Abstract
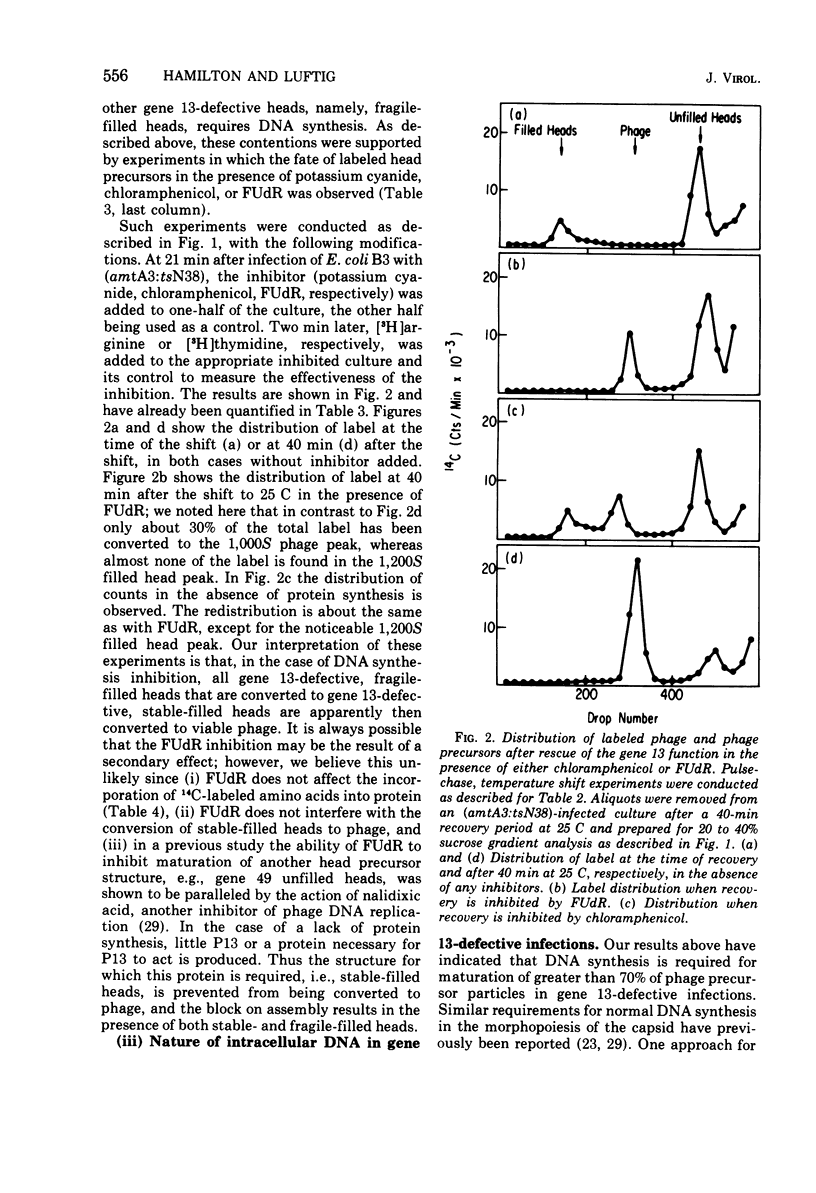
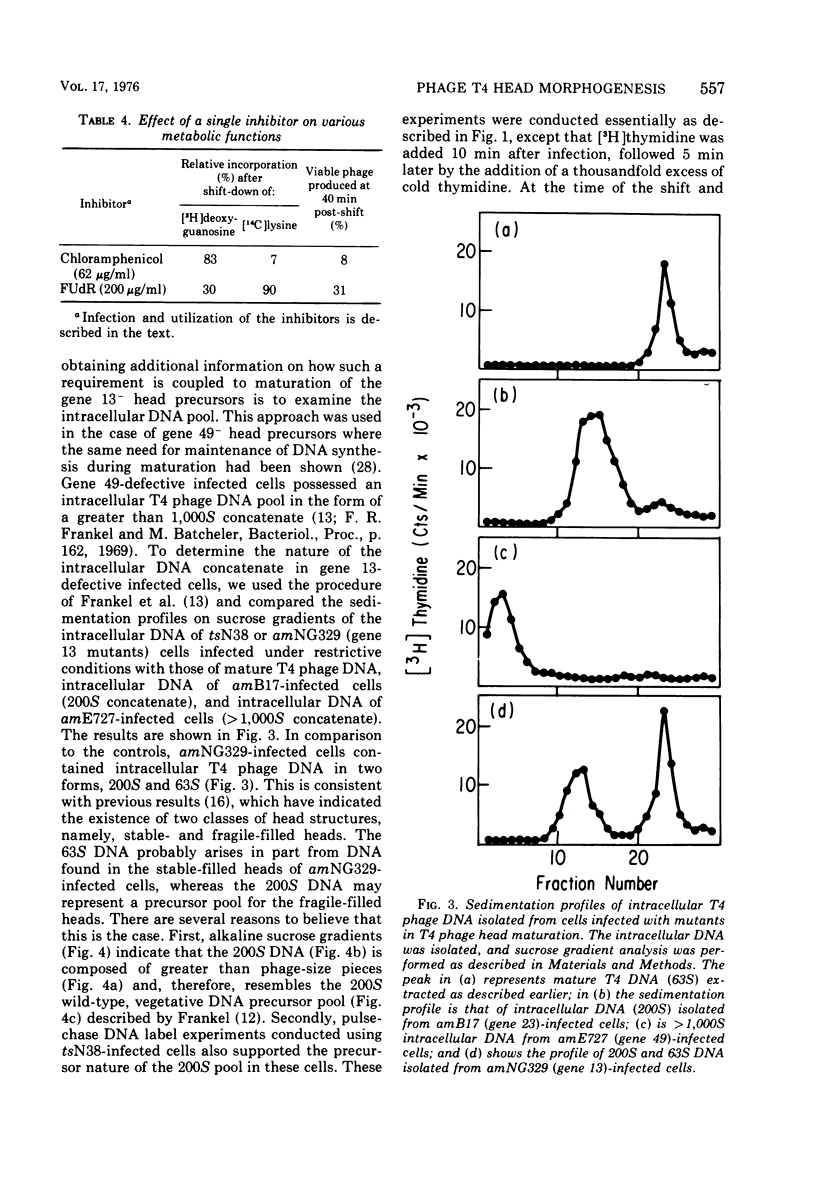
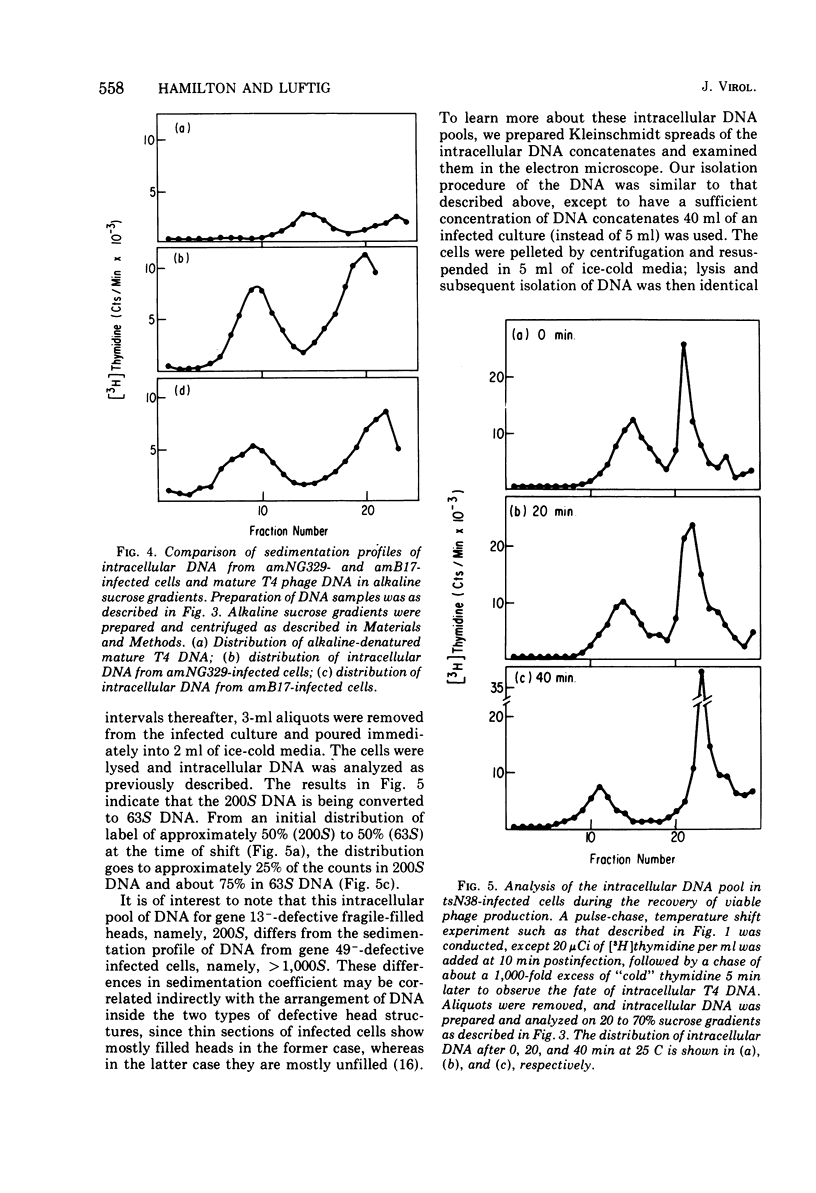
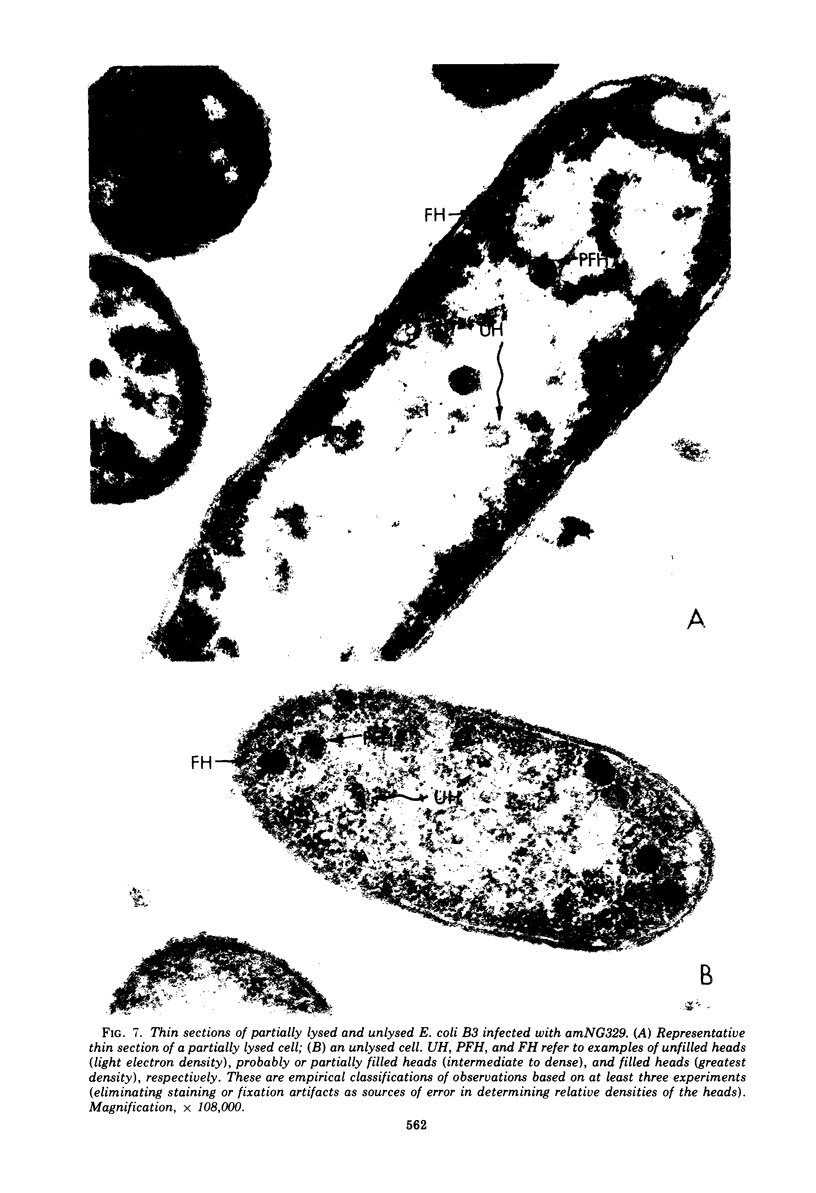
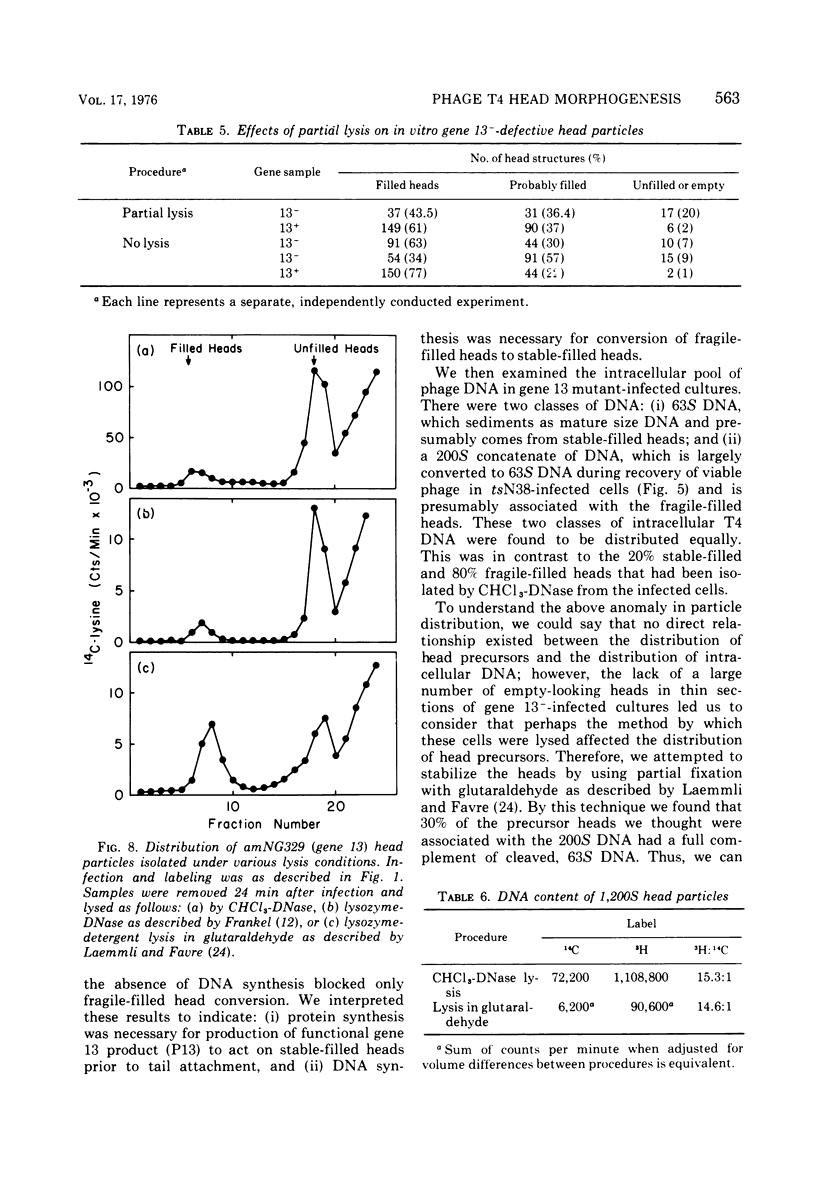
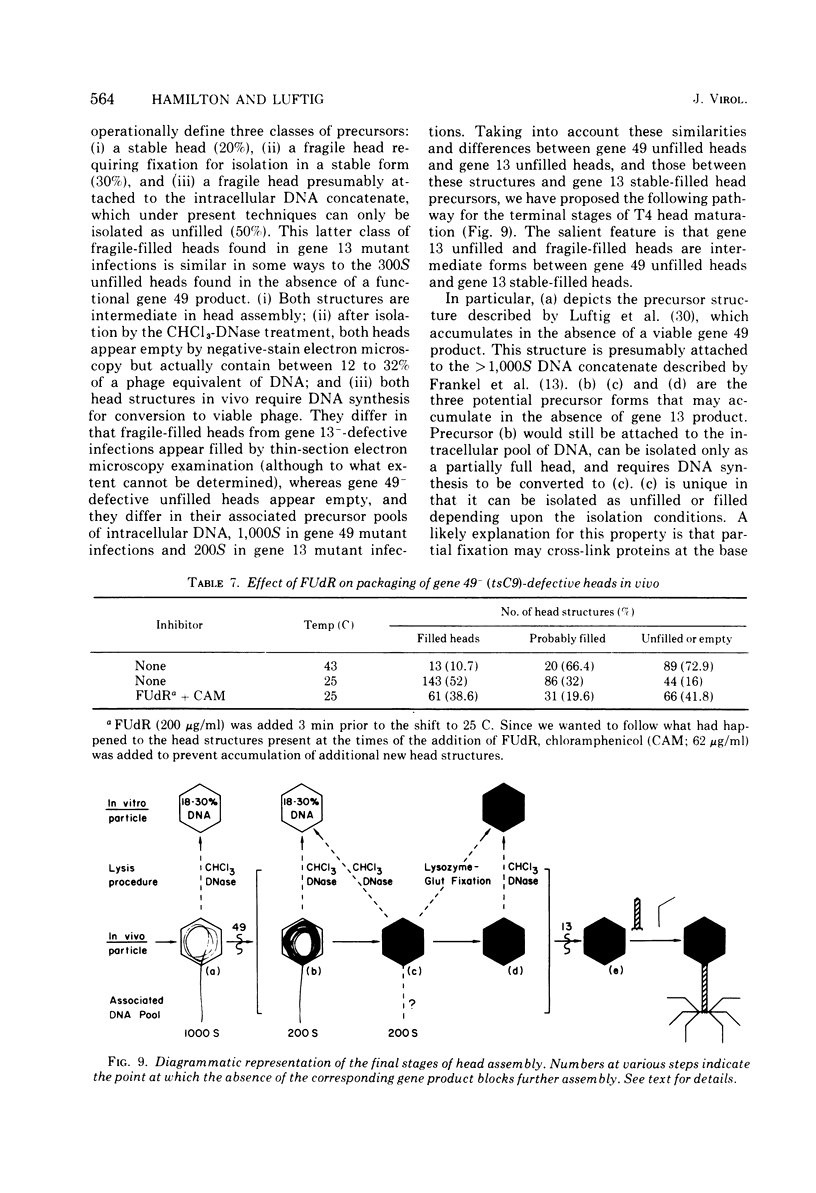
Several aspects of the terminal stages of T4 head maturation were investigated using ts and am mutants blocked at single steps of the assembly pathway. We had previously found that cells infected with mutants of gene 13, e.g., tsN38 and amE609, accumulated both stable (10 to 20%)- and fragile (80%)-filled head precursors (Hamilton and Luftig, 1972). Here we showed the following for such gene 13-defective, mutant-infected cells. (i) Using thin-section analysis the pool of phage precursor structures observed under nonpermissive conditions was one-third of that observed when the cells were cultured under permissive conditions. (ii) In order for complete conversion of the precursors into viable phage to occur, there were apparent requirements of metabolic energy, protein, and DNA synthesis. (iii) The intracellular DNA pool under nonpermissive conditions exhibited a 50% distribution between 63S (mature size) and 200 S (concatenate size) DNA, with