Abstract
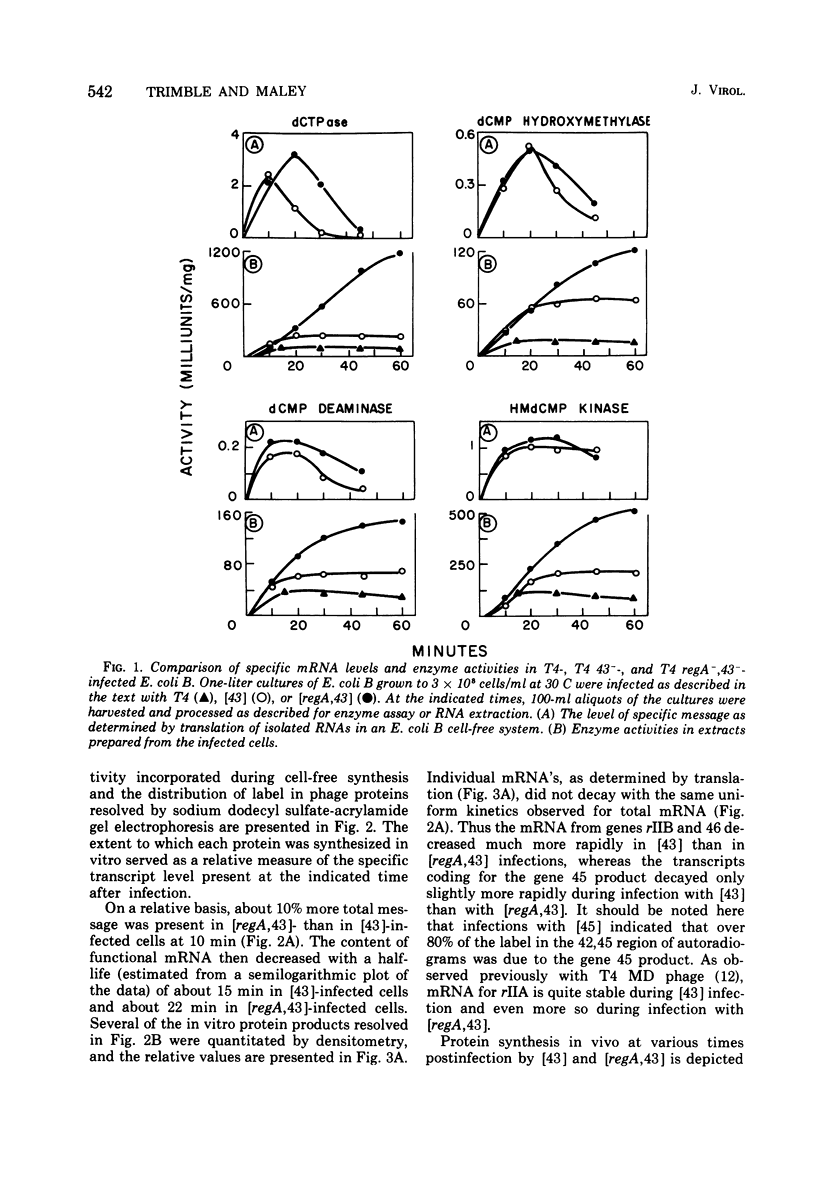
The role of the T4 bacteriophage regA gene in stabilizing early mRNA was investigated by assaying the level of functional mRNA from eight prereplicative genes (56 [dCMP hydroxymethylase], cd [dCMP deaminase], 1 [deoxynucleotide kinase], rIIA, rIIB, 46 [DNA arrest], and 45) during extended infection of Escherichia coli B with T4 regA-, 43- and T4 43- bacteriophage. The above gene-specific transcripts in RNA isolated from infected cells were quantitated by translation with an E. coli B cell-free system. Conditions were chosen to insure that the amount of gene product formed in vitro, measured either as an enzyme activity or as a radioactive band in acrylamide gel, was directly proportional to the level of mRNA present. The failure of T4 regA-, 43- phage to terminate prereplicative synthesis (Wiberg et al., 1973) resulted in an enhanced production of many early gene products over those formed during T4 43- infection. This increase did not appear to be associated with an increment in mRNA levels, since in the present study gene-specific early mRNA's were found to be only marginally elevated and slightly more stable in T4 regA-, 43-- than in T4 43--infected cells. Of interest was the observation that significant quantities of all of the mRNA's studied; with the exception of those from genes 45 and 46, could be isolated from T4 43--infected cells after synthesis of the respective gene products had ceased. On termination of normal prereplicative synthesis during infection with T4 43- phage, polyribosomes were found to be dissociated completely, a finding which suggests that the residual mRNA present in these cells is free in the cytoplasm. The persistence in T4 43--infected cells of translatable mRNA for many prereplicative genes after product synthesis ceased indicates that the impairment in protein synthesis is not due solely to regA-mediated messenger degradation or modification. Rather, the results suggest that the regA gene product may act either by interfering with early mRNA polypeptide chain initiation or by promoting prereplicative polysome dissociation.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cremer K., Silengo L., Schlessinger D. Polypeptide formation and polyribosomes in Escherichia coli treated with chloramphenicol. J Bacteriol. 1974 May;118(2):582–589. doi: 10.1128/jb.118.2.582-589.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S. K., Rudland P. S. Control of translation by T4 phage: altered binding of disfavoured messengers. Nature. 1970 May 30;226(5248):820–823. doi: 10.1038/226820a0. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Flessel C. P., Ralph P., Rich A. Polyribosomes of growing bacteria. Science. 1967 Nov 3;158(3801):658–660. doi: 10.1126/science.158.3801.658. [DOI] [PubMed] [Google Scholar]
