Abstract
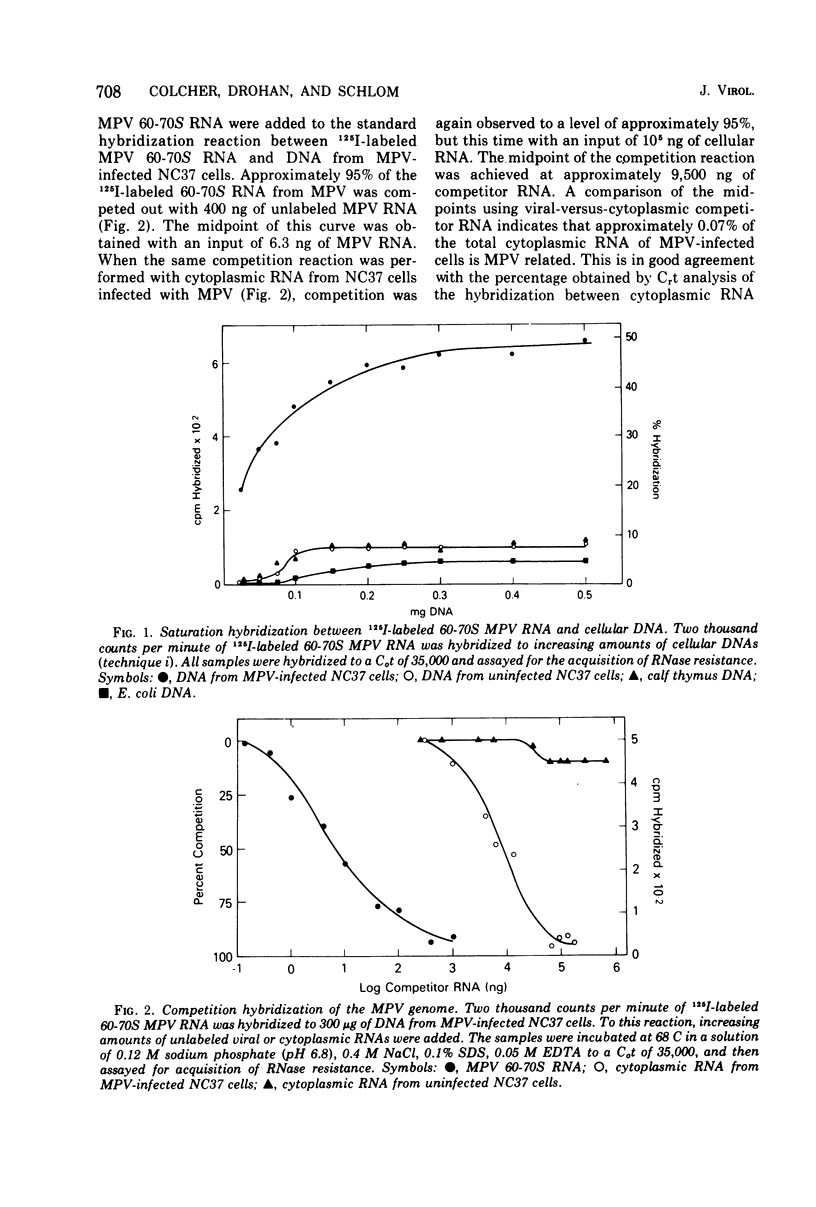
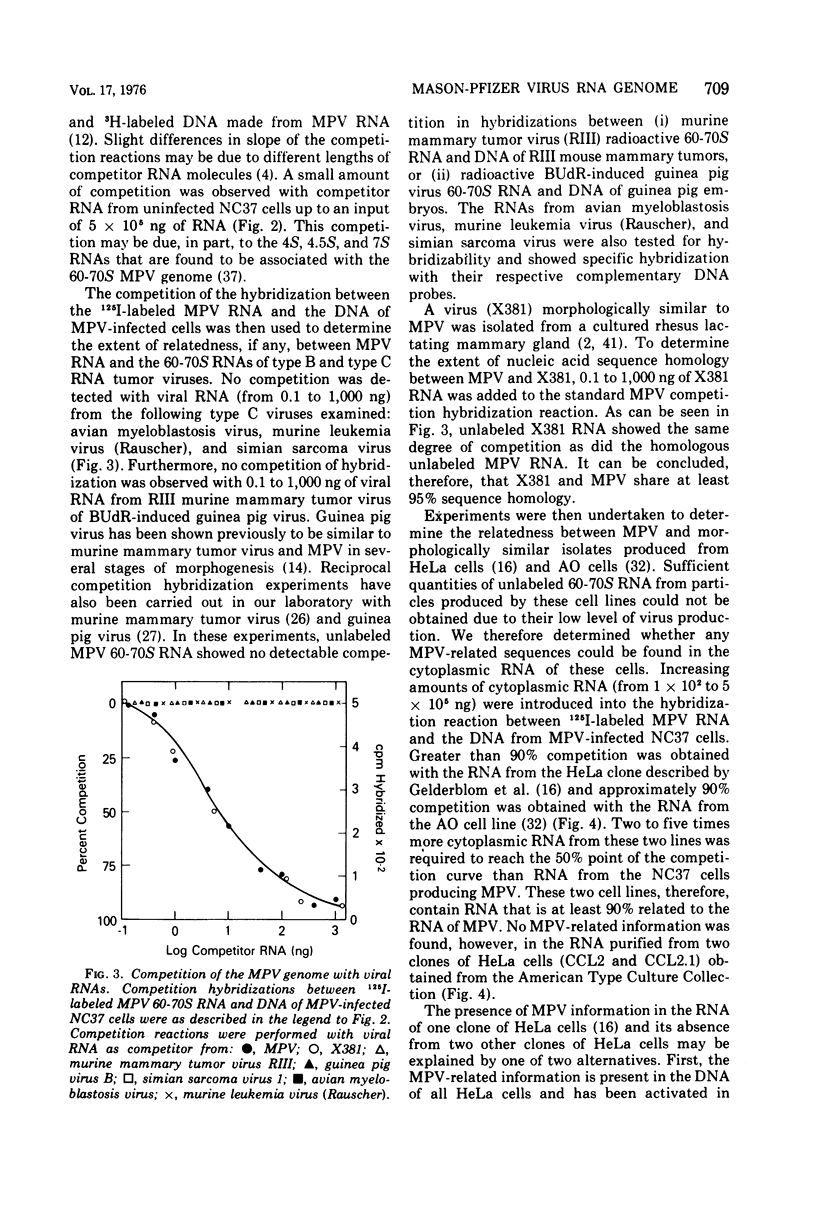
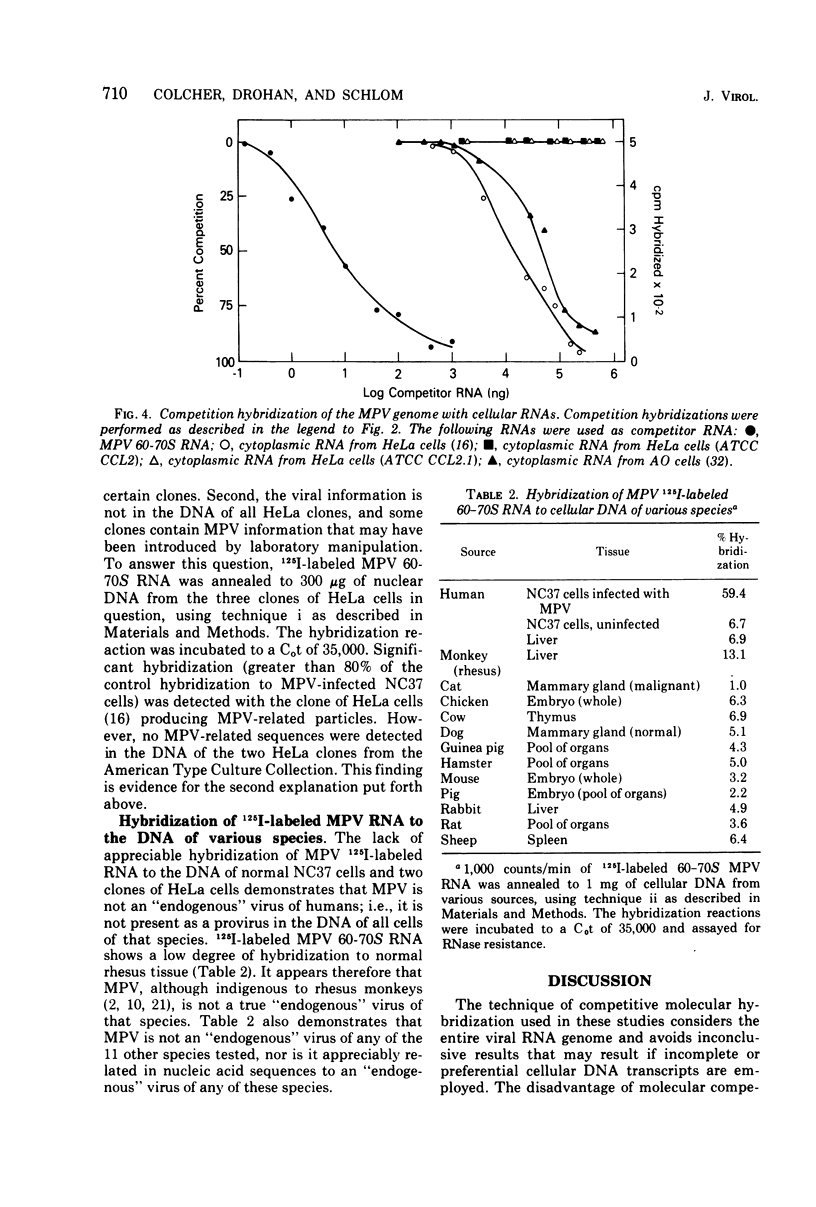
The 60-70S RNA of Mason-Pfizer virus (MPV) was iodinated in vitro and used in both direct and competitive molecular hybridization studies. MPV proviral sequences are present at a frequency of approximately one to two copies per haploid genome in the DNA of experimentally infected human cells. By nucleic acid competition hybridization, MPV RNA was found to be indistinguishable from the RNA of a virus (X381) isolated from a rhesus mammary gland and from RNA isolated from the cytoplasm of AO cells (Parks et al., 1973) and HeLa cells (Gelderblom et al., 1974), both previously reported to produce MPV-related particles. No homology was observed, however, between MPV RNA and the RNA, or the DNA, from two clones of HeLa cells obtained from the American Type Culture Collection. Hybridization of MPV 60-70S RNA to the DNA of normal tissues of humans and to the DNA of 11 other species revealed that MPV is not an endogenous virus of any of these species. Competition hybridization revealed no detectable sequence homology between the RNA of MPV and the RNAs of simian sarcoma virus, murine mammary tumor virus, murine leukemia virus, BUdR-induced guinea pig virus, or avian myeloblastosis virus. These nucleic acid studies substantiate previous ultrastructural and immunological findings that MPV and morphologically similar isolates constitute a distinct group of oncornavirus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Mayyasi S. A., Chopra H. C., Zelljadt I., Jensen E. M. Mason-Pfizer monkey virus isolated from spontaneous mammary carcinoma of a female monkey. I. Detection of virus antigens by immunodiffusion, immunofluorescent, and virus agglutination techniques. J Natl Cancer Inst. 1971 Jun;46(6):1325–1334. [PubMed] [Google Scholar]
- Ahmed M., Schidlovsky G., Korol W., Vidrine G., Cicmanec J. L. Occurrence of Mason-Pfizer monkey virus in healthy rhesus monkeys. Cancer Res. 1974 Dec;34(12):3504–3508. [PubMed] [Google Scholar]