the latter DNA serving as a precursor pool. Further, this DNA pool when spread onto a protein monolayer exhibited a dispersed array of DNA, strands around a core, which was less dense than that found for the greater than 1,000S DNA concatenate isolated from gene 49-defective infected cells. (iv) When precuations were taken to stabilize the head precursors, such as lysis of the cells into glutaraldehyde, there was a 30% increase in the yield of 1,200S filled heads. Correlating these results and previous results concerning gene 49-defective unfilled heads, we propose that there are several forms of gene 13 fragile head precursors which serve as intermediates between gene 49 unfilled heads and gene 13 stable filled heads. We cannot, however, rule out the possibility that all gene 13-defective heads represent a single class of unstable particles, which decay slowly. In either case, we have shown that gene 13-defective particles are unstable to some degree inside the cell and are highly unstable outside the cell; yet all particles can still be efficiently converted to phage in vivo.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bijlenga R. K., Broek R vd, Kellenberger E. The transformation of rho-particles into T4 heads. I. Evidence for the conservative mode of this transformation. J Supramol Struct. 1974;2(1):45–59. doi: 10.1002/jss.400020106. [DOI] [PubMed] [Google Scholar]
- Bode V. C., Gillin F. D. The arrangement of DNA in lambda phage heads. I. Biological consequences of micrococcal nuclease attack on a portion of the chromosome exposed in tailless heads. J Mol Biol. 1971 Dec 28;62(3):493–502. doi: 10.1016/0022-2836(71)90150-1. [DOI] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Chao J., Chao L., Speyer J. F. Bacteriophage T4 head morphogenesis: host DNA enzymes affect frequency of petite forms. J Mol Biol. 1974 May 5;85(1):41–50. doi: 10.1016/0022-2836(74)90127-2. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOERMANN A. H. The intracellular growth of bacteriophages. I. Liberation of intracellular bacteriophage T4 by premature lysis with another phage or with cyanide. J Gen Physiol. 1952 Mar;35(4):645–656. doi: 10.1085/jgp.35.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. S., Lielausis I. Some steps in the assembly of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):263–276. doi: 10.1016/0022-2836(68)90008-9. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. DNA replication after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:485–493. doi: 10.1101/sqb.1968.033.01.056. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Minagawa T. Genetic control of the DNA maturation in the process of phage morphogenesis. Virology. 1971 Jul;45(1):280–291. [PubMed] [Google Scholar]
- Hamilton D. L., Luftig R. B. Bacteriophage T4 head morphogenesis. 3. Some novel properties of gene 13-defective heads. J Virol. 1972 Jun;9(6):1047–1056. doi: 10.1128/jvi.9.6.1047-1056.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselkorn R., Vogel M., Brown R. D. Conservation of the rifamycin sensitivity of transcription during T4 development. Nature. 1969 Mar 1;221(5183):836–838. doi: 10.1038/221836a0. [DOI] [PubMed] [Google Scholar]
- Josslin R. Physiological studies on the t gene defect in T4-infected Escherichia coli. Virology. 1971 Apr;44(1):101–107. doi: 10.1016/0042-6822(71)90157-7. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- King J., Lenk E. V., Botstein D. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. II. Morphogenetic pathway. J Mol Biol. 1973 Nov 15;80(4):697–731. doi: 10.1016/0022-2836(73)90205-2. [DOI] [PubMed] [Google Scholar]
- Kühl P. W., Hofschneider P. H. Studies on the morphopoiesis of the head and phage T-even. 6. Maturation of T4 polyheads and T4 phage under conditions affecting protein-, DNAor ATP-synthesis. Eur J Biochem. 1969 Jan;7(3):353–359. doi: 10.1111/j.1432-1033.1969.tb19616.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Paulson J. R., Hitchins V. Maturation of the head of bacteriophage T4. V. A possible DNA packaging mechanism: in vitro cleavage of the head proteins and the structure of the core of the polyhead. J Supramol Struct. 1974;2(2-4):276–301. doi: 10.1002/jss.400020219. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Teaff N., D'Ambrosia J. Maturation of the head of bacteriophage T4. III. DNA packaging into preformed heads. J Mol Biol. 1974 Oct 5;88(4):749–765. doi: 10.1016/0022-2836(74)90397-0. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. II. Studies on the maturation of gene 49-defective head intermediates. J Virol. 1972 Feb;9(2):377–389. doi: 10.1128/jvi.9.2.377-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Lundh N. P. Bacteriophage T4 head morphogenesis. V. The role of DNA synthesis in maturation of an intermediate in head assembly. Virology. 1973 Feb;51(2):432–442. doi: 10.1016/0042-6822(73)90442-x. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- Matthews C. K. Deoxyribonucleic acid metabolism and virus-induced enzyme synthesis in a thymine-requiring bacterium infected by a thymine-requiring bacteriophage. Biochemistry. 1966 Jun;5(6):2092–2100. doi: 10.1021/bi00870a042. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P., Speyer J. Rifampicin inhibition of ribonucleic acid and protein synthesis in normal and ethylenediaminetetraacetic acid-treated Escherichia coli. J Bacteriol. 1970 Oct;104(1):376–389. doi: 10.1128/jb.104.1.376-389.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D. Infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope: T4 head morphogenesis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):907–911. doi: 10.1073/pnas.69.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P. Dominance interactions in Escherichia coli cells mixedly infected with bacteriophage T4D wild-type and amber mutants and their possible implications as to type of gene-product function: catalytic vs. stoichiometric. Virology. 1968 Aug;35(4):550–563. doi: 10.1016/0042-6822(68)90285-7. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]