Abstract
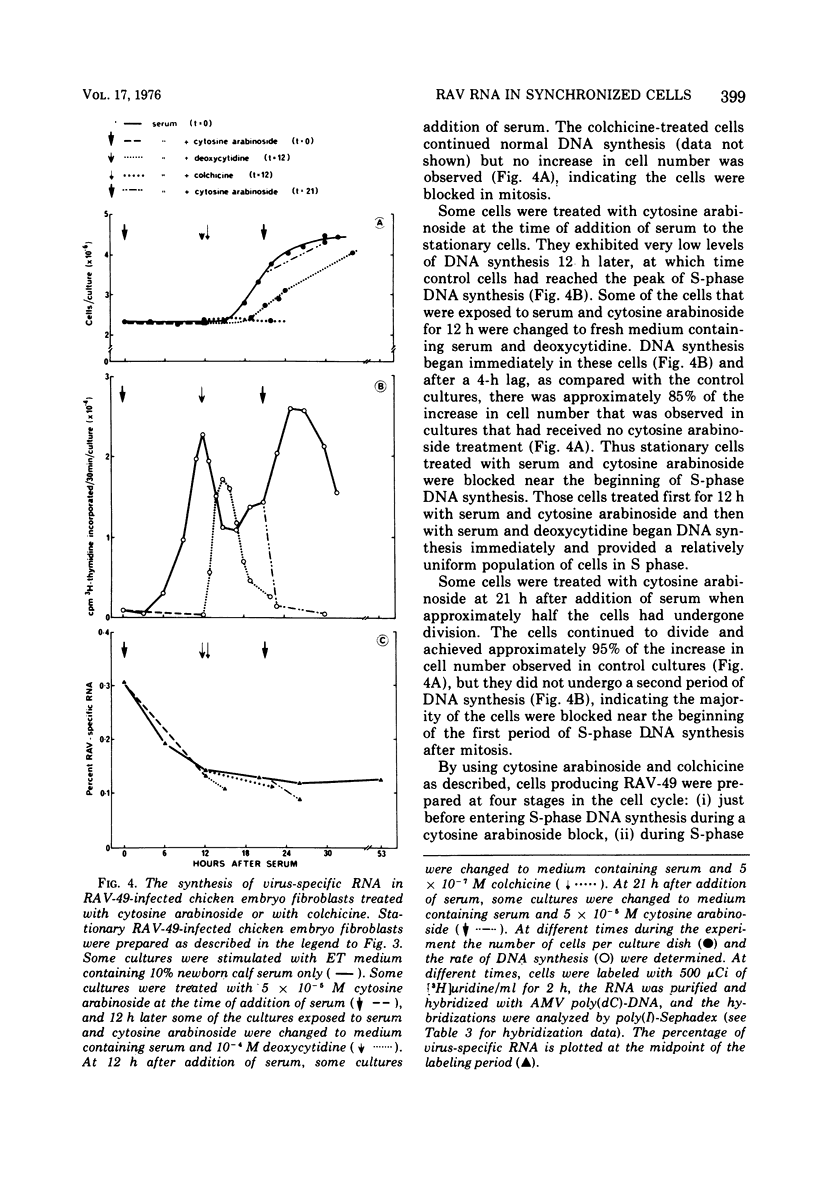
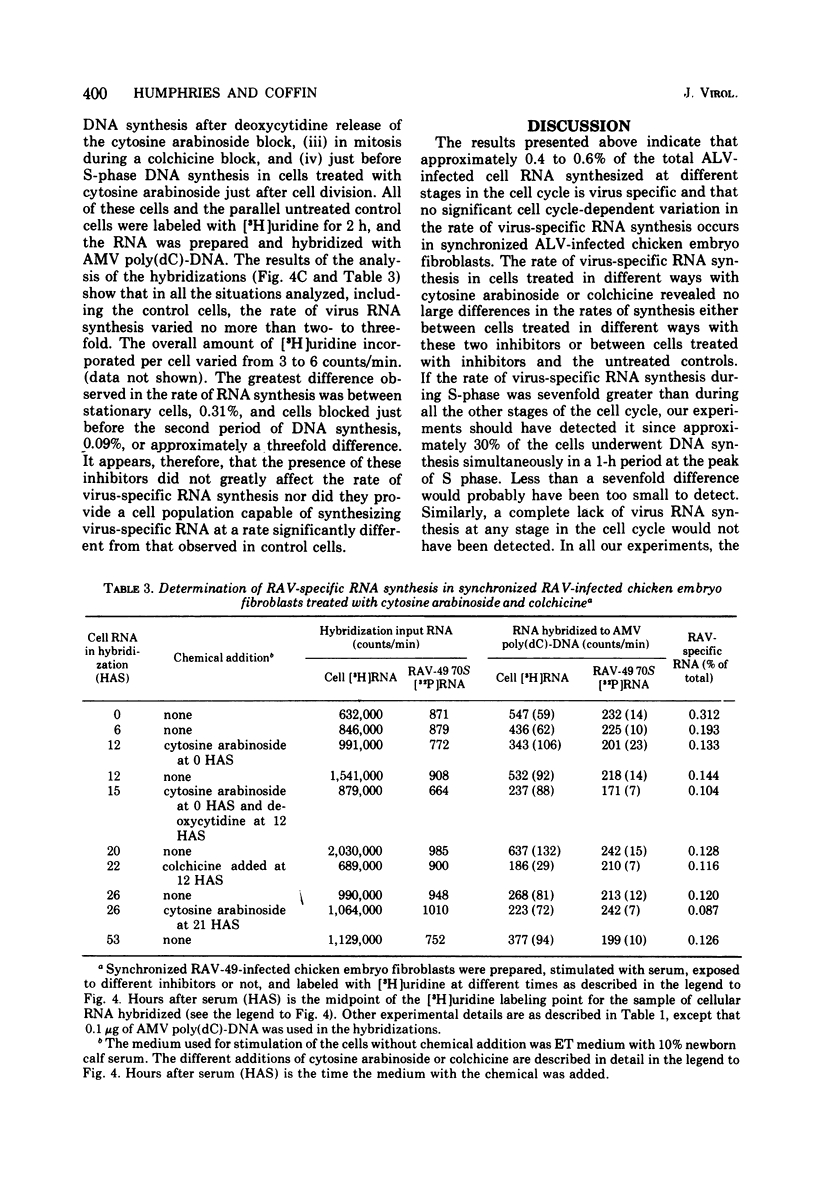
The rate of avian leukosis virus (ALV)-specific RNA synthesis has been examined in bot- uninfected and ALV-infected synchronized chicken embryo fibroblasts. RNA from cells labeled for 2h with [3H]uridine was hybridized with avian myeloblastosis virus poly(dC)-DNA, and the hybridized RNA was analyzed with poly(I)-spephadex chromatography. Approximately 0.5% of the RNA synthesized in ALV-infected cells was detected as virus specific, and no more than a twofold variation in the rate of synthesis was detected at different times in the cell cycle. In synchronized uninfected chicken embryo fibroblasts, approximately 0.03% of the RNA synthesized was detected as virus specific, and no significant variation in the rate of synthesis was observed during the cell cycle. Treatment of ALV-infected chicken embryo fibroblasts with cytosine arabinoside or colchicine was used to block cells at different stages in the cell cycle. The rates of virus-specific RNA synthesis in cells so treated did not differ significantly from the rates in either stationary or unsynchronized virus-infected chicken embryo fibroblasts. These findings support the conclusion that after the initial division of an ALV-infected chicken embryo fibroblast and the initiation of virus RNA synthesis, the rate of virus-specific RNA synthesis is independent of the cell cycle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coffin J. M., Parsons J. T., Rymo L., Haroz R. K., Weissmann C. A new approach to the isolation of RNA-DNA hybrids and its application to the quantitative determination of labeled tumor virus RNA. J Mol Biol. 1974 Jun 25;86(2):373–396. doi: 10.1016/0022-2836(74)90026-6. [DOI] [PubMed] [Google Scholar]
