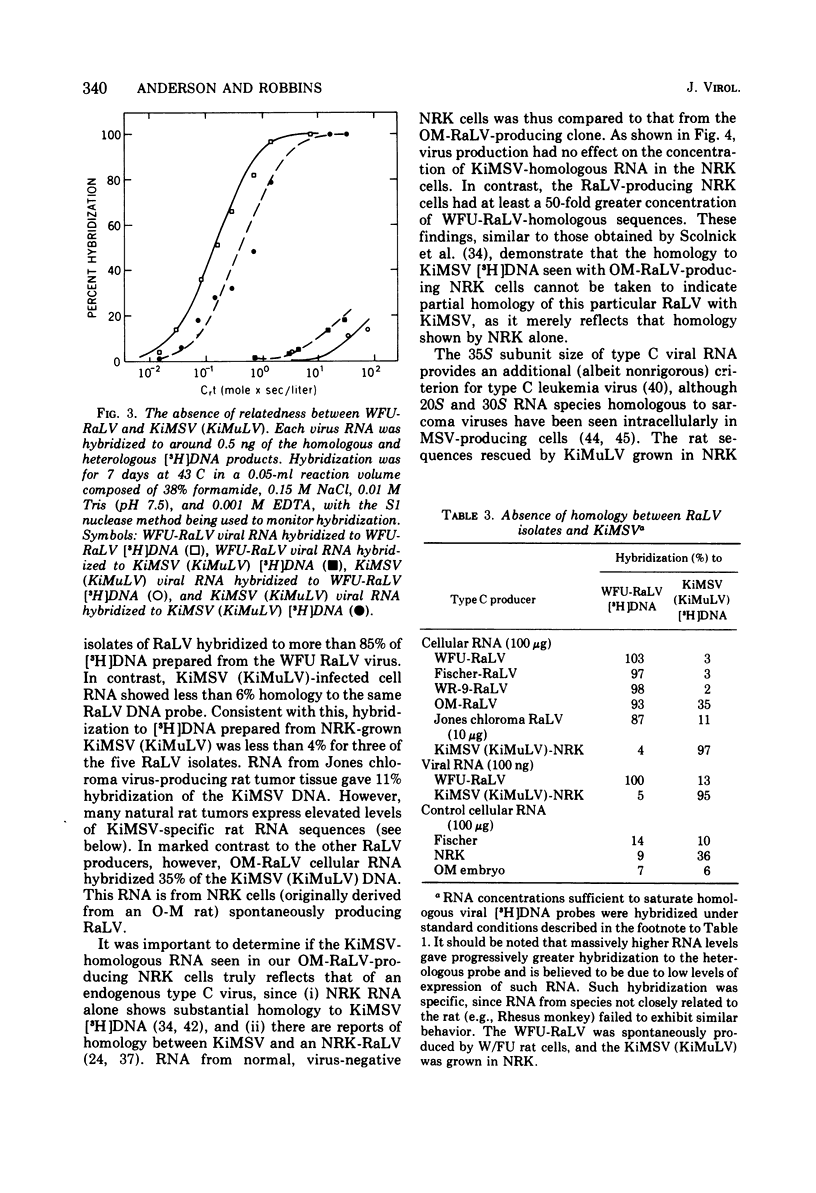
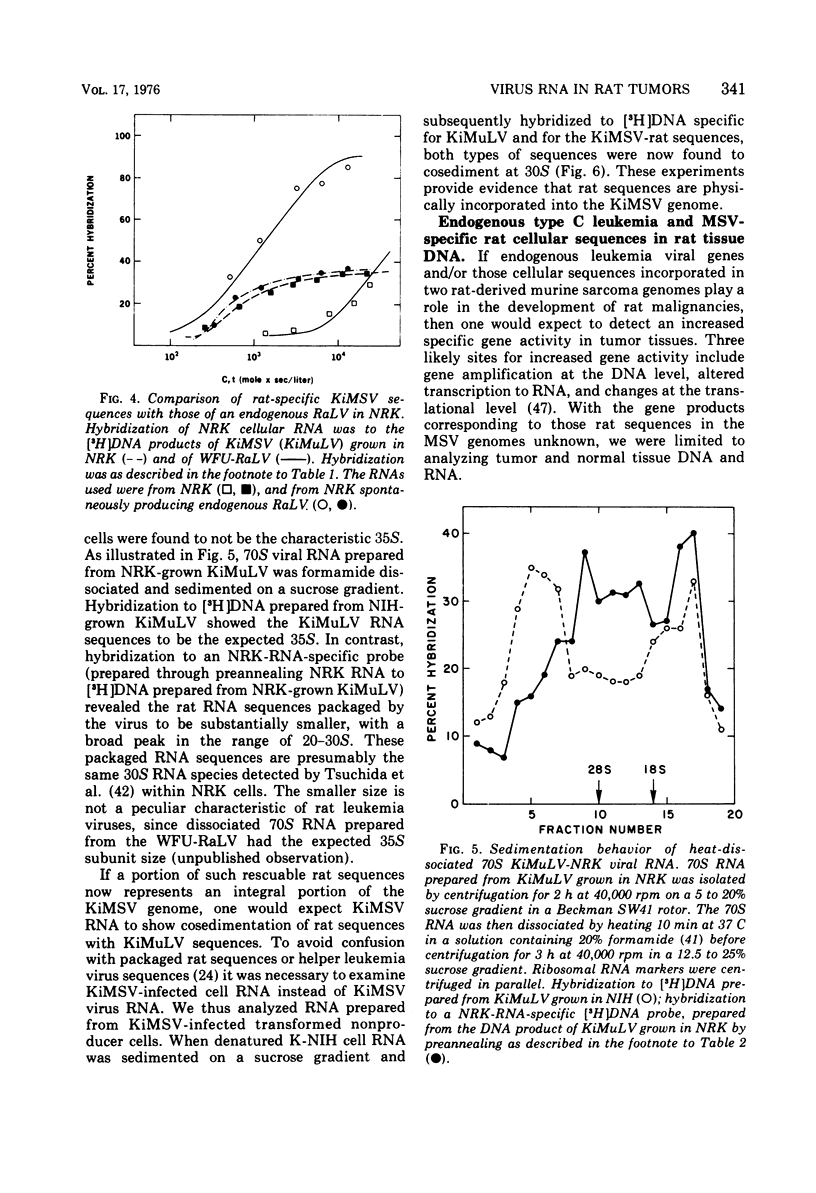
Abstract
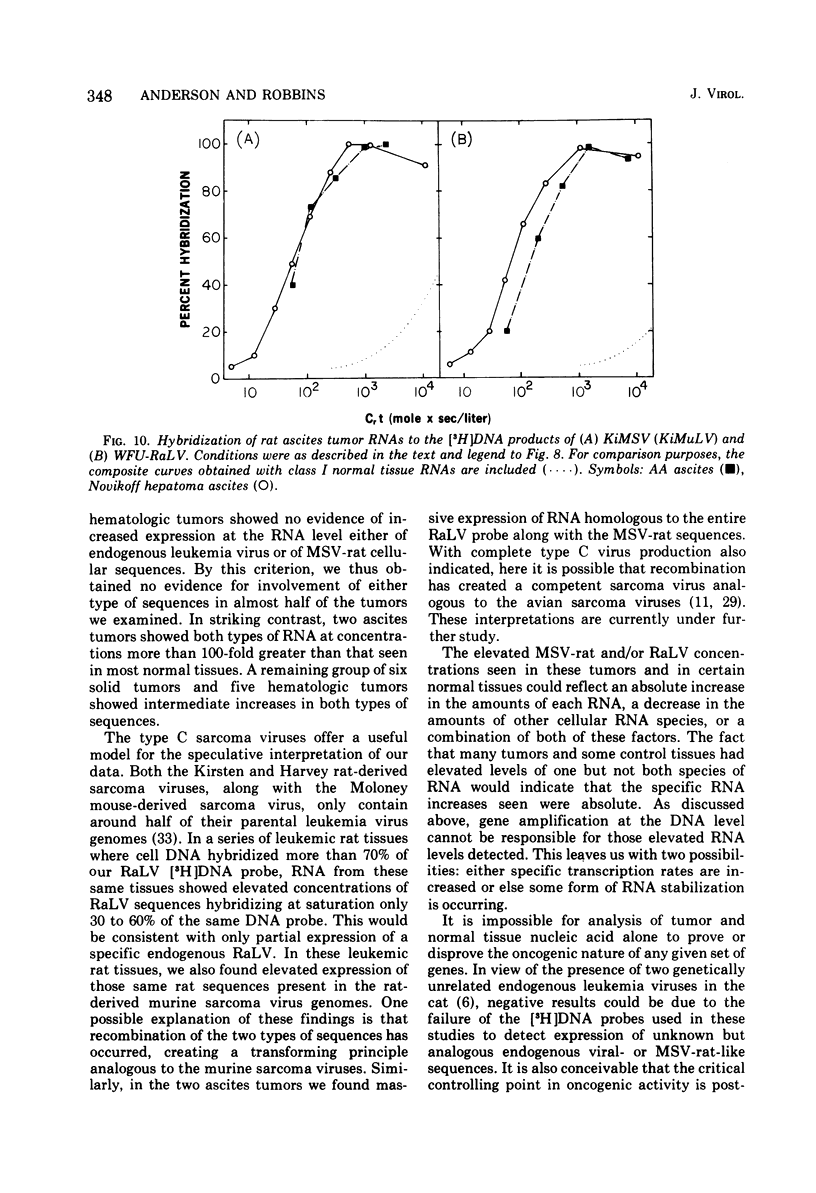
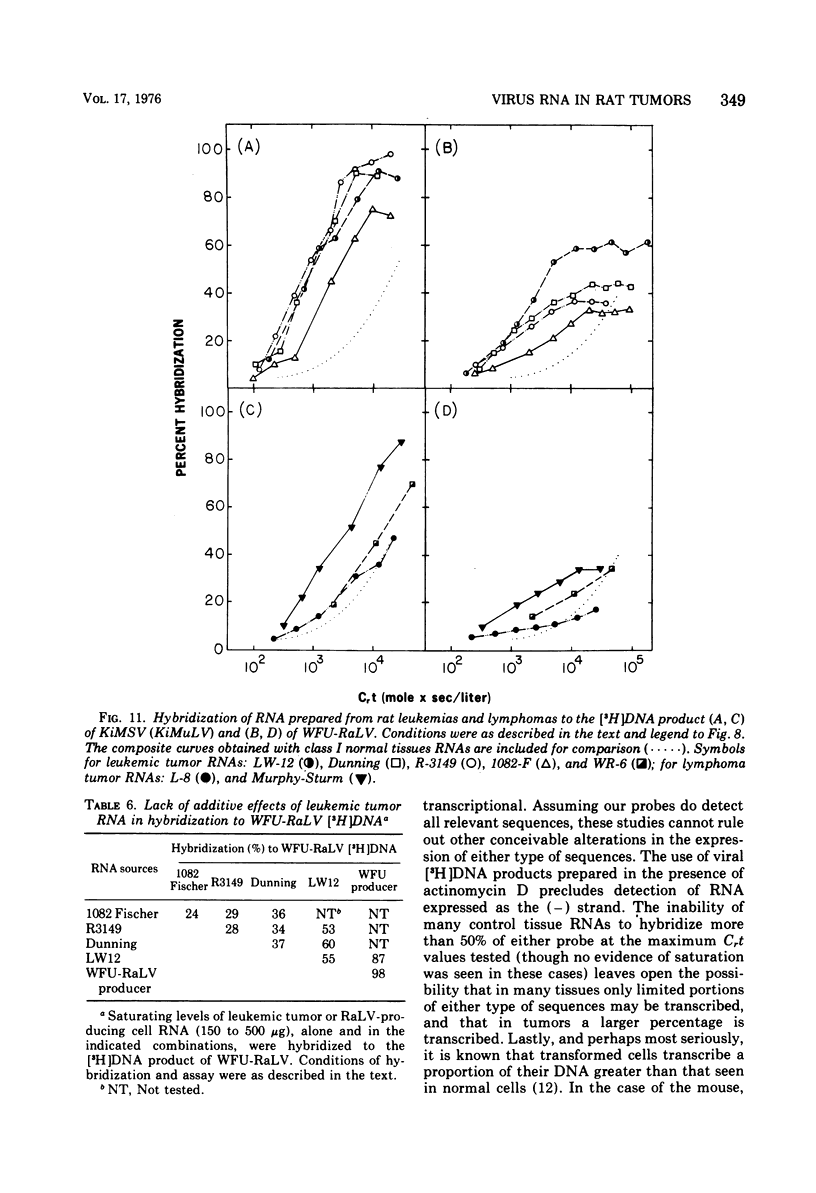
Two murine sarcoma viruses, the Kirsten and the Harvey, were isolated by passage of mouse type C leukemia viruses through rats. These sarcoma viruses have genomes containing portions of their parental type C mouse leukemia virus genomes, in stable association with specific rat cellular sequences that we find to be quite likely not those of a rat type C leukemia virus. To determine if these murine sarcoma viruses provide a model relevant to the events occurring in spontaneous tumors, we have hybridized DNA and RNA prepared from rat tumors and normal rat tissues to [3H]DNA prepared from the Kirsten murine sarcoma virus. We have also hybridized these rat tissue nucleic acids to [3H]DNA prepared from a respresentative endogenous rat type C leukemia virus, the WFU (Wistar-Furth). Sarcoma-viral rat cellular sequences and endogenous rat leukemia viral sequences were detected in the DNA of both tumor and normal tissues, with no evidence of either gene amplification or additional sequences being present in tumor DNA. Sarcoma-viral rat cellular sequences and endogenous rat leukemia viral sequences were detected at elevated concentrations in the RNA of many rat tumors and in specific groups of normal tissues.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Isolation of a rat-tropic helper virus from M-MSV-O stocks. Virology. 