Abstract
Juvenile male rhesus macaques received therapeutic doses of fluoxetine daily from one to three years of age and were compared to vehicle-treated controls (N=16/group). Genotyping for monoamine oxidase A (MAOA) polymorphisms was used to form subgroups (N=8) with high and low expression of the gene. Behavioral responses were scored during 30-second exposures to pictures differing in affective content. As expected from its therapeutic effect, fluoxetine decreased the behavioral response to emotionally evocative pictures. A 44% reduction in number of expressive behaviors was seen, but only in subjects with low expression MAOA polymorphisms. In general, this effect occurred for pictures of varying affective content and was not due to altered occurrence of one specific behavior or type of behavior. The drug*genotype interaction was seen after one and two years of treatment and did not reverse one year after discontinuation of dosing. Two potential translational implications are suggested: (1) MAOA genetic polymorphisms may be the source of some of the variability in response to fluoxetine treatment in children; (2) extended fluoxetine treatment during juvenile brain development may result in persistent effects on emotional regulation.
Keywords: fluoxetine, children, nonhuman primates, emotion, behavior, MAOA
1.0 Introduction
Fluoxetine was approved in the US in 2003 and in the EU in 2006 for treatment of children with MDD (major depressive disorder), and it continues to be the first line pharmacological treatment for this disorder (Pfalzgraf et al., 2012; Birmaher et al., 2007). The use of fluoxetine in treating depression in children is consistent with its interaction with brain circuits regulating emotional response. In adults and adolescents, regulation of emotional response, as reflected in activation of amygdala and associated brain areas (Delaveau et al., 2011), is shown to be elevated in depression and normalized by treatment with antidepressants (Fu et al., 2013) including fluoxetine (Rizvi et al., 2013; Tao et al., 2012). Antidepressants including fluoxetine also affect emotional brain activation in normal adults (Norbury et al., 2009; Pringle and Harmer, 2015). There is some information on the brain activation response to emotional stimuli in depressed children (Gaffrey et al., 2013), but no studies of antidepressant effects. In addition to its use in depression, fluoxetine therapy is approved for use in OCD, but it is also used “off-label” in a variety of other childhood disorders such as anxiety (Birmaher et al., 2003; Strawn et al., 2015), autism (Williams et al., 2013), obesity (Rezvanian et al., 2010), social phobia (Davidson et al., 2004), and Down’s syndrome (Costa and Scott-McKean, 2013).
While the efficacy of fluoxetine therapy for depression in children is supported by published studies (Hetrick et al., 2007), many safety issues arise when considering psychoactive drug use in children. The demonstration of increased risk of suicidal ideation in adolescents treated with antidepressants raised the question of whether side effects in children can be anticipated from experience with adults or whether unique unwanted effects can occur. Another issue, more difficult to study in children, is whether developmental treatment can alter the trajectory of brain development with unfavorable long-term consequences. We have addressed these issues in juvenile nonhuman primate model for childhood treatment with fluoxetine at therapeutic doses. The ages of the rhesus macaque subjects in the present study (one to four years of age) correspond roughly to four to twelve year-old children. Previous reports from this project have described dose selection (Golub and Hogrefe, 2014), metabolomic biomarkers of drug action (He et al., 2014), bone growth (Golub et al., 2015), sleep disturbance (Golub and Hogrefe, 2016), and social interaction (Golub et al., 2016).
Macaque monkeys have long been studied as models for emotional response during infant development (Kalin and Shelton, 2003) and are becoming widely employed as suitable animal models for studying psychoactive drugs during juvenile and adolescent brain development (Soto et al., 2012; Shrestha et al., 2014; Rodriguez et al., 2010; Popke et al., 2001; Paule et al., 1992; Patterson et al., 2010; Mattison et al., 2011; Mandell et al., 2011; Gill et al., 2012). The extended period of postnatal brain development, the specialization of higher cortical areas and top-down regulation of lower centers are all common characteristics of primate species’ brains. Parallel technical evaluations of brain function can be used in human and nonhuman primates, particularly the noninvasive imaging techniques and structured behavioral evaluations. Single offspring pregnancies, complex social structures, and extensive use of visual information are other valuable parallels to humans that improve translation of this animal model.
The data reported here are from a test paradigm using response to emotionally evocative pictures. This technique is becoming widely used with fMRI to study the brain circuits mediating emotional response and modification of their activation in humans (Delaveau et al., 2011) including children (Perlman et al., 2014). In the current study, response to pictures differing in affective content was recorded as the frequency of occurrence of vocalizations, facial expressions and simple actions used as expressive behaviors in young monkeys. It was hypothesized that fluoxetine would affect regulation of the frequency of these expressive behaviors in comparison with vehicle-treated controls.
Genetic polymorphisms are another factor known to modify brain regulation of emotional response in humans. The influence of genetic polymorphisms can also be studied in nonhuman primates due to sharing of genetic variants among evolutionarily related species. Our study design included subgroups with high- and low-transcription VNTR polymorphisms of the monoamine oxidase A (MAOA) gene. These polymorphisms have been associated with risk for impulsivity, violence/criminality and psychopathology in adolescents and adults, particularly in interaction with early experiences (Byrd and Manuck, 2014; Enoch et al., 2010; Kim-Cohen et al., 2006). They have also been shown to influence brain circuits regulating emotional response (Meyer-Lindenberg et al., 2006). Rhesus monkeys have uVNTR polymorphisms homologous to those seen in humans and behavioral differences have been reported between high- and low-expression genotypes in interaction with environmental variables in monkeys (Newman et al., 2005; Karere et al., 2009). Using emotionally evocative pictures, we have previously shown interactions between developmental iron deficiency and MAOA polymorphisms in our nonhuman primate model of juvenile behavior (Golub et al., 2012).