- Colby C., Edlin G. Nucleotide pool levels in growing, inhibited, and transformed chick fibroblast cells. Biochemistry. 1970 Feb 17;9(4):917–920. doi: 10.1021/bi00806a029. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Temin H. M. Cell cycle-dependent activation of rous sarcoma virus-infected stationary chicken cells: avian leukosis virus group-specific antigens and ribonucleic acid. J Virol. 1972 Jul;10(1):82–87. doi: 10.1128/jvi.10.1.82-87.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Temin H. M. Requirement for cell division for initiation of transcription of Rous sarcoma virus RNA. J Virol. 1974 Sep;14(3):531–546. doi: 10.1128/jvi.14.3.531-546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Levinson W., Bishop M. J. Synchronization of Rous sarcoma virus production in chick embryo cells. Virology. 1972 Jan;47(1):133–141. doi: 10.1016/0042-6822(72)90246-2. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Coffin J. M., Haroz R. K., Bromley P. A., Weissmann C. Quantitative determination and location of newly synthesized virus-specific ribonucleic acid in chicken cells infected with Rous sarcoma virus. J Virol. 1973 May;11(5):761–774. doi: 10.1128/jvi.11.5.761-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymo L., Parsons J. T., Coffin J. M., Weissmann C. In vitro synthesis of Rous sarcoma virus-specific RNA is catalyzed by a DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2782–2786. doi: 10.1073/pnas.71.7.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol. 1971 Oct;78(2):161–170. doi: 10.1002/jcp.1040780202. [DOI] [PubMed] [Google Scholar]
- Temin H. M. The cellular and molecular biology of RNA tumor viruses, especially avian leukosis-sarcoma viruses, and their relatives. Adv Cancer Res. 1974;19(0):47–104. doi: 10.1016/s0065-230x(08)60052-4. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Rubin H. Uridine transport and RNA synthesis in growing and in density-inhibited animal cells. J Cell Physiol. 1971 Apr;77(2):157–168. doi: 10.1002/jcp.1040770205. [DOI] [PubMed] [Google Scholar]