1971 Apr;44(1):29–36. doi: 10.1016/0042-6822(71)90149-8. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3316–3320. doi: 10.1073/pnas.70.12.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Sells B. H., Purdom I. F. Kinetic complexity of RNA molecules. J Mol Biol. 1972 Jan 14;63(1):21–39. doi: 10.1016/0022-2836(72)90519-0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. The effect of genetic complexity on the time-course of ribonucleic acid-deoxyribonucleic acid hybridization. Biochem J. 1969 Aug;113(5):805–811. doi: 10.1042/bj1130805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Evidence that the AKR murine-leukemia-virus genome is complete in DNA of the high-virus AKR mouse and incomplete in the DNA of the "virus-negative" NIH mouse. Proc Natl Acad Sci U S A. 1974 Jan;71(1):167–171. doi: 10.1073/pnas.71.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady L. J., Campbell W. P. Non-repetitive DNA transcription in mouse cells grown in tissue culture. Nat New Biol. 1973 Jun 13;243(128):195–198. doi: 10.1038/newbio243195a0. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Stephenson J. R., Moloney W. C., Aaronson S. A. Different hematological diseases induced by type C viruses chemically activated from embryo cells of different mouse strains. Cancer Res. 1975 Jan;35(1):245–252. [PubMed] [Google Scholar]