The present study included low- and high-expression MAOA polymorphisms as an independent variable in the design. The potential for interaction between fluoxetine and MAOA polymorphisms in adults has been demonstrated by the finding that both MAOA inhibitors and SSRIs are effective therapies for depression in adults (Thase, 2012), that MAOA polymorphism genotype is associated with therapeutic response to fluoxetine (Yu et al., 2005), and that both fluoxetine and MAOA polymorphisms can influence the brain circuits mediating response to emotion-evoking stimuli (Tao et al., 2012; Dannlowski et al., 2009). There are few studies of the MAOA polymorphism influences on the behavior of infants and children (Pickles et al., 2013; Zhang et al., 2011; Zhang et al., 2014) and no studies of interactions with psychoactive drugs. The study reported here assessed interactions both during dosing and after discontinuation of dosing.
2.0 Experimental Procedures
2.1 Assurance of compliance with animal codes
All animal procedures followed the Guide for the Care and Use of Laboratory Animals of the US National Research Council (National Research Council, 2011). Protocols were reviewed and approved by the UC Davis Institutional Animal Care and Use Committee prior to implementation.
2.2 Subjects
Thirty-two male rhesus macaques (Macaca mulatta) were selected at ten months of age from the outdoor colony of the California National Primate Research Center (CNPRC) and relocated indoors in pair housing with a compatible peer. Selection criteria have been previously described (Golub and Hogrefe, 2016) and allowed for treatment groups balanced for cage of origin, age, size, maternal reproductive history, and infant biobehavioral response. Cagemates lived in a standard double cage with a pairing door to allow separation for behavioral testing and drug dosing. Monkeys were fed commercial monkey chow (LabDiet #5047, St. Louis, MO, USA), had continual access to an automatic water system, and were given twice weekly fresh produce and daily forage and supplemental enrichment. Housing rooms were maintained on a 12:12 light cycle and were continually monitored for temperature and humidity.
Subjects participated in a comprehensive study of juvenile fluoxetine exposure examining effects on growth, activity, impulsivity, social interaction and cognitive development throughout the two year dosing period and the one year post-dosing follow-up period. The full battery of assessments is listed in Supplementary Table 1. During the first year of the study, subjects were gradually adapted to separation for behavioral testing, to relocation to testing environments and to equipment used for testing. This adaptation minimized stress during the testing sessions reported here.
2.3 Design
Table 1 shows the design of the study as determined by two independent variables (fluoxetine, MAOA genotype) and the within-group variable (dosing session).
Table 1.
Design and group size
| Test Sessions | |||||
|---|---|---|---|---|---|
| Treatment | Genotype | Sex | 1 year dosing |
2 year dosing | 1 year post- dosing |
| Vehicle | hi-MAOA | M | N=8 | N=8 | N=8 |
| low-MAOA | M | N=8 | N=8 | N=7* | |
| Fluoxetine | hi-MAOA | M | N=8 | N=8 | N=8 |
| low-MAOA | M | N=8 | N=8 | N=8 | |
One monkey did not complete the post-dosing session
2.4 Drug Treatment
Daily drug treatment with fluoxetine was initiated at one year of age (N=16) and continued for two years until animals reached three years of age. Fluoxetine (Webster Veterinary Supply, Devens, MA, USA) was mixed with flavored syrup or liquefied baby food and delivered via oral syringe directly into the mouth. Monkeys were trained to come forward and place the tip of the syringe in their mouths to initiate dosing. Controls (N=16) received only the flavored vehicle.
The fluoxetine variable corresponds to a dose of ∼2.0 mg/kg over the two-year treatment period, a dose based on preliminary pharmacokinetic studies (Golub and Hogrefe, 2014), as well as previous experience with this drug in macaques (Anderson, 2004; Clarke et al., 1999; Clarke et al., 1998; Fontenot et al., 2009; Fontenot et al., 2005; Sawyer and Howell, 2011; Shrestha et al., 2014). For 11 months prior to behavior data collection, a dose of 1.6 mg/kg was administered. This allowed adaptation of the subjects to the new housing and testing environments, training for consistent compliance with dosing and induction of metabolizing enzymes. After analysis of steady state serum levels, the dose was adjusted to 2.4 mg/kg, the daily dose used during the Dosing 1 and Dosing 2 elicited emotion test sessions reported here.
2.5 MAOA genotype
Most three-month old rhesus at CNPRC are genotyped for MAOA polymorphisms and this information is available in their electronic record. VNTR polymorphism (rhMAOA-LPR) genotyping was performed by the Veterinary Diagnostic Laboratory using PCR with forward and reverse primers. VNTR repeat lengths of 5 and 6 were classified as “hi-MAOA” while repeat length 7 was classified as “low-MAOA” based on 26% higher transcription rate for the 5 and 6 repeats (Newman et al., 2005). Allele frequencies in rhesus have been estimated at 60% “hi-MAOA” and 40% “low-MAOA” (Newman et al., 2005). Three-month old rhesus were also genotyped for SERT polymorphisms, but 5HTTLPR genotypes were not found to predict behavior in the picture-elicited emotion test. The fluoxetine groups were balanced for 5HTTLPR genotypes so that confounding with MAOA genotype did not occur.
2.6 Picture-elicited emotional response
Emotional responsiveness to pictures with varying affective content was assessed at the end of the first year of dosing (two years of age), the end of the second year of dosing (three years of age) and one year after the conclusion of dosing (four years of age). A series of eight pictures were presented on a monitor via a PowerPoint slide show. Animals were transferred to a familiar test cage with a clear plexiglass front and placed approximately 40 cm away from the video monitor in a darkened room. A video camera and light placed above the monitor recorded the session for later coding of behavior. Each slide was presented for 30 sec followed by a 1 min interslide interval of a black screen. Behavior was coded during the slide presentations. The eight slides were: a plain light green colored slide; fruit (apple slice and half peeled banana); a snake; a cage (identical to the home cage); an adult male monkey with an open mouth stare; a mother and infant monkey; two monkeys grooming; and a technician dressed in protective clothing wearing leather gauntlet/hand catching gloves (see Figure 2 and Supplementary Figure 1).
