Abstract
Objective
The low regenerative potential of cartilage contributed to the development of different cell therapies aimed to improve the clinical outcome in young patients with Osteochondral Lesions of the Talus (OLT). This study is designed to assess the regenerative potential of autologous matrix-induced Bone Marrow Aspirate Concentrate (mBMAC) and matrix-induced Autologous Chondrocyte Implantation (mACI) evaluating, on a small number of osteochondral biopsies, the expression of some catabolic, inflammatory, and pain mediators.
Design
Twenty-two patients with OLT were analyzed in this study; 7 were treated with mACI and 15 with mBMAC. Informed consent was obtained from all the patients. Clinical assessments were performed pre-operatively and at 12, 24, and 36 months after surgery using the American Orthopedic Foot and Ankle Society (AOFAS). Histology and immunohistochemistry were used to assess cartilage repair at 24 months. Data were analyzed using non-parametric Wilcoxon-Mann-Whitney and Spearman tests.
Results
A remarkable improvement in AOFAS score was noticed for both treatments up to 36 months; however, patients treated with mACI reported the best AOFAS score. Various degrees of tissue remodeling were observed by histological analysis for both cell strategies. However, mBMAC treatment showed a higher expression of some fibrous and hypertrophic markers compared to mACI group. A mild positivity for nerve growth factor, as pain mediator, was noticed for both treatments.M
Conclusions
Our findings demonstrated the best histological and clinical results following mACI treatment since different fibrotic and hypertrophic features were evident in the mBMAC group at 24-month follow-up.
Keywords: chondrocytes, stromal cells, scoring systems, ankle, cartilage repair
Introduction
Osteochondral lesions of the talus (OLT), often resulting from sport injuries in young patients, are associated with local inflammatory and catabolic reactions and represent one of the main risk factors for the development of post-traumatic osteoarthritis (OA).1-5 Concurrent with the impairment of cartilage and bone homeostasis, the sensitization of nociceptive pathways lead to the onset of acute and chronic pain states during OA.6,7 The ideal therapeutic strategy for OLT should be able to provide biological and biomechanical properties necessary for the regeneration of the entire osteochondral unit. From a biological point of view, the integrity of the calcified cartilage layer is an important criterion to consider as it plays a crucial role in preventing vascularization and migration of cells resulting in bone ingrowth.8 However, a number of other key aspects have to be monitored during osteochondral repair, such as fibrosis, catabolic and inflammatory processes, responsible for the production of several cytokines and oxygen-derived free radicals, which lead to joint destruction and pain.2,3
From a clinical point of view, therapeutic strategies for OLT are influenced by a variety of factors, including patient characteristics, the anatomical site, and the size of the lesion.9 Besides marrow stimulating techniques such as abrasion, drilling, or microfractures,10,11 great clinical success was obtained in 1994 through the use of Autologous Chondrocyte Implantation (ACI).12-15 The advances in the field of tissue engineering moved more quickly toward the use of bioactive biomaterials, the research of new cell sources, and the identification of innovative therapeutic strategies to improve cartilage repair. With regard to biomaterials, they were first used in combination with chondrocytes, giving rise to the second-/third-generation ACI,16,17 resulting in good clinical results at mid–long-term follow-ups.18-20 Subsequently, more attention has been directed toward the importance of using different cell types, wherein the application of mesenchymal stem cells (MSC)21-24 covered a noticeable interest because of their ability to differentiate towards chondrogenic and osteogenic lineages as well as their ability of secreting a wide amount of growth factors and cytokines.25 Lately, increasing research studies have been focused on the use of “one-step” surgical techniques, such as Bone Marrow Aspirate Concentration (BMAC),26-28 thus allowing easy handling, no cell manipulation, and a large number of cells for the repair of large cartilage defects, resulting in a less expensive strategy compared to other therapeutic strategies.
This study aimed to assess the regenerative potential of autologous matrix-induced Bone Marrow Aspirate Concentrate (mBMAC) and matrix-induced Autologous Chondrocyte Implantation (mACI) techniques in evaluating, on a small number of osteochondral biopsies, the expression of some catabolic, inflammatory, and pain mediators in a small number of patients affected by OLT, treated at the Rizzoli Orthopedic Institute.
Materials and Methods
Twenty-two patients affected by OLT were evaluated through clinical, histological, and immunohistochemical analyses at the Rizzoli Orthopedic Institute. Clinical analyses were carried out preoperatively and at 12, 24, and 36 months following cell treatments, whereas all the other assessments were performed only at 24-month follow-up. Seven patients underwent to an arthroscopic ACI technique (3 women and 4 men; mean age = 31.2 ± 8 years) using chondrocytes seeded onto HYAFF-11 (Fidia Advanced Biopolymers SRL, Abano Terme, Italy), a derivative of hyaluronic acid. Fifteen patients underwent to an arthroscopic BMAC procedure (5 women and 10 men; mean age = 31 ± 7.8 years) obtained with IORG-1 kit (Novagenit, Mezzolombardo, Italy), seeded onto either a collagen scaffold (Novagenit) or a hyaluronic acid autoadhesive membrane (HYAFF-11). All the ankle joints had a mean lesion size of 1.8 ± 0.7 cm2 (range = 1-2.5 cm2). Arthritis of the ankle joint and rheumatoid arthritis were considered exclusion criteria to the surgical procedure. Clinical assessments were performed at 12, 24, and 36 months with American Orthopedic Foot and Ankle Society Ankle-Hindfoot Score (AOFAS).29 The biopsy samples were taken from all treated patients at 24-month follow-up after having obtained informed consent. Histological and immunohistochemical analyses were performed on osteochondral tissues. The study was approved by the Ethical Committee of the Rizzoli Orthopedic Institute (Numbers: 29877 and 0008310).