- HARVEY J. J. AN UNIDENTIFIED VIRUS WHICH CAUSES THE RAPID PRODUCTION OF TUMOURS IN MICE. Nature. 1964 Dec 12;204:1104–1105. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa Y., Ross J., Leder P. An association between globin messenger RNA and 60S RNA derived from Friend leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1154–1158. doi: 10.1073/pnas.71.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalter S. S., Panigel M., Kraemer D. C., Heberling R. L., Helmke R. J., Smith G. C., Hellman A. C-type particles in baboon (Papio cynocephalus) preimplantation embryos. J Natl Cancer Inst. 1974 Jun;52(6):1927–1928. doi: 10.1093/jnci/52.6.1927. [DOI] [PubMed] [Google Scholar]
- Karasaki S. Virus particles associated with the transplantable Novikoff hepatoma in the rat. Cancer Res. 1969 Jun;29(6):1313–1315. [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel J., Klement V., Lai M. M., Ostertag W., Duesberg P. Ribonucleic acid components of murine sarcoma and leukemia viruses. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3536–3540. doi: 10.1073/pnas.70.12.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel J., Scolnick E. M., Duesberg P. Base sequence differences between the RNA components of Harvey sarcoma virus. J Virol. 1975 Sep;16(3):749–753. doi: 10.1128/jvi.16.3.749-753.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney W. C., Boschetti A. E., King V. P. Spontaneous leukemia in Fischer rats. Cancer Res. 1970 Jan;30(1):41–43. [PubMed] [Google Scholar]
- Neiman P. E., Wright S. E., McMillin C., MacDonnell D. Nucleotide sequence relationships of avian RNA tumor viruses: measurement of the deletion in a transformation-defective mutant of Rous sarcoma virus. J Virol. 