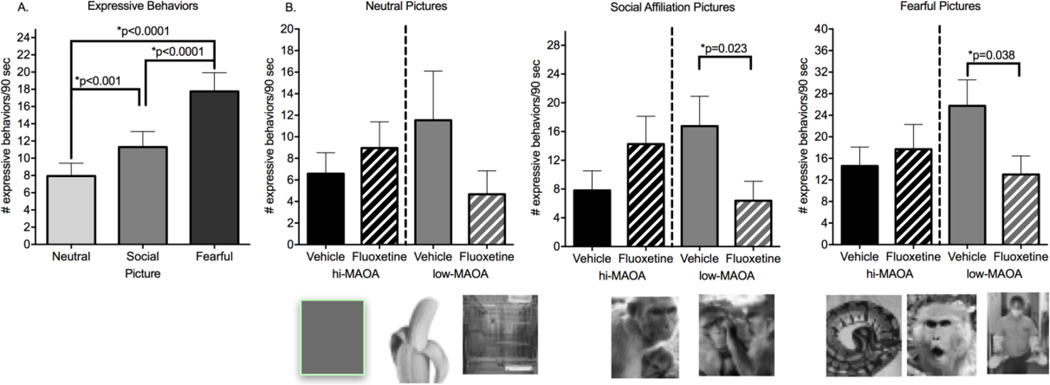
Figure 2.
Response to categories of slides. A. Comparison of the response (all expressive behaviors) of all animals to three picture categories. B. Comparison of the response of Treatment and Genotype subgroups to each behavior category. P values are for planned comparisons conducted after identification of significant Treatment*Genotype interactions. Representations of the slides included in each category are shown below the graphs. Actual slides are provided in Supplementary Figure 1.
Videos were scored with The Observer (Noldus Information Technology, Wageningen, The Netherlands) using an ethogram that included a number of expressive behaviors including facial expressions, vocalizations and simple behaviors known to reflect emotional response in rhesus (Supplementary Table 2). Motor activity, and time spent looking at the pictures were also included in the ethogram and analyzed (Supplementary Table 2). All videos were scored blind by the same observer (AMB), with an average intra-observer reliability of 89%.
2.7 Data Analysis
Prior to analysis, each dataset was screened for covariates (Supplementary Table 3), normality and outliers. No covariates were consistently associated with endpoints to be analyzed, normality assumptions were adequate for endpoints examined by ANOVA, and no outliers were identified that required exclusion.
The data analyses examined the following hypotheses:
Fluoxetine alters the response to emotionally evocative pictures
Fluoxetine effects can depend on MAOA genotype
Fluoxetine effects progress from one to two year of dosing
Fluoxetine effects reverse after discontinuation of dosing for one year
Fluoxetine effects are specific to pictures with high affective content
Fluoxetine influences clusters of behavior
Fluoxetine effects are independent of general changes in activity
All analyses were conducted in JMP (SAS, Inc., Carey, NC). Major statistics were RMANOVA and ANOVA. For RMANOVA, the repeated measure (Sessions) was evaluated to see if an initial fluoxetine effect was augmented with continuing dosing (Dosing 1 vs Dosing 2) and/or reversed upon discontinuation of dosing (Dosing 2 vs Post-dosing). The Treatment variable (fluoxetine vs. vehicle) evaluated the drug effect. The genotype variable (hi-MAOA vs low-MAOA) evaluated the effect of polymorphisms with low-MAOA and hi-MAOA expression. The interaction between fluoxetine and MAOA polymorphism category (Treatment*Genotype) was also tested. If the interaction was significant, the effect of Treatment was examined within each of the two genotype groups with planned comparisons. Second level two-way ANOVAs were also conducted for each of the three sessions separately. This was particularly important because one animal did not complete the post-dosing session, and thus his data were not included in the RMANOVA. Statistical significance was recognized at p<0.05. Marginal p-values (0.06>p>0.05) were reported as nonsignificant but were further investigated. For interactions, trending p-values (p<0.12) were also reported and further investigated.
2.8 Endpoints for analysis
Observational datasets yielded a large number of individual behaviors (see Supplementary Table 2) with low frequencies of occurrence (see Supplementary Figure 2). A hierarchical strategy was employed, first examining effects on the sum of all expressive behaviors across all pictures and sessions. If effects or trends (p<0.12) were detected, multivariate analyses were conducted to identify clusters of behaviors that might be more sensitive. Behaviors that were associated with the first three principal components from a Principal Components analysis (PCA behavior categories) were summed separately for analysis. These behavior clusters, termed “negative/aggressive”, “positive/fearful” and “distress”, were analyzed by RMANOVA. Response to the different categories of pictures was also evaluated by RMANOVA. In each session, eight pictures differing in affective valence were shown (Figure 2). A different random order of presentation was used in each session. Pictures were combined in three groups (neutral, social affiliation, fearful) and submitted to RMANOVA analysis. If interactions between the main variables and picture category were identified, separate second level two-way ANOVAs were conducted for each picture category.
3.0 Results
3.1 Fluoxetine decreased expressive behaviors in the low-MAOA genotype subjects
RMANOVA of the sum of all expressive behaviors across Sessions show significant Treatment*Genotype interaction (F=5.13, p=0.032). The drug effect was seen in the low-MAOA group (low-MAOA fluoxetine vs. vehicle, F= 5.68, p=0.024), with lower numbers of behaviors in the fluoxetine subgroup (Figure 1).