Matrix-Autologous Chondrocyte Implantation
The mACI was performed through 2-step surgical procedures to obtain the biopsy for chondrocyte isolation followed by a second phase where the cells were implanted. In particular, 3 patients were treated using the classical approach of seeding cultured cells onto a periosteal flap, whereas 4 patients were treated with a third-generation ACI, consisting of chondrocytes grown onto HYAFF-11, a derivative of hyaluronic acid. The average time lag between the first and secondary arthroscopies was approximately 3 weeks. The surgical approach used to carry out the ACI technique has been described in our previous work.30
Matrix-Autologous Bone Marrow Aspirate Concentrate Implantation
Bone marrow was harvested from the iliac crest in small fractions from multiple sites into plastic syringes, internally coated with calcium-heparin solution. This procedure was repeated through the same skin opening to obtain a total of 60 mL bone marrow aspirate. The harvested bone marrow was directly concentrated in the operating theater with a cell separator-concentrator (IORG-1). In particular, 6 patients were treated with seeding cultured cells onto a collagen membrane (Novagenit), whereas in 9 patients cells were seeded onto HYAFF-11 (Fidia), a derivative of hyaluronic acid. The details related to the technical procedure used for BMAC together with the surgical approach have been described in our previous article.1
Post-operative Treatment
Active and passive ankle motions were suggested to all treated patients the day after the surgery. The range of motion was increased gradually according to pain tolerance during the first few weeks. No weight bearing on the affected ankle was recommended for the first 6 weeks following the surgery, and partial weight bearing was permitted starting from this time point. Low-impact sport activities were allowed 4 months after surgery, whereas high-impact activities such as tennis and soccer were permitted after 10 to 12 months after surgery. Patients were followed-up at 12, 24, and 36 months after surgery.
Clinical Evaluation
Patients were clinically evaluated with the AOFAS system as described by Kitaoka et al.29 to monitor their progress preoperatively and at 12, 24, and 36 months after surgery. AOFAS score is based on a scale from 0 (poor clinical outcomes) to 100 (excellent clinical outcomes). Results were rated as follows: excellent, 90 to 100; good, 80 to 89; fair, 60 to 79; and poor, <60.
Histological Analyses
Specimens were fixed in 10% buffered formalin and decalcified in 4% hydrochloric acid and 5% formic acid at room temperature for approximately 4 days and processed until their paraffin embedding. Thin sections (5 µm) were stained with Gill III hematoxylin-eosin (Bioptica, Milano, Italy) and 0.1% Safranin-O/0.02% Fast Green (Sigma Aldrich, St Louis, MO) to evaluate the general morphology and proteoglycan/collagen content, respectively. Histological assessments were supported by specific scoring systems: a modified International Cartilage Repair Society (ICRS)-I score31 to investigate the level of cartilage repair following the different treatments. This score is composed of 5 parameters—surface, matrix organization, cell distribution, cell viability, and cartilage mineralization with a scoring system from 0 (no repair) up to 15 (complete repair of cartilage tissue). All the evaluations were performed by 2 blinded researchers with Eclipse 90i microscope (Nikon, Melville, NY).
Catabolic, Inflammatory, and Pain Mediators through Immunohistochemical Analyses
Protein expressions of collagens I and X; metalloproteinase (MMP)-1, MMP-3, MMP-13; caspase-3 and inducible-Nitric Oxide Synthase (iNOS); Tumor Necrosis Factor-α (TNF-α); interleukin (IL)-1β; S100 calcium-binding protein A9 (S100A9); Nerve Growth Factor (NGF); and Substance P (SP) were evaluated on tissue slides by immunohistochemistry. After having performed antigen retrieval of specimens with 0.1% proteinase (Sigma) at 37°C for 20 minutes, the sections were blocked with 2% bovine serum albumin (Sigma) in phosphate-buffered saline for 30 minutes and then incubated with mouse monoclonal antibodies against human collagen type I (2 µg/mL; Chemicon International, Temecula, CA) and type X (0.5 µg/mL; Sigma), MMP-1, MMP-13 (5 mg/mL; R&D Systems, Minneapolis, MN), caspase-3 (5 mg/mL; R&D Systems), i-NOS (2 mg/mL; Chemicon), IL-1β (1 µg/mL; R&D Systems), TNF-α (2 µg/mL; R&D Systems), S100A9 (1 µg/mL; Abcam, Cambridge, UK), NGF (1 µg/mL; Chemicon), and SP (2 µg/mL; R&D Systems). Sections were incubated with a biotinylated secondary antibody and then reactions were developed using Vulcan Fast Red Chromogen Kit (Biocare Medical, Concord, CA). Negative controls were performed by omitting the primary antibodies or using an isotype-matched control. At the end, the nuclear component was counterstained using CAT hematoxylin (Biocare Medical) and tap-water activation. Image acquisition and processing with Eclipse 90i microscope and NIS-Elements Software allowed the semiquantitative analysis by two blinded investigators using Hue/Saturation/Intensity (HSI). Because all negative-control pixels showed values ranging from 0 to less than 215, we set Hue (H) thresholds for positive pixels at 215 to 255. Ranges of 0 to 150 were established as threshold values for Saturation (S) and Intensity (I). The percentage of positive cells and/or area for each marker was carried out on the whole of the osteochondral sample (10× objective lens) and reported as percentage of positive cells and/or area on a scale from 0 (no protein expression) to 100 (the highest protein expression).
