Abstract
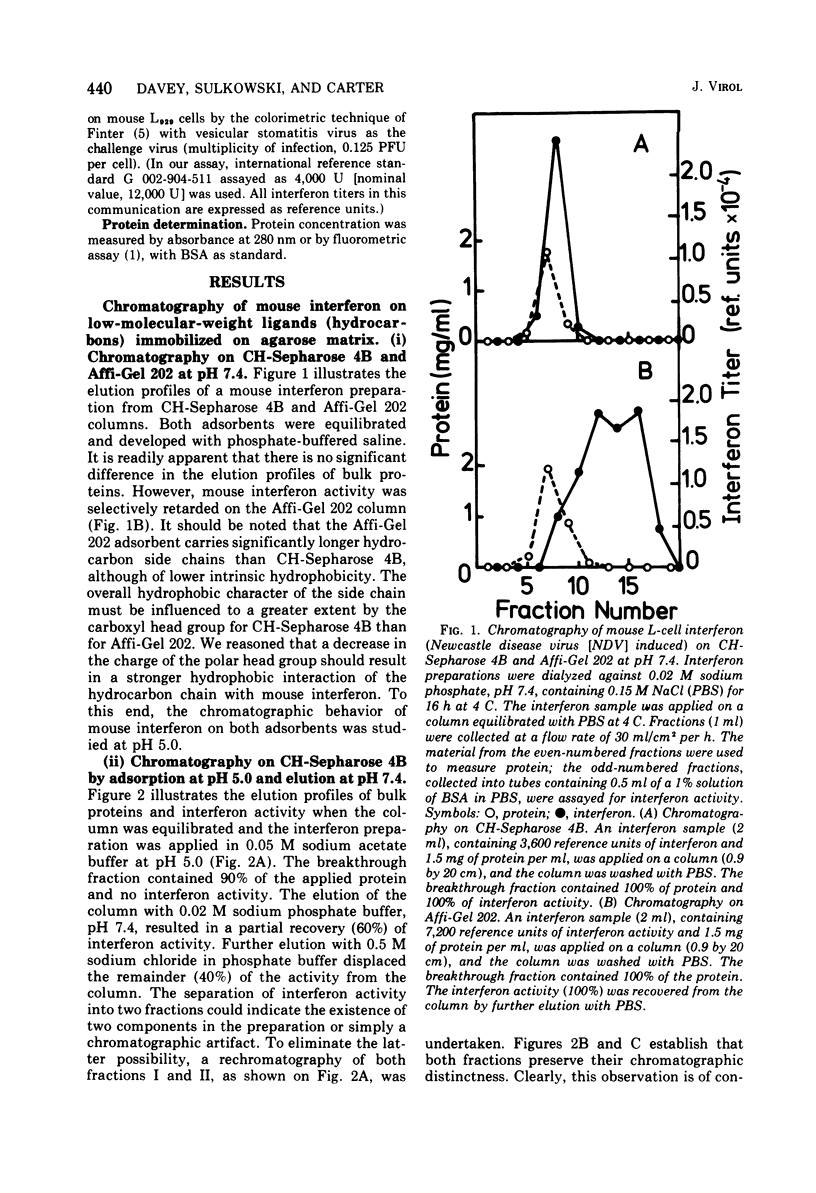
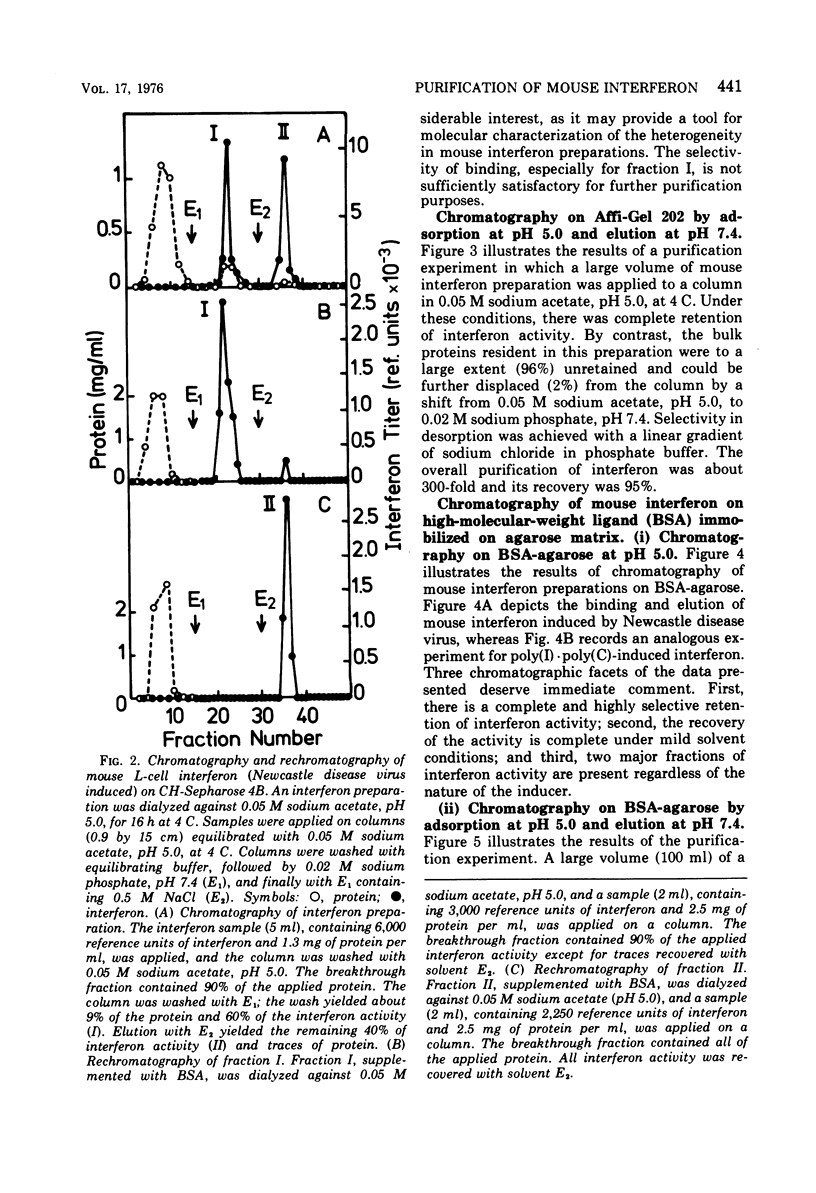
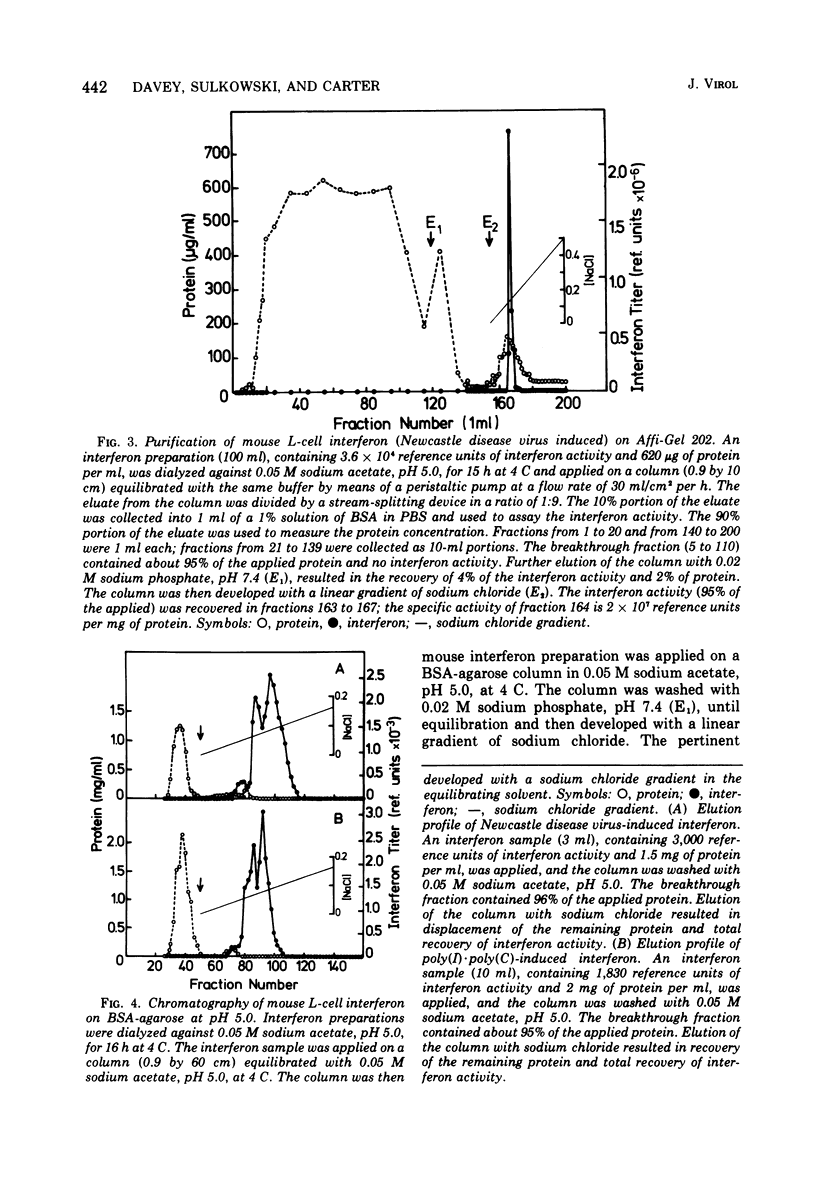
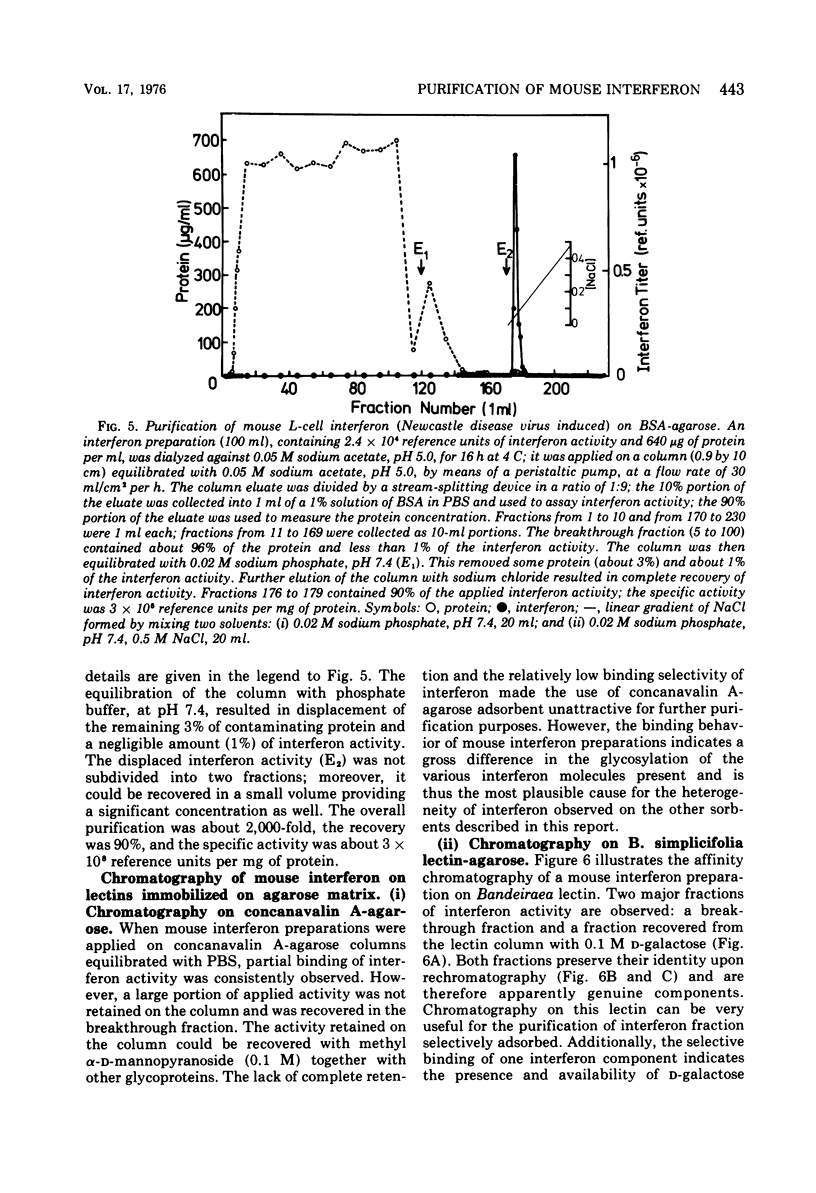
Several novel selective sorbents for mouse interferon are described that exploit the hydrophobic property and glycoprotein nature of this molecule. Low-molecular-weight ligands (hydrocarbons) and high-molecular-weight ligands (bovine serum albumin) immobilized on agarose bind selectively mouse L-cell interferon. The high selectivity of binding is due primarily to a hydrophobic effect, although electrostatic forces are also apparently involved. Mouse L-cell interferon binds to immobilized serum albumin and can be completely recovered by raising the ionic strength of the eluant. The specific activity of interferon preparations can be increased 2,000-fold to a value of 3 x 10(8) reference units per mg of protein in a single step with full recovery of the antiviral activity. A selective