Figure 1.
Response to slides across three sessions. The average of the sum of all expressive behaviors for animals in each Treatment and Genotype subgroup is shown. The “Dosing 1” session was conducted after one year of fluoxetine administration; “Dosing 2” was conducted after two years of fluoxetine administration at the end of the dosing period; “Post-dosing” session was conducted one year after the conclusion of fluoxetine dosing. P values are for planned comparisons conducted after identification of significant Treatment*Genotype interactions in RMANOVA. See Table 1 for study design.
3.2 The Treatment*Genotype interaction was consistent across dosing and post-dosing sessions
There was also a significant effect of session (p<0.0001); the sum of all expressive behaviors declined from the first to second dosing session (p<0.001) but did not further change in the post-dosing session (p=0.42). The interaction (Session*Genotype*Treatment) was not significant but a trend was suggested (p=0.12). In second level ANOVAs, the Treatment*Genotype interaction was significant for the one-year dosing session (Dosing 1, p=0.038), and post-dosing session (p=0.041), but not for the two-year dosing session (Dosing 2, p=0.25). For the two-year dosing session there was a trend for a Treatment effect (p=0.12).
3.3 Fluoxetine’s effect on expressive behavior was more apparent for more evocative pictures
More information on the basis of the Treatment*Genotype interaction was sought by looking at response to three different categories of pictures: neutral, social affiliation and fearful (Figure 2). These picture categories differed in the number of behaviors elicited, lowest for neutral, greater for social affiliation, and fearful (Figure 2A). In RMANOVA across categories, there was a significant effect of picture category (F=24.32, p<0.0001).
In pairwise post hoc comparisons, all categories differed from one another (p<0.001). The same pattern of group means was seen for all picture categories (summed across sessions) (Figure 2B). Separating the picture categories for second level analysis, the Treatment*Genotype interaction was significant for social affiliation (F=7.08, p=0.013), and fearful (F=4.96, p=0.034) categories, but not significant for the neutral picture category (p=0.13). (Figure 2B). In planned comparisons, the fluoxetine effect was significant in the low-MAOA subgroup for social affiliation (p=0.023) and fearful (p=0.038) but not neutral (p=0.11) picture categories.
As regards individual pictures, the two social affiliation pictures, the grooming and nursing pictures, were the only pictures that individually showed significant Treatment*Genotype interactions (grooming F=4.52, p=0.042, planned comparison low-MAOA vehicle vs fluoxetine p=0.034, nursing F=6.10, p=0.020, planned comparison low-MAOA vehicle vs fluoxetine p=0.09). This analysis suggests that the interaction seen for all pictures had the same pattern for individual picture categories, but was stronger for the pictures that elicited the most behaviors and had positive social content.
3.4 Different types of expressive behaviors were not differentially affected by fluoxetine
Another secondary analysis was done to identify the categories of expressive behaviors most affected by genotype interaction. The contribution of individual behaviors to the total elicited behaviors differed by Session (Table 2). Because the subjects matured from infancy to prepubertal status during this time, the behaviors expressed in response to emotionally evocative pictures would be expected to change. The frequencies of most individual behaviors were too low for analysis as continuous variables and clusters of related variables were constructed as informed by Principal Components analysis. Multivariate analysis of the individual behaviors in each session also revealed principle components that differed by session. Three clusters emerged with significant loadings in at least two of the three sessions (Table 2). Based on the commonly recognized associations in adult rhesus, the three clusters could be characterized as “positive/fearful”, “negative/aggressive”, and “distress”. Treatment, Genotype and Treatment*Genotype effects were not significant for any of these clusters individually.
Table 2.
Individual expressive behaviors included in the ethogram. Behaviors contributing to principal components derived from individual session multivariate analysis are shown in the first three columns. Occurrences summed across all sessions and in each 240 second session are shown in the last four columns.
| PC1 Negative/ Aggressive |
PC2 Positive/ Fearful |
PC3 Distress |
All Occurrences |
Dosing 1 | Dosing 2 | Post- Dosing |
|
|---|---|---|---|---|---|---|---|
| Bark | X | - | - | 49 | 0 | 12 | 37 |
| Cage shake | X | - | - | 195 | 61 | 47 | 87 |
| Threat | X | - | - | 239 | 147 | 44 | 48 |
| Grunt | X | - | - | 622 | 340 | 195 | 87 |
| Coo | X | - | - | 1035 | 702 | 244 | 89 |
| Crouch | - | - | - | 331 | 107 | 44 | 180 |
| Fear grimace | - | X | - | 73 | 44 | 15 | 14 |
| Lipsmack | - | X | - | 450 | 245 | 188 | 17 |
| Rump present | - | X | - | 18 | 9 | 2 | 7 |
| Jerk | - | - | X | 10 | 1 | 4 | 5 |
| Scratch | - | - | X | 7 | 1 | 3 | 3 |
| Self-clasp | - | - | X | 20 | 8 | 7 | 5 |
| Self-directed stereotypy | - | - | - | 36 | 20 | 4 | 12 |
| Tooth grind | - | - | - | 8 | 0 | 8 | 0 |
| Scream | - | - | - | 13 | 10 | 3 | 0 |
| Yawn | - | - | - | 13 | 1 | 9 | 3 |
3.5 Fluoxetine effects in the post-dosing session
The lack of a Session*Treatment*Genotype interaction indicated that the effects on expressive behaviors seen during and after dosing were consistent. However, independent second level analyses of the post-dosing session were conducted to support this conclusion. The interaction term (Treatment*Genotype) was significant for sum of all expressive behaviors in the session (F=4.58, p=0.042). The pattern of means is shown in Figure 3A. Treatment effects within Genotype groups were not significant, in contrast to analyses during dosing. Fluoxetine appeared to decrease the number of expressive behaviors in the low-MAOA group, as was the case during dosing, but also to increase it in the hi-MAOA group. Also, an effect of genotype on expressive behaviors appeared in the post-dosing session. For the control (vehicle) group, the low-MAOA subgroup had marginally higher numbers of expressive behaviors than the hi-MAOA group (p=0.046).