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism software. The Kolmogorov-Smirnov test was used to assess whether data have a normal distribution. The non-parametric Wilcoxon-Mann-Whitney test was used to evaluate differences between preoperative and various clinical follow-up data and to assess the effectiveness of mACI and mBMAC treatments in favoring cartilage repair. Spearman rank correlation test was used to study relationships between clinical, histological, and immunohistochemical data. For all tests, P < 0.05 was considered significant.
Results
Clinical Assessment Performed at the Rizzoli Orthopaedic Institute
The mean preoperative AOFAS score was 47.17 ± 17.10 and 56.07 ± 16.10 in mACI and mBMAC groups, respectively. Both cell-treated groups reported an improvement of AOFAS score from preoperative to 12, 24- and 36-month follow-ups (P < 0.05). In general, the mACI group showed fair clinical results at 12 months and excellent results at long-term follow-ups; mBMAC treatment showed fair results at the short term and good results at 24- and 36-month-follow-ups ( Table 1 ).
Table 1.
Global Clinical Results for AOFAS Score of Patients Treated with mACI (n = 7) and Those Treated with BMAC (n = 15).
| Patient Group | AOFAS Score |
||||
|---|---|---|---|---|---|
| Time | Mean | SD | Median | P Value * | |
| mACI | Preoperative | 47.17 | 17.10 | 54.00 | |
| 12 months | 75.70 | 24.30 | 79.50 | 0.04 | |
| 24 months | 92.40 | 10.04 | 96.00 | 0.001 | |
| 36 months | 92.40 | 10.04 | 96.00 | 0.001 | |
| mBMAC | Preoperative | 56.07 | 16.10 | 54.00 | |
| 12 months | 78.50 | 11.00 | 78.00 | 0.03 | |
| 24 months | 81.92 | 12.81 | 85.00 | 0.001 | |
| 36 months | 84.22 | 14.64 | 90.00 | 0.001 | |
AOFAS = American Orthopedic Foot and Ankle Society; mACI = matrix-induced Autologous Chondrocyte Implantation; mBMAC = matrix-induced Bone Marrow Aspirate Concentrate.
P values < 0.05 were considered significant.
No Differences in Cartilage Repair Were Observed Between mACI and mBMAC Treatments
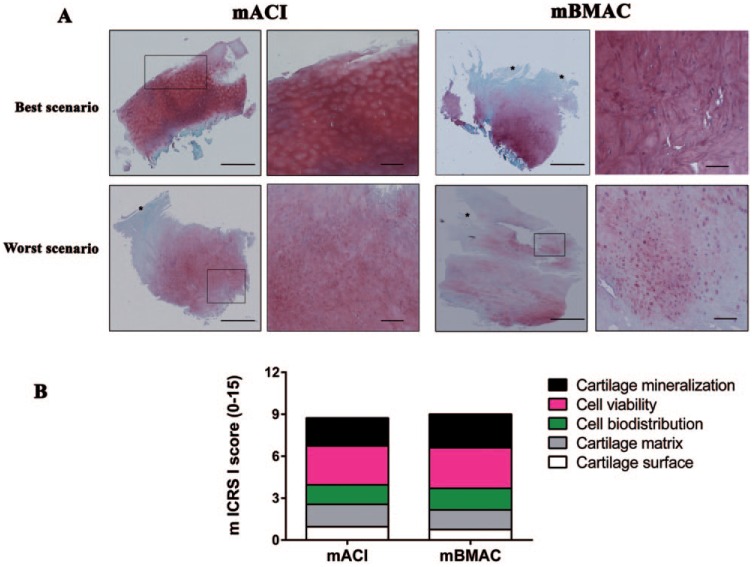
Similar histological findings were observed for specimens following mACI and mBMAC treatments at 24-month follow-up. The best histological scenario with the lowest score, reported in 5 out of 7 patients in the mACI group and 10 out of 15 in the mBMAC group, displayed a well-organized cartilage matrix with nearly regular cellular arrangement and good proteoglycan content with a defined tidemark. Conversely, a small number of patients from the mACI and mBMAC groups showed the presence of various fibrillation processes, individual cells distributed within the cartilage matrix, and proteoglycan depletion ( Fig. 1A ). On the whole, both cell therapies showed various degrees of tissue remodeling, resulting in the formation of a hyaline-like cartilage tissue in both mACI and mBMAC treatments, with mean values for ICRS-I score of 7.6 ± 1.2 and 9.1 ± 0.6, respectively ( Fig. 1B ).