- Bauer H., Daams J. H., Watson K. F., Mölling K., Gelderblom H., Schäfer W. Oncornavirus-like particles in HeLa cells. II. Immunological characterization of the virus. Int J Cancer. 1974 Feb 15;13(2):254–261. doi: 10.1002/ijc.2910130213. [DOI] [PubMed] [Google Scholar]
- Beckmann J. S., Daniel V. Relative stabilities of RNA/DNA hybrids: effect of RNA chain length in competitive hybrization. J Mol Biol. 1974 Oct 25;89(2):355–362. doi: 10.1016/0022-2836(74)90524-5. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. DNA-RNA hybridization. Acta Endocrinol Suppl (Copenh) 1972;168:247–276. doi: 10.1530/acta.0.071s247. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Smith G. P. The determination of RNA homogeneity by molecular hybridization. Cell. 1974 Dec;3(4):341–346. doi: 10.1016/0092-8674(74)90048-8. [DOI] [PubMed] [Google Scholar]
- Bykovsky A. F., Miller G. G., Yershov F. I., Ilyin K. V., Zhdanov V. M. B-type oncornaviruses isolated from continuous human cancer cell lines. Arch Gesamte Virusforsch. 1973;42(1):21–35. doi: 10.1007/BF01250504. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D., Blair P. B., Nakayama P. In vitro cultivation of mouse mammary tumor virus: detection of MTV production by radioisotope labeling and identification by immune precipitation. Proc Natl Acad Sci U S A. 1968 Mar;59(3):895–902. doi: 10.1073/pnas.59.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Chopra H. C., Zelljadt I., Jensen E. M., Mason M. M., Woodside N. J. Infectivity of cell cultures by a virus isolated from a mammary carcinoma of a rhesus monkey. J Natl Cancer Inst. 1971 Jan;46(1):127–137. [PubMed] [Google Scholar]
- Colcher D., Spiegelman S., Schlom J. Sequence homology between the RNA of Mason-Pfizer monkey virus and the RNA of human malignant breast tumors. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4975–4979. doi: 10.1073/pnas.71.12.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Perk K., Dalton A. J. Virus-like particles induced in guinea pig cells by 5-bromo-2'-deoxyuridine are morphologically similar to murine B-type virus. Nature. 1974 Jun 28;249(460):828–830. doi: 10.1038/249828a0. [DOI] [PubMed] [Google Scholar]