1974 Apr;13(4):837–846. doi: 10.1128/jvi.13.4.837-846.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Burman P., Klement V. Derivation of mouse sarcoma virus (Kirsten) by acquisition of genes from heterologous host. J Gen Virol. 1975 Aug;28(2):193–198. doi: 10.1099/0022-1317-28-2-193. [DOI] [PubMed] [Google Scholar]
- Scolnic E. M., Goldberg R. J., Parks W. P. A biochemical and genetic analysis of mammalian RNA-containing sarcoma viruses. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):885–895. doi: 10.1101/sqb.1974.039.01.103. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Maryak J. M., Parks W. P. Levels of rat cellular RNA homologous to either Kirsten sarcoma virus or rat type-C virus in cell lines derived from Osborne-Mendel rats. J Virol. 1974 Dec;14(6):1435–1444. doi: 10.1128/jvi.14.6.1435-1444.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P. Harvey sarcoma virus: a second murine type C sarcoma virus with rat genetic information. J Virol. 1974 Jun;13(6):1211–1219. doi: 10.1128/jvi.13.6.1211-1219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W., Kawakami T., Kohne D., Okabe H., Gilden R., Hatanaka M. Primate and murine type-C viral nucleic acid association kinetics: analysis of model systems and natural tissues. J Virol. 1974 Feb;13(2):363–369. doi: 10.1128/jvi.13.2.363-369.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Rands E., Williams D., Parks W. P. Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. J Virol. 1973 Sep;12(3):458–463. doi: 10.1128/jvi.12.3.458-463.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Murine sarcoma and leukemia viruses: genetic differences determined by RNA-DNA hybridization. Virology. 1971 Nov;46(2):480–484. doi: 10.1016/0042-6822(71)90048-1. [DOI] [PubMed] [Google Scholar]