Figure 1.
(A) Safranin-O/Fast Green staining of representative osteochondral samples stained with Safranin-O/Fast Green, treated with matrix-induced Autologous Chondrocyte Implantation (mACI) and matrix-induced Bone Marrow Aspirate Concentrate (mBMAC) procedures at 24-month follow-up. Red indicates proteoglycan content and green indicates collagen content. Scale bars = 100 and 200 µm. (B) Graphical representation of a modified ICRS-I score to assess cartilage repair from mACI (n = 7) and mBMAC (n = 15) groups. Arrows show cell positivity. Data were reported as 95% confidence interval with standard deviation.
Various Levels of Fibrotic, Hypertrophic, and Catabolic Markers Were Noticed Especially Following mBMAC Treatment
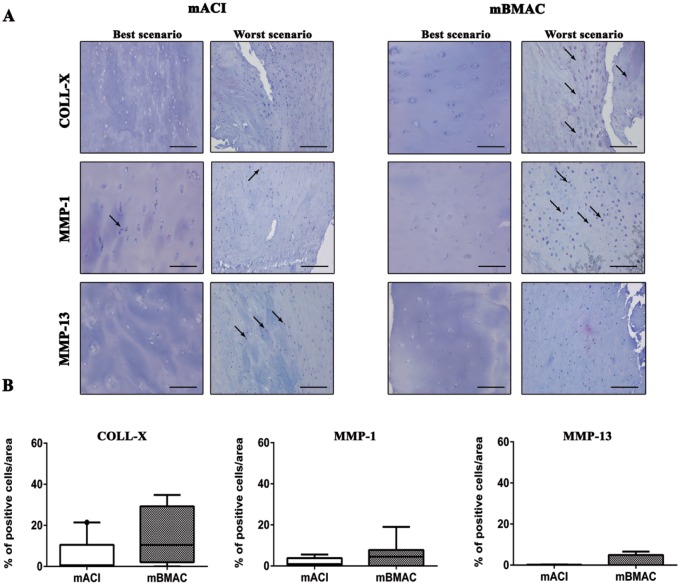
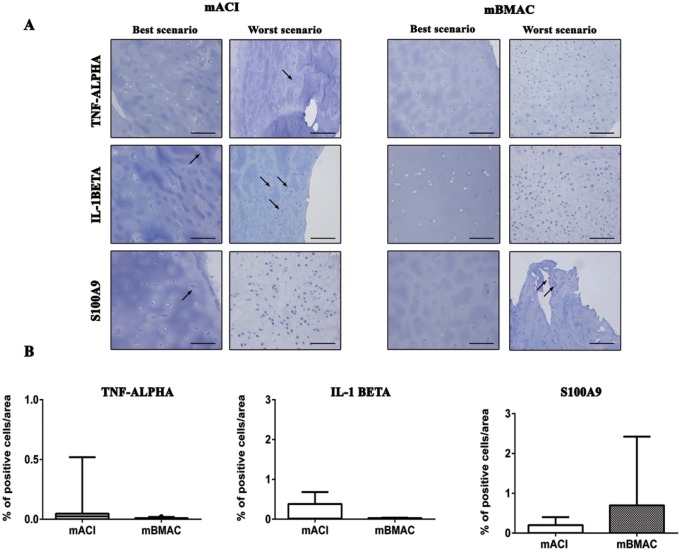
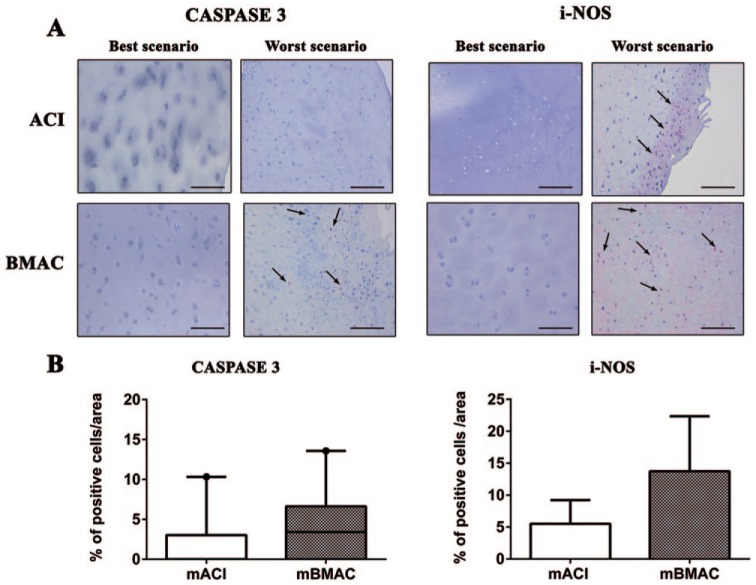
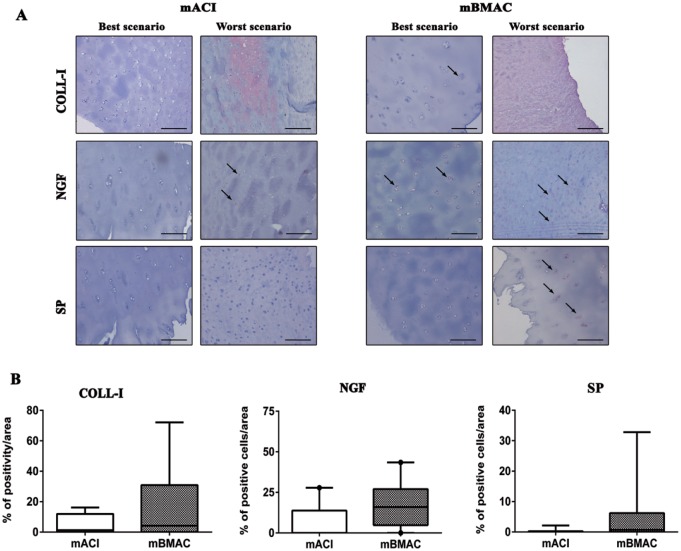
The protein expression for collagen type X was more pronounced for both cell treatments in the worst histological scenarios, reporting positivity at cellular level in the superior and mid-layers of articular cartilage. The percentage of positivity for this hypertrophic marker was slightly higher in mBMAC compared to mACI treatment but without reporting significant evidence. The levels of protein expression for MMP-1 and MMP-13 were lower than collagen type X in both cell therapies; however, mBMAC treatment showed the highest protein expression at the cellular level, especially in the worst histological scenarios ( Fig. 2A and B ). Among the inflammatory markers associated with OA we analyzed, TNF-α, IL-1β, and S100A9 in cartilage biopsies, we observed very low levels of protein expression after both cell treatments ( Fig. 3A and B ). Although mBMAC treatment showed the highest percentages of positive cells for caspase-3 and i-NOS, located at the cellular level near the cartilage surface, no statistical differences between the two treatments were noticed ( Fig. 4A and B ). Fibrotic processes in terms of expression of collagen type I were slightly more pronounced following mBMAC rather than mACI treatment, with a pattern of positivity at the cellular level as well as at the extracellular matrix. Similar levels of expression for NGF were noticed for both cell treatments with an increased number of positive cells in mBMAC treatment. The percentage of protein expression for Substance P was very low, except for bioptic samples from the mBMAC treatment, where there were very low levels of positivity ( Fig. 5A and B ).