Figure 3.
Persistence of fluoxetine effects in the post-dosing session conducted one year after discontinuation of fluoxetine administration. A. Comparison of the response (all expressive behaviors) of Treatment and Genotype subgroups to all slides in the post-dosing session. A treatment*Genotype interaction was identified with no significant planned comparisons. B. Comparison of response to social affiliation pictures in the postdosing session. N=8/subgroup except for low-MAOA vehicle subgroup N=7.
Effects on the separate PCA behavior categories (positive, negative/aggressive, distress) in the post-dosing session as was the case during dosing. For picture categories significant Treatment*Genotype interaction was seen for the social affiliation pictures (F=8.55, p=0.007)(Figure 3B), but not the fearful (p=0.08) and neutral (p=0.12) pictures. In planned comparisons for the social affiliation category, the fluoxetine effect in the low-MAOA group was marginal (p=0.056) and a fluoxetine effect also appeared in the hi-MAOA group (p=0.042). .
3.6 Fluoxetine effects on looking, activity and stereotypy
As background for interpretation of expressive behavior, the amount of time spent looking at the pictures, the duration of activity (walking, moving, rapid position changes, rapid torso movement), and the number and duration of stereotypy episodes were summarized and analyzed (Figure 4). The average time spent looking at pictures (Figure 4A) and being active (Figure 4B) did not differ across sessions. There were no treatment or genotype effect, or interactions, for looking time. For activity duration, neither fluoxetine treatment nor MAOA genotype were significant in the RMANOVA, but the Treatment*Genotype interaction showed a nonsignificant trend (p=0.12). When sessions were examined separately, activity was significantly lower in the fluoxetine than vehicle group at the end of dosing (F=4.72, p=0.038). Five fluoxetine-treated monkeys and two vehicle monkeys had no activity in that session. There was also a trend (p=0.07) for an effect of MAOA genotype, with the low-MAOA animals having less activity in that session.
Figure 4.
Behavior states during slide presentations. A. Duration of looking at the monitor during slide presentations. No Treatment or Genotype effects were identified. B. Duration of motor activity during slide presentations. ANOVA analysis of Dosing 2 session identified lower activity in the fluoxetine treated group (Treatment effect) (see text). C. Number of subjects displaying episodes of stereotypy. No treatment effect was identified with nonparametric analysis.
Juvenile monkeys display motor and self-directed stereotypies in stress-inducing situations. Thirteen monkeys (13/31) demonstrated motor stereotypy at least once during the three slide show presentation sessions with an average episode duration of 6.6 ± 0.4 sec. Six were in the fluoxetine group and seven in the vehicle group. Five monkeys had at least one incident of self-directed stereotypy when summed across sessions; four of these were in the vehicle treated group. The episodes of stereotypy were not frequent enough to allow multivariate analyses by session, but there were no apparent differences in number or duration by Treatment group, Genotype, picture category or session (Figure 4C).
4.0 Discussion
In terms of the hypotheses under consideration in the design, the following were supported:
Fluoxetine affects responding to emotionally evocative pictures
The fluoxetine effect can be modified by MAOA genotype
The fluoxetine decreased the frequency of emotional behaviors, but this effect occurred only in the low-MAOA genotype subgroup during dosing. After dosing one specific behavior was also significantly affected in the hi-MAOA group. Appearance of the effect only in the MAOA subgroup with a low expression allele suggests that this genetic polymorphism may be the source of some of the variability in response to treatment for this drug in children. Notably, one study found that women patients with low-MAOA showed a better response to four weeks of fluoxetine treatment than their high-MAOA genotype counterparts (Yu et al., 2005). However, the high-MAOA expression allele was more prevalent in the MDD patients than in controls of this study. There have been a number of studies of MAOA VNTR polymorphisms as risk factors for MDD, but metaanalysis confirmed this association only in Asian populations (Fan et al., 2010). In preschool boys (N=97), increased caregiver depression or family conflict was associated with increased depression symptoms in boys with low-MAOA polymorphism genotypes (Lavigne et al., 2013). Our study did not use an animal model of a childhood behavior disorder, but, using the RDoC framework for integrating basic research and diagnostic criteria (Garvey et al., 2016; Cuthbert and Insel, 2013; Maestripieri and Lilienfeld, 2016), the results can be seen applicable to the “negative valence” construct dimension relevant to depression, as well as other developmental diagnoses.
Two other hypotheses were not supported:
Fluoxetine effects are specific to pictures with high affective content
Fluoxetine influences specific behaviors or clusters of behavior
Fluoxetine effects were most clearly seen in response to pictures with high affective content, particularly pictures of monkeys. However the same pattern of decreased response in low-MAOA subgroup treated with fluoxetine was seen for all categories of pictures. Additionally, fluoxetine did not appear to specifically influence behaviors commonly associated with aggression, affiliation, anxiety or distress. It should be mentioned that the relatively minor level of stress imposed by the repeated picture viewing sessions would not be expected to evoke a strong emotional response as other paradigms such as maternal-infant separation or stranger intrusion.
Two hypotheses concerning length and termination of dosing were not supported:
Fluoxetine effects progress from one to two years of dosing
The fluoxetine effect reverses after discontinuation of dosing for one year
Perhaps the unique contribution of this study is evaluation of effects after discontinuation of a long period of therapeutic exposure in childhood. The analysis was oriented toward persistence of effects in a longitudinal study. For generalization to human pediatric patients, more work would need to be done varying the age at initiation and duration of treatment and age at post-treatment assessment to understand the implications of the persistent effects in terms of sensitive periods in brain development. However, the finding of persistent effects does reinforce the caution that pediatric treatment with psychoactive agents could affect brain development as well as providing a therapeutic effect.