- Trávnícek M., Ríman J. Subunits of oncornavirus high-molecular-weight RNA. I. Stepwise conversion of 60 S AMV (avian myeloblastosis virus) RNA to subunits. Biochem Biophys Res Commun. 1973 Jul 2;53(1):217–223. doi: 10.1016/0006-291x(73)91422-8. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Gilden R. V., Hatanaka M. Sarcoma-virus-related RNA sequences in normal rat cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4503–4507. doi: 10.1073/pnas.71.11.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Gilden R. V., Hatanaka M. Size of virus-specific RNA in B-34, a hamster tumor cell producing nucleic acids of type C viruses from three species. J Virol. 1975 Oct;16(4):832–837. doi: 10.1128/jvi.16.4.832-837.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Shih M. S., Gilden R. V., Hatanaka M. Sarcoma and helper-specific RNA tumor virus subunits in transformed nonproducer mouse cells activated to produce virus by treatment with bromodeoxyuridine. J Virol. 1974 Nov;14(5):1262–1267. doi: 10.1128/jvi.14.5.1262-1267.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon M. L., Lane W. T., Huebner R. J. Prevalence of type-C particles in visceral tissues of embryonic and newborn mice. J Natl Cancer Inst. 1973 Oct;51(4):1171–1175. doi: 10.1093/jnci/51.4.1171. [DOI] [PubMed] [Google Scholar]