Figure 2.
(A) Immunostainings for collagen-X, MMP-1, and MMP-13 of representative osteochondral samples that underwent to mACI and mBMAC procedures at 24-month follow-up. Scale bar = 100 µm. Arrows indicate the presence of positive cells. (B) Graphical representation of percentages of positivity for the aforementioned markers at the cartilage level in patients treated with mACI (n = 7) and mBMAC (n = 15). Arrows report cell positivity. Data were reported as 95% confidence interval with standard deviation. P < 0.05: collagen X for mBMAC versus mACI.
Figure 3.
(A) Representative micrographs of immunohistochemical analyses for TNF-α, IL1β, and S100A9 of osteochondral samples that underwent to mACI and mBMAC procedures at 24-month follow-up. Scale bar = 100 µm. Arrows indicate the presence of positive cells. (B) Graphical representation of percentages of positivity for the aforementioned markers at the cartilage level in patients treated with mACI (n = 7) and mBMAC (n = 15). Arrows report cell positivity. Data were reported as 95% confidence interval with standard deviation.
Figure 4.
(A) Representative micrographs of immunohistochemical analyses for caspase 3 and i-NOS of osteochondral samples that underwent to mACI and mBMAC procedures at 24-month follow-up. Scale bar = 100 µm. Arrows indicate the presence of positive cells. (B) Graphical representation of percentages of positivity for the aforementioned markers at the cartilage level in patients treated with mACI (n = 7) and mBMAC (n = 15). Arrows report cell positivity. Data were reported as 95% confidence interval with standard deviation.
Figure 5.
(A) Representative micrographs of immunohistochemical analyses for collagen, NGF, and SP of osteochondral samples that underwent to mACI and mBMAC procedures at 24-month follow-up. Scale bar = 100 µm. Arrows indicate the presence of positive cells. (B) Graphical representation of percentages of positivity for the aforementioned markers at the cartilage level in patients treated with mACI (n = 7) and mBMAC (n = 15) treatments. Arrows report cell positivity. Data were reported as 95% confidence interval with standard deviation.