adsorption, although to a lesser degree, can be also obtained on hydrocarbon-coated agarose (Affi-Gel 202), resulting in 300-fold purification on desorption. The existence of two major components of mouse interferon was revealed upon its chromatography on the following sorbents: (i) bovine serum albumin-agarose, (ii) omega-carboxypentyl-agarose; and (iii) Bandeiraea simplicifolia lectin-agarose. This report thus provides for the first time a means for efficient and clear-cut separation of interferon components, thus enabling their further characterization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Chadha K. C., Davey M. W., Byrd D. M., Carter W. A. Differential production of interferon and refractoriness inducing principle in L cells. Infect Immun. 1974 Nov;10(5):1057–1061. doi: 10.1128/iai.10.5.1057-1061.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. W., Huang J. W., Sulkowski E., Carter W. A. Hydrophobic binding sites on human interferon. J Biol Chem. 1975 Jan 10;250(1):348–349. [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J., Vandeputte M. Inhibition by interferon of delayed-type hypersensitivity in the mouse. Proc Natl Acad Sci U S A. 1975 May;72(5):1753–1757. doi: 10.1073/pnas.72.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes C. E., Goldstein I. J. An alpha-D-galactosyl-binding lectin from Bandeiraea simplicifolia seeds. Isolation by affinity chromatography and characterization. J Biol Chem. 1974 Mar 25;249(6):1904–1914. [PubMed] [Google Scholar]
- Hofstee B. H. Hydrophobic affinity chromatography of proteins. Anal Biochem. 1973 Apr;52(2):430–448. doi: 10.1016/0003-2697(73)90046-8. [DOI] [PubMed] [Google Scholar]
- Huang J. W., Davey M. W., Hejna C. J., Von Muenchhausen W., Sulkowski E., Carter W. A. Selective binding of human interferon to albumin immoblized on agarose. J Biol Chem. 1974 Jul 25;249(14):4665–4667. [PubMed] [Google Scholar]
- Knight E., Jr Heterogeneity of purified mouse interferons. J Biol Chem. 1975 Jun 10;250(11):4139–4144. [PubMed] [Google Scholar]
- Ogburn C. A., Berg K., Paucker K. Purification of mouse interferon by affinity chromatography on anti-interferon globulin-sepharose. J Immunol. 1973 Oct;111(4):1206–1218. [PubMed] [Google Scholar]
- Sipe J. D., de Maeyer-Guignard J., Fauconnier B., de Maeyer E. Purification of mouse interferon by affinity chromatography on a solid-phase immunoadsorbent. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1037–1040. doi: 10.1073/pnas.70.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. E., 2nd Distinct molecular species of interferons. Virology. 1974 Sep;61(1):80–86. doi: 10.1016/0042-6822(74)90243-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Tsukui K., Ohwaki M., Kawade Y. Electrophoretic characterization of purified mouse L cell interferon of high specific activity. J Gen Virol. 1974 Apr;23(1):23–32. doi: 10.1099/0022-1317-23-1-23. [DOI] [PubMed] [Google Scholar]