In general the study confirmed the effect of fluoxetine in reducing emotional response as measured here behaviorally. A new and important finding was that this fluoxetine effect appeared only in monkeys with low-MAOA polymorphisms, at least at younger ages during dosing. These findings suggest that MAOA is an important candidate gene for studies of children’s response to fluoxetine treatment. Controlled clinical trials with fluoxetine found response to treatment rates of 56% (Emslie et al., 1997) and 65% (Emslie et al., 2002) but in the context of substantial placebo effects (33% and 53% respectively) resulting in a modest improvement. Notably, in human studies with mixed sex populations, the occurrence of various polymorphism and a mixture of male and female heterozygotes and homozygotes (23% low-high, 23% low-low, 54% high-high alleles) (Enoch et al., 2010) would support a response rate of 20% if only low-MAOA subjects responded.
In translating to humans, it is important to note that MAOA VNTR polymorphisms in monkeys are not structurally identical to those in humans although they are associated with different transcription rates. Other limitations in translation require consideration. In humans, MDD diagnoses do occur in preschoolers (Hopkins et al., 2013), but peak childhood (excluding adolescent) rates of diagnosis are just prior to puberty (Kessler et al., 2005), much later than treatment was initiated in the present study. Antidepressant treatment, once initiated, can continue for several years in “maintenance” mode in children as in adults. A chart review of SSRI use in children reported an average duration of 27±20 months (mean ± SD) (Wilens et al., 2003). In the present study, even adjusted for the shorter duration of juvenile development in monkeys vs. humans, the two-year duration of continuous treatment was longer than typically experienced by children. Finally the post-dosing follow-up session provided ample time for drug clearance but was still prior to puberty and adolescence which occur at about 4.5 years of age, 6 months after the conclusion of the post dosing evaluations (Mann et al., 1998). Thus carryover of drug effects into that vulnerable life-stage could not be evaluated.
In conclusion, this study demonstrates the potential value of nonhuman primate model for informing safe and effective use of fluoxetine in children. Two potential translational implications are suggested: (1) MAOA genetic polymorphisms may be the source of some of the variability in response to fluoxetine treatment in children; (2) extended fluoxetine treatment during juvenile brain development may result in persistent effects on emotional regulation.
Supplementary Material
Acknowledgments
The authors acknowledge the contributions of CNPRC Research Services, Veterinary Medicine and Colony Management in maintaining the health and well-being of the monkeys during the study and facilitating the experimental protocol. Laura del Rosso provided insights into interpretation of behavioral patterns. Supported by NIH grants R01 HD065862 (Mari Golub, PI), and OD011107 (Harris Lewin, PI). NIH had no role in conduct, analysis or interpretation of the study.
Role of Funding Source
Funding for this study was provided by NIH Grant HD065862; the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MSG designed and supervised the study, analyzed the data and wrote the manuscript. CEP developed protocols, and performed and supervised technical work. AMB developed video scoring protocols, conducted video scoring and prepared figures. All authors contributed to and have approved the final manuscript.
References
- Anderson GM. Peripheral and central neurochemical effects of the selective serotonin reuptake inhibitors (SSRIs) in humans and nonhuman primates: assessing bioeffect and mechanisms of action. Int J Dev Neurosci. 2004;22:397–404. doi: 10.1016/j.ijdevneu.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson DA, Monk K, Kalas C, Clark DB, Ehmann M, Bridge J, Heo J, Brent DA. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42:415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent D, Bernet W, Bukstein O, Walter H, Benson RS, Chrisman A, Farchione T, Greenhill L, Hamilton J, Keable H, Kinlan J, Schoettle U, Stock S, Ptakowski KK, Medicus J. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- Byrd AL, Manuck SB. MAOA, childhood maltreatment, and antisocial behavior: meta-analysis of a gene-environment interaction. Biol Psychiatry. 2014;75:9–17. doi: 10.1016/j.biopsych.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AS, Ebert MH, Schmidt DE, McKinney WT, Kraemer GW. Biogenic amine activity in response to fluoxetine and desipramine in differentially reared rhesus monkeys. Biol Psychiatry. 1999;46:221–228. doi: 10.1016/s0006-3223(99)00027-x. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Kraemer GW, Kupfer DJ. Effects of rearing condition on HPA axis response to fluoxetine and desipramine treatment over repeated social separations in young rhesus monkeys. Psychiatry Res. 1998;79:91–104. doi: 10.1016/s0165-1781(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Costa AC, Scott-McKean JJ. Prospects for improving brain function in individuals with Down syndrome. CNS Drugs. 2013;27:679–702. doi: 10.1007/s40263-013-0089-3. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:e126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, Hohoff C, Schoning S, Kersting A, Baune BT, Mortensen LS, Arolt V, Zwitserlood P, Deckert J, Heindel W, Suslow T. Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol. 2009;12:11–22. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Foa EB, Huppert JD, Keefe FJ, Franklin ME, Compton JS, Zhao N, Connor KM, Lynch TR, Gadde KM. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61:1005–1013. doi: 10.1001/archpsyc.61.10.1005. [DOI] [PubMed] [Google Scholar]
- Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J Affect Disord. 2011;130:66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, Nilsson M, Jacobson JG. Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2002;41:1205–1215. doi: 10.1097/00004583-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Hughes CW, Carmody T, Rintelmann J. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry. 1997;54:1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Steer CD, Newman TK, Gibson N, Goldman D. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes Brain Behav. 2010;9:65–74. doi: 10.1111/j.1601-183X.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Liu B, Jiang T, Jiang X, Zhao H, Zhang J. Meta-analysis of the association between the monoamine oxidase-A gene and mood disorders. Psychiatr Genet. 2010;20:1–7. doi: 10.1097/YPG.0b013e3283351112. [DOI] [PubMed] [Google Scholar]
- Fontenot MB, Musso MW, McFatter RM, Anderson GM. Dose-finding study of fluoxetine and venlafaxine for the treatment of self-injurious and stereotypic behavior in rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2009;48:176–184. [PMC free article] [PubMed] [Google Scholar]
- Fontenot MB, Padgett EE, 3rd, Dupuy AM, Lynch CR, De Petrillo PB, Higley JD. The effects of fluoxetine and buspirone on self-injurious and stereotypic behavior in adult male rhesus macaques. Comp Med. 2005;55:67–74. [PubMed] [Google Scholar]
- Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Gaffrey MS, Barch DM, Singer J, Shenoy R, Luby JL. Disrupted amygdala reactivity in depressed 4- to 6-year-old children. J Am Acad Child Adolesc Psychiatry. 2013;52:737–746. doi: 10.1016/j.jaac.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey M, Avenevoli S, Anderson K. The National Institute of Mental Health Research Domain Criteria and Clinical Research in Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry. 2016;55:93–98. doi: 10.1016/j.jaac.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KE, Pierre PJ, Daunais J, Bennett AJ, Martelle S, Gage HD, Swanson JM, Nader MA, Porrino LJ. Chronic treatment with extended release methylphenidate does not alter dopamine systems or increase vulnerability for cocaine self-administration: a study in nonhuman primates. Neuropsychopharmacology. 2012;37:2555–2565. doi: 10.1038/npp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Bulleri AM, Hogrefe CE, Sherwood RJ. Bone growth in juvenile rhesus monkeys is influenced by 5HTTLPR polymorphisms and interactions between 5HTTLPR polymorphisms and fluoxetine. Bone. 2015;79:162–169. doi: 10.1016/j.bone.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE. Fluoxetine: juvenile pharmacokinetics in a nonhuman primate model. Psychopharmacology (Berl) 2014;231:4041–4047. doi: 10.1007/s00213-014-3537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE. Sleep disturbance as detected by actigraphy in pre-pubertal juvenile monkeys receiving therapeutic doses of fluoxetine. Neurotoxicol Teratol in press. 2016 doi: 10.1016/j.ntt.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Bulleri AM. Peer social interaction is facilitated in juvenile rhesus monkeys treated with fluoxetine. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.02.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Unger EL. Influence of prenatal iron deficiency and MAOA genotype on response to social challenge in rhesus monkey infants. Genes Brain Behav. 2012;11:278–290. doi: 10.1111/j.1601-183X.2012.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Hogrefe CE, Grapov D, Palazoglu M, Fiehn O, Turck CW, Golub MS. Identifying individual differences of fluoxetine response in juvenile rhesus monkeys by metabolite profiling. Transl Psychiatry. 2014;4:e478. doi: 10.1038/tp.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetrick S, Merry S, McKenzie J, Sindahl P, Proctor M. Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents. Cochrane Database Syst RevCD004851. 2007 doi: 10.1002/14651858.CD004851.pub2. [DOI] [PubMed] [Google Scholar]
- Hopkins J, Lavigne JV, Gouze KR, LeBailly SA, Bryant FB. Multi-domain models of risk factors for depression and anxiety symptoms in preschoolers: evidence for common and specific factors. J Abnorm Child Psychol. 2013;41:705–722. doi: 10.1007/s10802-013-9723-2. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “adverse” environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biol Psychiatry. 2009;65:770–777. doi: 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Lavigne JV, Herzing LB, Cook EH, Lebailly SA, Gouze KR, Hopkins J, Bryant FB. Gene x environment effects of serotonin transporter, dopamine receptor D4, and monoamine oxidase A genes with contextual and parenting risk factors on symptoms of oppositional defiant disorder, anxiety, and depression in a community sample of 4-year-old children. Dev Psychopathol. 2013;25:555–575. doi: 10.1017/S0954579412001241. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Lilienfeld SO. Using the NIMH Research Domain Criteria (RDoC) in human and nonhuman primate research. Psychophysiology. 2016;53:367–371. doi: 10.1111/psyp.12552. [DOI] [PubMed] [Google Scholar]
- Mandell DJ, Unis A, Sackett GP. Post-drug consequences of chronic atypical antipsychotic drug administration on the ability to adjust behavior based on feedback in young monkeys. Psychopharmacology (Berl) 2011;215:345–352. doi: 10.1007/s00213-010-2147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DR, Akinbami MA, Gould KG, Paul K, Wallen K. Sexual maturation in male rhesus monkeys: importance of neonatal testosterone exposure and social rank. J Endocrinol. 1998;156:493–501. doi: 10.1677/joe.0.1560493. [DOI] [PubMed] [Google Scholar]
- Mattison DR, Plant TM, Lin HM, Chen HC, Chen JJ, Twaddle NC, Doerge D, Slikker W, Jr, Patton RE, Hotchkiss CE, Callicott RJ, Schrader SM, Turner TW, Kesner JS, Vitiello B, Petibone DM, Morris SM. Pubertal delay in male nonhuman primates (Macaca mulatta) treated with methylphenidate. Proc Natl Acad Sci U S A. 2011;108:16301–16306. doi: 10.1073/pnas.1102187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, A RH, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 2011 [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Norbury R, Taylor MJ, Selvaraj S, Murphy SE, Harmer CJ, Cowen PJ. Short-term antidepressant treatment modulates amygdala response to happy faces. Psychopharmacology (Berl) 2009;206:197–204. doi: 10.1007/s00213-009-1597-1. [DOI] [PubMed] [Google Scholar]