A Direct Correlation Between AOFAS and ICRS-I Scores Was Noticed for ACI and BMAC Treatments
A direct relationship between AOFAS and ICRS-I scores was observed for the ACI and BMAC groups, indicating that the improvement of clinical outcome (high AOFAS score) is related to the reorganization of cartilage structure (high ICRS score; P < 0.05) ( Table 2 ). An inverse correlation was instead observed between AOFAS score and fibrosis and pain mediators for both cell strategies, indicating how both treatments contribute to downregulate these degenerative reactions.
Table 2.
Relationship Between Histological Score, Hypertrophy, Fibrosis, Inflammation, Pain, and AOFAS Score for mACI and mBMAC Treatments at 24 Months.
| Variables | Spearman Correlation Between ICRS-I Score and Variables |
Spearman Correlation Between Collagen X and Variables |
Spearman Correlation Between Collagen I and Variables |
Spearman Correlation Between S100A9 and Variables |
Spearman Between NGF and Variables |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| P Value | Rho Value | P Value | Rho Value | P Value | Rho Value | P Value | Rho Value | P Value | Rho Value | |
| AOFAS score for mACI (24 months) | *P < 0.001 | 0.9 | P = 0.9 | −0.3 | *P < 0.001 | −1 | P = 0.8 | 0.3 | *P < 0.001 | −0.9 |
| AOFAS score for mBMAC (24 months) | *P < 0.001 | 1 | P = 0.9 | 0.4 | *P < 0.001 | −0.9 | P = 0.9 | 0.4 | *P < 0.001 | −0.8 |
AOFAS = American Orthopedic Foot and Ankle Society; mACI = matrix-induced Autologous Chondrocyte Implantation; mBMAC = matrix-induced Bone Marrow Aspirate Concentrate; ICRS = International Cartilage Repair Society.
P values < 0.05 were considered significant.
An inverse relationship between AOFAS score and hypertrophy was noticed only in the group treated with mACI. In general, AOFAS score does not show any correlation with the examined inflammatory processes in both treated groups in our case series ( Table 2 ).
Discussion
Autologous chondrocyte implantation is still considered a good therapeutic strategy for cartilage repair, showing encouraging results due to its ability to recreate a cartilage tissue with similar histological features to the native tissue at mid- and long-term follow-up.15,32,33 Our group demonstrated satisfactory clinical results using the ACI technique in the ankle joint up to 7 years.25 However, the limitations of this strategy, such as the great number of cells required that needs laboratory expansion and manipulation, as well as the patient burden and high costs, moved the field of the research toward the development of single-stage approaches. To this end, the use of bone marrow aspirate concentrate, obtained directly in the operating theatre via the use of specific devices,26,27 opened new perspectives in the orthopedic field, reducing the aforementioned problems.34-36 Indeed, the promising results obtained by in vitro and in vivo studies on BMC, indicating its ability to differentiate cartilage and osteochondral lineage37 and to contribute to cartilage repair,38,39 support the idea of using mBMAC in filling osteochondral defects promoting tissue repair.
The present study is a prospective case series aimed to assess the clinical and biological features observed following the use of mACI and mBMAC for OLT in a small number of young patients. Clinical assessment through AOFAS score demonstrated cartilage improvement by the use of both cell strategies with optimal to excellent clinical outcomes up to 36-month follow-up. These data are in agreement with findings from Gobbi’s group that demonstrated, especially through various clinical assessments, the cartilage healing potential of both cell strategies for the treatment of patellofemoral chondral lesions.40
Based on the findings obtained by Peterson et al.,41 who underlined the importance of considering patient status at 2 years as an indicator of future outcome, we harvested osteochondral biopsies at this time, in order to assess the maturation status of the neo-formed tissue following the different treatments. Histological assessments confirmed the clinical results, showing a good cartilage repair with low signs of OA changes according to the ICRS-I score overall following mACI treatment. In general, the majority of cases displayed a well-structured articular cartilage with an adequate cellular arrangement and proteoglycan content; however, some cases displayed some degenerative features, including fibrosis. There is clear evidence that the AOFAS score has a direct correlation with ICRS-I score at 24 months: this means that the best AOFAS score was related to a high histological score, index of good cartilage repair.