- Fry M., Israeli-Reches M., Artman M. Stabilization and breakdown of Escherichia coli messenger ribonucleic acid in the presence of chloramphenicol. Biochemistry. 1972 Aug 1;11(16):3054–3059. doi: 10.1021/bi00766a017. [DOI] [PubMed] [Google Scholar]
- Hsu W. T., Weiss S. B. Selective translation of T4 template RNA by ribosomes from T4-infected Escherichia coli. Proc Natl Acad Sci U S A. 1969 Sep;64(1):345–351. doi: 10.1073/pnas.64.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihler G., Nakada D. Role of initiation factors in the control of T4-specific protein synthesis. J Mol Biol. 1971 Dec 14;62(2):419–421. doi: 10.1016/0022-2836(71)90439-6. [DOI] [PubMed] [Google Scholar]
- Johnson J. R., Hall D. H. Characterization of new regulatory mutants of bacteriophage T4. J Virol. 1974 Mar;13(3):666–676. doi: 10.1128/jvi.13.3.666-676.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam J. D., Bowles M. G. Mutation to overproduction of bacteriophage T4 gene products. J Virol. 1974 Feb;13(2):428–438. doi: 10.1128/jvi.13.2.428-438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam J. D., O'Donnell P. V. Suppression of amber mutations of bacteriophage T4 gene 43 (DNA polymerase) by translational ambiguity. J Virol. 1973 Jun;11(6):933–945. doi: 10.1128/jvi.11.6.933-945.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee-Huang S., Ochoa S. Messenger discriminating species of initiation factor F3. Nat New Biol. 1971 Dec 22;234(51):236–239. doi: 10.1038/newbio234236a0. [DOI] [PubMed] [Google Scholar]
- Maley F., Maley G. F. Tetrahydrodeoxyuridylate: a potent inhibitor of deoxycytidylate deaminase. Arch Biochem Biophys. 1971 Jun;144(2):723–729. doi: 10.1016/0003-9861(71)90379-1. [DOI] [PubMed] [Google Scholar]
- Maley G. F., Guarino D. U., Maley F. T2r + bacteriophage-induced enzymes. I. The purification and properties of deoxycytidylate deaminase. J Biol Chem. 1972 Feb 10;247(3):931–939. [PubMed] [Google Scholar]
- Sauerbier W., Hercules K. Control of gene function in bacteriophage T4. IV. Post-transcriptional shutoff of expression of early genes. J Virol. 1973 Sep;12(3):538–547. doi: 10.1128/jvi.12.3.538-547.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff N., Miller M. J., Wahba A. J. Purification and properties of chain initiation factor 3 from T4-infected and uninfected Escherichia coli MRE 600. Stimulation of translation of synthetic and natural messengers. J Biol Chem. 1974 Jun 25;249(12):3797–3802. [PubMed] [Google Scholar]
- Singer R. E., Conway T. W. Defective initiation of f2 RNA translation by ribosomes from bacteriophage T4-infected cells. Biochim Biophys Acta. 1973 Nov 26;331(1):102–116. doi: 10.1016/0005-2787(73)90423-1. [DOI] [PubMed] [Google Scholar]