- Dalton A. J., Melnick J. L., Bauer H., Beaudreau G., Bentvelzen P., Bolognesi D., Gallo R., Graffi A., Haguenau F., Heston W. The case for a family of reverse transcriptase viruses: Retraviridae. Intervirology. 1974;4(4):201–206. doi: 10.1159/000149963. [DOI] [PubMed] [Google Scholar]
- Gelderblom H., Bauer H., Ogura H., Wigand R., Fischer A. B. Detection of oncornavirus-like particles in HeLa cells. I. Fine structure and comparative morphological classification. Int J Cancer. 1974 Feb 15;13(2):246–253. doi: 10.1002/ijc.2910130212. [DOI] [PubMed] [Google Scholar]
- Hooks J., Gibbs C. J., Jr, Chopra H., Lewis M., Gajdusek D. C. Spontaneous tranformation of human brain cells grown in vitro and description of associated viurs particles. Science. 1972 Jun 30;176(4042):1420–1422. doi: 10.1126/science.176.4042.1420. [DOI] [PubMed] [Google Scholar]
- Il'in K. V., Bykovskii A. F., Zhdanov V. M. Onkornavirus tipa B iz kletok kartsinomy gortani cheloveka. Vopr Virusol. 1972 Jul-Aug;17(4):494–499. [PubMed] [Google Scholar]
- Ilyin K. V., Bykovsky A. F., Zhdanov V. M. An oncornavirus isolated from human cancer cell line. Cancer. 1973 Jul;32(1):89–96. doi: 10.1002/1097-0142(197307)32:1<89::aid-cncr2820320112>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Jensen E. M., Zelljadt I., Chopra H. C., Mason M. M. Isolation and propagation of a virus from a spontaneous mammary carcinoma of a rhesus monkey. Cancer Res. 1970 Sep;30(9):2388–2393. [PubMed] [Google Scholar]
- Kramarsky B., Sarkar N. H., Moore D. H. Ultrastructural comparison of a virus from a Rhesus-monkey mammary carcinoma with four oncogenic RNA viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1603–1607. doi: 10.1073/pnas.68.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: sequence components of heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Feb;4(2):77–93. doi: 10.1016/0092-8674(75)90113-0. [DOI] [PubMed] [Google Scholar]
- Manning J. S., Hackett A. J. Morphological and biophysical properties of the Mason-Pfizer monkey virus. J Natl Cancer Inst. 1972 Feb;48(2):417–422. [PubMed] [Google Scholar]