To further assess the characteristics of the newly formed tissue, analyses were carried out to investigate collagen I expression and some catabolic and hypertrophic markers. It is well known that one of the major limitations during cartilage repair is the formation of fibrocartilage with large quantities of disorganized collagen I, filling the gap in damaged tissue that lead to an altered tissue function with inferior biomechanical properties.42 Histological analysis supported by ICRS-I score gave evidence of very good histological scenarios in the mACI group, whereas higher levels of fibrotic, hypertrophic processes, and proteins involved in the oxidative stress pathway were evident following mBMAC treatment. We believe that the presence of these features might reflect a late stage of cartilage maturation that is not reported with the use of ACI in which differentiated cells are able to secrete early cartilage matrix proteins in loco. In this regard, we have previously demonstrated by in vitro studies a delay in chondrogenic differentiation following the use of BMAC compared to MSC.37
To properly consider which biological processes are involved in tissue regeneration, we examined some important inflammatory mediators such as IL-1β and TNF-α acting during OA and responsible for pain and joint destruction. Immunohistochemical analyses of these cytokines gave evidence of a very low inflammatory response, suggesting how both cell treatments likely counteract in the first instance this state, well evident during OLT. After having stated the low inflammatory profile in both treatments, we decided to examine what were the effects of both cell strategies on some pain mediators, such as NGF and SP, involved in the annoying pain symptoms during OA. In general, we observed that NGF showed higher expression of SP in both cell treatments. Besides the validated role of NGF in the pain context, various authors supported the idea that NGF is also involved in favoring the cartilage repair processes during OA through the interaction with other growth factors.43,44 From this perspective, the presence of NGF could be seen as an important regulator, since it could modulate transforming growth factor-β1, thus influencing tissue repair.
This study presents some limitations that render difficult to achieve a clear conclusion on the biological effects of the two cell strategies proposed. In particular, the difference on cell precursor percentage in mBMAC group, the use of two different kinds of biomaterials, and most importantly the low number of patients enrolled are the main critical points.
However, our findings would indicate both mACI and mBMAC as valid candidates to regenerate cartilage in patients affected by OLT even if this last presents major drawbacks compared to mACI. Indeed, the use of a point-of-care device that allows the concentration of total nucleated cells and platelets26 with the lack of cell manipulation and expansion, as well as the release of cytokines and growth factors and low costs, would render for some aspects the mBMAC strategy more attractive for orthopedic surgeons. Thus, we believe that further studies are necessary to gain knowledge about the stability of mBMAC approach over time in a larger patient cohort to verify whether the neo-formed cartilage tissue evolves toward more hyaline features.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Regione Emilia-Romagna, Programma di Ricerca Regione-Università 2010-2012 “Regenerative Medicine of Cartilage and Bone.”
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BG, SG, and RB are inventors of the European Patent No. EP 2 129 384, “Composition containing a medullary concentrate supported by a scaffold,” in the name of Istituto Ortopedico Rizzoli, Bologna, and licensed to Novagenit, Mezzolombardo (Trento), Italy. GD, IB, FV, CC, FC, and EM disclose no potential conflicts of interest.
Ethical Approval: The study was approved by the Ethical Committee of Rizzoli Orthopedic Institute (Numbers: 29877 and 0008310).
Informed Consent: Written informed consent was obtained from all subjects before the study.
References
- 1. Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467:3307-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pritsch M, Horoshovski H, Farine I. Arthroscopic treatment of osteochondral lesions of the talus. Surgery. 1986;68:862-65. [PubMed] [Google Scholar]
- 3. Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stevens AL, Wishnok JS, White FM, Grodzinsky AJ, Tannenbaum SR. Mechanical injury and cytokines cause loss of cartilage integrity and upregulate proteins associated with catabolism, immunity, inflammation, and repair. Mol Cell Proteomics. 2009;8:1475-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kraus VB. Osteoarthritis year 2010 in review: biochemical markers. Osteoarthritis Cartilage. 2011;19:346-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perrot S. Osteoarthritis pain. Best Pract Res Clin Rheumatol. 2015;29(1):90-7. [DOI] [PubMed] [Google Scholar]
- 7. Salaffi F, Ciapetti A, Carotti M. The sources of pain in osteoarthritis: a pathophysiological review. Reumatismo. 2014;66(1):57-71. [DOI] [PubMed] [Google Scholar]
- 8. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432-63. [DOI] [PubMed] [Google Scholar]
- 9. Lee KT, Lee YK, Young KW, Park SY, Kim JS. Factors influencing result of autologous chondrocyte implantation in osteochondral lesion of the talus using second look arthroscopy. Scand J Med Sci Sports. 2012;22(4):510-5. [DOI] [PubMed] [Google Scholar]
- 10. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-54. [DOI] [PubMed] [Google Scholar]
- 11. Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1999;81:1229-35. [DOI] [PubMed] [Google Scholar]