- Patterson TA, Li M, Hotchkiss CE, Mauz A, Eddie M, Greischel A, Stierstorfer B, Deschl U, Paule MG. Toxicity assessment of pramipexole in juvenile rhesus monkeys. Toxicology. 2010;276:164–171. doi: 10.1016/j.tox.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Paule MG, Allen RR, Bailey JR, Scallet AC, Ali SF, Brown RM, Slikker W., Jr Chronic marijuana smoke exposure in the rhesus monkey. II: Effects on progressive ratio and conditioned position responding. J Pharmacol Exp Ther. 1992;260:210–222. [PubMed] [Google Scholar]
- Perlman SB, Hein TC, Stepp SD. Emotional reactivity and its impact on neural circuitry for attention-emotion interaction in childhood and adolescence. Dev Cogn Neurosci. 2014;8:100–109. doi: 10.1016/j.dcn.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalzgraf AR, Scott V, Makela E, Kavookjian J, Hartsock SL, Miller LA. Child psychiatrists’ self-reported treatment and monitoring of children and adolescents with major depressive disorder. J Psychiatr Pract. 2012;18:253–261. doi: 10.1097/01.pra.0000416015.60838.a5. [DOI] [PubMed] [Google Scholar]
- Pickles A, Hill J, Breen G, Quinn J, Abbott K, Jones H, Sharp H. Evidence for interplay between genes and parenting on infant temperament in the first year of life: monoamine oxidase A polymorphism moderates effects of maternal sensitivity on infant anger proneness. J Child Psychol Psychiatry. 2013;54:1308–1317. doi: 10.1111/jcpp.12081. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Allen RR, Pearson EC, Hammond TG, Paule MG. Differential effects of two NMDA receptor antagonists on cognitive-behavioral development in nonhuman primates I. Neurotoxicol Teratol. 2001;23:319–332. doi: 10.1016/s0892-0362(01)00156-8. [DOI] [PubMed] [Google Scholar]
- Pringle A, Harmer CJ. The effects of drugs on human models of emotional processing: an account of antidepressant drug treatment. Dialogues Clin Neurosci. 2015;17:477–487. doi: 10.31887/DCNS.2015.17.4/apringle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvanian H, Hashemipour M, Kelishadi R, Tavakoli N, Poursafa P. A randomized, triple masked, placebo-controlled clinical trial for controlling childhood obesity. World J Pediatr. 2010;6:317–322. doi: 10.1007/s12519-010-0232-x. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Salomons TV, Konarski JZ, Downar J, Giacobbe P, McIntyre RS, Kennedy SH. Neural response to emotional stimuli associated with successful antidepressant treatment and behavioral activation. J Affect Disord. 2013;151:573–581. doi: 10.1016/j.jad.2013.06.050. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, Morris SM, Hotchkiss CE, Doerge DR, Allen RR, Mattison DR, Paule MG. The effects of chronic methylphenidate administration on operant test battery performance in juvenile rhesus monkeys. Neurotoxicol Teratol. 2010;32:142–151. doi: 10.1016/j.ntt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer EK, Howell LL. Pharmacokinetics of fluoxetine in rhesus macaques following multiple routes of administration. Pharmacology. 2011;88:44–49. doi: 10.1159/000329417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha SS, Nelson EE, Liow JS, Gladding R, Lyoo CH, Noble PL, Morse C, Henter ID, Kruger J, Zhang B, Suomi SJ, Svenningsson P, Pike VW, Winslow JT, Leibenluft E, Pine DS, Innis RB. Fluoxetine administered to juvenile monkeys: effects on the serotonin transporter and behavior. Am J Psychiatry. 2014;171:323–331. doi: 10.1176/appi.ajp.2013.13020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto PL, Wilcox KM, Zhou Y, Kumar A, Ator NA, Riddle MA, Wong DF, Weed MR. Long-term exposure to oral methylphenidate or dl-amphetamine mixture in peri-adolescent rhesus monkeys: effects on physiology, behavior, and dopamine system development. Neuropsychopharmacology. 2012;37:2566–2579. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32:149–157. doi: 10.1002/da.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Calley CS, Hart J, Mayes TL, Nakonezny PA, Lu H, Kennard BD, Tamminga CA, Emslie GJ. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. Am J Psychiatry. 2012;169:381–388. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME. MAOIs and depression treatment guidelines. J Clin Psychiatry. 2012;73:e24. doi: 10.4088/JCP.11096tx4c. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Kwon A, Chase R, Greenberg L, Mick E, Spencer TJ. A systematic chart review of the nature of psychiatric adverse events in children and adolescents treated with selective serotonin reuptake inhibitors. J Child Adolesc Psychopharmacol. 2003;13:143–152. doi: 10.1089/104454603322163862. [DOI] [PubMed] [Google Scholar]
- Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2013;8:Cd004677. doi: 10.1002/14651858.CD004677.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YW, Tsai SJ, Hong CJ, Chen TJ, Chen MC, Yang CW. Association study of a monoamine oxidase a gene promoter polymorphism with major depressive disorder and antidepressant response. Neuropsychopharmacology. 2005;30:1719–1723. doi: 10.1038/sj.npp.1300785. [DOI] [PubMed] [Google Scholar]
- Zhang M, Chen X, Deng H, Lu Z. Identifying the interaction of maternal sensitivity and two serotonin-related gene polymorphisms on infant self-regulation. Infant Behav Dev. 2014;37:606–614. doi: 10.1016/j.infbeh.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Zhang M, Chen X, Way N, Yoshikawa H, Deng H, Ke X, Yu W, Chen P, He C, Chi X, Lu Z. The association between infants’ self-regulatory behavior and MAOA gene polymorphism. Dev Sci. 2011;14:1059–1065. doi: 10.1111/j.1467-7687.2011.01047.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.