- Mason M. M., Bogden A. E., Ilievski V., Esber H. J., Baker J. R., Chopra H. C. History of a rhesus monkey adenocarcinoma containing virus particles resembling oncogenic RNA viruses. J Natl Cancer Inst. 1972 May;48(5):1323–1331. [PubMed] [Google Scholar]
- Michalides R., Schlom J., Dahlberg J., Perk K. Biochemical properties of the bromodeoxyuridine-induced guinea pig virus. J Virol. 1975 Oct;16(4):1039–1050. doi: 10.1128/jvi.16.4.1039-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. G., Zhdanov V. M., Lozinsky T. F., Volkova M. Y., Ilyin K. V., Golubev D. B., Irlin I. S., Bykovsky A. F. Production of an oncornavirus by the continuous human cell line, Detroit-6. J Natl Cancer Inst. 1974 Feb;52(2):357–364. doi: 10.1093/jnci/52.2.357. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Wright S. E., McMillin C., MacDonnell D. Nucleotide sequence relationships of avian RNA tumor viruses: measurement of the deletion in a transformation-defective mutant of Rous sarcoma virus. J Virol. 1974 Apr;13(4):837–846. doi: 10.1128/jvi.13.4.837-846.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson-Rees W. A., Zhdanov V. M., Hawthorne P. K., Flandermeyer R. R. HeLa-like marker chromosomes and type-A variant glucose-6-phosphate dehydrogenase isoenzyme in human cell cultures producing Mason-Pfizer monkey virus-like particles. J Natl Cancer Inst. 1974 Sep;53(3):751–757. doi: 10.1093/jnci/53.3.751. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Edynak E., Sarkar N. H. Serological and structural properties of Mason-Pfizer monkey virus isolated from the mammary tumor of a Rhesus monkey. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1608–1612. doi: 10.1073/pnas.68.7.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Gilden R. V., Bykovsky A. F., Miller G. G., Zhdanov V. M., Soloviev V. D., Scolnick E. M. Mason-Pfizer virus characterization: a similar virus in a human amniotic cell line. J Virol. 1973 Dec;12(6):1540–1547. doi: 10.1128/jvi.12.6.1540-1547.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Bishop J. O., Milstein C., Brownlee G. G. Comparative hybridization studies iwth an immunoglobulin light chain mRNA fraction and non-immunoglobulin mRNA of mouse. FEBS Lett. 1974 Mar 15;40(1):157–160. doi: 10.1016/0014-5793(74)80917-8. [DOI] [PubMed] [Google Scholar]