- 12. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. [DOI] [PubMed] [Google Scholar]
- 13. Nam EK, Ferkel RD, Applegate GR. Autologous chondrocyte implantation of the ankle: a 2- to 5-year follow-up. Am J Sport Med. 2009;37:274-84. [DOI] [PubMed] [Google Scholar]
- 14. Giannini S, Buda R, Grigolo B, Vannini F. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int. 2001;22:513-17. [DOI] [PubMed] [Google Scholar]
- 15. Petersen L, Brittberg M, Lindahl A. Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin. 2003;8:291-303. [DOI] [PubMed] [Google Scholar]
- 16. Cherubino P, Grassi FA, Bulgheroni P, Ronga M. Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg (Hong Kong). 2003;11(1):10-5. [DOI] [PubMed] [Google Scholar]
- 17. Zhang C, Cai YZ, Lin XJ. Autologous chondrocyte implantation: is it likely to become a saviour of large sized and full-thickness cartilage defect in young adult knee? Knee Surg Sports Traumatol Arthrosc. Epub 2015. May 19. [DOI] [PubMed] [Google Scholar]
- 18. Filardo G, Kon E, Andriolo L, Di Matteo B, Balboni F, Marcacci M. Clinical profiling in cartilage regeneration: prognostic factors for midterm results of matrix-assisted autologous chondrocyte transplantation. Am J Sports Med. 2014;42(4):898-905. [DOI] [PubMed] [Google Scholar]
- 19. Filardo G, Kon E, Perdisa F, Balboni F, Marcacci M. Autologous osteochondral transplantation for the treatment of knee lesions: results and limitations at two years’ follow-up. Int Orthop. 2014;38(9):1905-12. [DOI] [PubMed] [Google Scholar]
- 20. Brix MO, Stelzeneder D, Chiari C, Koller U, Nehrer S, Dorotka R, et al. Treatment of full-thickness chondral defects with Hyalograft C in the knee: long-term results. Am J Sports Med. 2014;42(6):1426-32. [DOI] [PubMed] [Google Scholar]
- 21. Grässel S, Lorenz J. Tissue-engineering strategies to repair chondral and osteochondral tissue in osteoarthritis: use of mesenchymal stem cells. Curr Rheumatol Rep. 2014;16(10):452. doi: 10.1007/s11926-014-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orozco L, Munar A, Soler R, Alberca M, Soler F, Huguet M, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: two-year follow-up results. Transplantation. 2014;97:66-8. [DOI] [PubMed] [Google Scholar]
- 23. Kim YS, Lee HJ, Choi YJ, Kim YI, Koh YG. Does an injection of a stromal vascular fraction containing adipose-derived mesenchymal stem cells influence the outcomes of marrow stimulation in osteochondral lesions of the talus? A clinical and magnetic resonance imaging study. Am J Sports Med. 2014;42:2424-34. [DOI] [PubMed] [Google Scholar]
- 24. Wei CC, Lin AB, Hung SC. Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: bench, bedside, and industry. Cell Transplant. 2014;23:505-12. [DOI] [PubMed] [Google Scholar]
- 25. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;15:e45-e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hermann PC, Huber SL, Herrler T, von Hesler C, Andrassy J, Kevy SV, et al. Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant. 2008;16(10):1059-69. [PubMed] [Google Scholar]
- 27. Ridgway J, Butcher A, Chen PS, Horner A, Curran S. Novel technology to provide an enriched therapeutic cell concentrate from bone marrow aspirate. Biotechnol Prog. 2010;26:1741-48. [DOI] [PubMed] [Google Scholar]
- 28. Yamasaki S, Mera H, Itokazu M, Hashimoto Y, Wakitani S. Cartilage repair with autologous bone marrow mesenchymal stem cell transplantation: review of preclinical and clinical studies. Cartilage. 2014;5(4):196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349-53. [DOI] [PubMed] [Google Scholar]
- 30. Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36(5):873-80. [DOI] [PubMed] [Google Scholar]
- 31. Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85:45-57. [PubMed] [Google Scholar]
- 32. Rosa D, Balato G, Ciaramella G, Soscia E, Improta G, Triassi M. Long-term clinical results and MRI changes after autologous chondrocyte implantation in the knee of young and active middle aged patients. J Orthop Traumatol. Epub 2015. October 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akgun I, Unlu MC, Erdal OA, Ogut T, Erturk M, Ovali E, et al. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135(2):251-63. [DOI] [PubMed] [Google Scholar]
- 34. Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87(7):896-902. [DOI] [PubMed] [Google Scholar]
- 35. Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise review: bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32:1713-23. [DOI] [PubMed] [Google Scholar]
- 36. Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;15(22):181-92. [DOI] [PubMed] [Google Scholar]
- 37. Cavallo C, Desando G, Columbaro M, Ferrari A, Zini N, Facchini A, et al. Chondrogenic differentiation of bone marrow concentrate grown onto a hylauronan scaffold: rationale for its use in the treatment of cartilage lesions. J Biomed Mater Res Part A. 2013;101:1559-70. [DOI] [PubMed] [Google Scholar]
- 38. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927-37. [DOI] [PubMed] [Google Scholar]
- 39. Song F, Tang J, Geng R, Hu H, Zhu C, Cui W, et al. Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. Int J Clin Exp Pathol. 2014;7:1415-26. [PMC free article] [PubMed] [Google Scholar]
- 40. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12. [DOI] [PubMed] [Google Scholar]
- 42. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cosgaya JM, Aranda A. Nerve growth factor regulates transforming growth factor-beta 1 gene expression by both transcriptional and post-transcriptional mechanisms in PC12 cells. J Neurochem. 1995;65(6):2484-90. [DOI] [PubMed] [Google Scholar]
- 44. Lindholm D, Hengerer B, Zafra F, Thoenen H. Transforming growth factor-beta 1 stimulates expression of nerve growth factor in the rat CNS. Neuroreport. 1990;1(1):9-12. [DOI] [PubMed] [Google Scholar]