- Spremulli L. L., Haralson M. A., Ravel J. M. Effect of T4 infection on initiation of protein synthesis and messenger specificity of initiation factor 3. Arch Biochem Biophys. 1974 Dec;165(2):581–587. doi: 10.1016/0003-9861(74)90285-9. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Dube S. K., Rudland P. S. Control of translation of T4 phage: altered ribosome binding at R17 initiation sites. Nature. 1970 May 30;226(5248):824–827. doi: 10.1038/226824a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Specific recognition of the isolated R17 replicase initiator region by R17 coat protein. Nature. 1974 Mar 15;248(445):223–225. doi: 10.1038/248223a0. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Galivan J., Maley F. The temporal expression of T2r + bacteriophage genes in vivo and in vitro. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1659–1663. doi: 10.1073/pnas.69.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. The influence of potassium and rifampicin on the expression of bacteriophage T2 prereplicative genes in vitro and in vivo. Arch Biochem Biophys. 1975 Mar;167(1):377–387. doi: 10.1016/0003-9861(75)90474-9. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Maley G. F., Maley F. Relationship between Escherichia coli B titer and the level of deoxycytidylate deaminase activity induced on bacteriophage T2r + infection. J Virol. 1972 Mar;9(3):454–464. doi: 10.1128/jvi.9.3.454-464.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley G. F., Maley F. The in vitro synthesis of T2 bacteriophage-induced deoxycytidylate deaminase and its regulation by allosteric effectors. Arch Biochem Biophys. 1972 Dec;153(2):515–525. doi: 10.1016/0003-9861(72)90370-0. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Barnes J. E. Evidence for a dual role for the bacteriophage T4-induced deoxycytidine triphosphate nucleotidohydrolase. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1233–1240. doi: 10.1073/pnas.56.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Lewis N. The synthesis of deoxycytidylate deaminase and dihydrofolate reductase and its control in Escherichia coli infected with bacteriophage T4 and T-4 amber mutants. Virology. 1966 May;29(1):172–175. doi: 10.1016/0042-6822(66)90208-x. [DOI] [PubMed] [Google Scholar]
- Wiberg J. S., Mendelsohn S., Warner V., Hercules K., Aldrich C., Munro J. L. SP62, a viable mutant of bacteriophage T4D defective in regulation of phage enzyme synthesis. J Virol. 1973 Oct;12(4):775–792. doi: 10.1128/jvi.12.4.775-792.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. T., 2nd, van Houwe G. Control of synthesis of glucosyl transferase and lysozyme messengers after T4 infection. J Mol Biol. 1970 Aug;51(3):605–619. doi: 10.1016/0022-2836(70)90011-2. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN S. B., KORNBERG A. Deoxycytidine di- and triphosphate cleavage by an enzyme formed in bacteriophage-infected Eschrichia coli. J Biol Chem. 1961 May;236:1480–1486. [PubMed] [Google Scholar]