- Schlom J., Colcher D., Spiegelman S., Gillespie S., Gillespie D. Quantitation of RNA tumor viruses and viruslike particles in human milk by hybridization to polyadenylic acid sequences. Science. 1973 Feb 16;179(4074):696–698. doi: 10.1126/science.179.4074.696. [DOI] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. DNA polymerase activities and nucleic acid components of virions isolated from a spontaneous mammary carcinoma from a rhesus monkey. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1613–1617. doi: 10.1073/pnas.68.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Schlom J. RNA subunit structure of Mason-Pfizer monkey virus. J Virol. 1975 Feb;15(2):423–427. doi: 10.1128/jvi.15.2.423-427.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick S. R., Stephenson J. R., Aaronson S. A. Immunological properties of two polypeptides of Mason-Pfizer monkey virus. J Virol. 1974 Jul;14(1):125–132. doi: 10.1128/jvi.14.1.125-132.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K. F., Mölling K., Gelderblom H., Bauer H. Oncornavirus-like particles in HeLa cells. 3. Biochemical characterization of the virus. Int J Cancer. 1974 Feb 15;13(2):262–267. doi: 10.1002/ijc.2910130214. [DOI] [PubMed] [Google Scholar]
- Yaniv A., Ono T., Kacian D., Colcher D., Witkin S., Schlom J., Spiegelman S. Serological analysis of reverse transcriptase of the Mason-Pfizer monkey virus. Virology. 1974 May;59(1):335–338. doi: 10.1016/0042-6822(74)90233-5. [DOI] [PubMed] [Google Scholar]
- Yen J., Ahmed M., Lyles J., Larson D., Mayyasi S. A. Competition radioimmunoassay for mason-pfizer monkey virus: comparison with recent isolates. Int J Cancer. 1975 Apr 15;15(4):632–639. doi: 10.1002/ijc.2910150412. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. M., Soloviev V. D., Bektemirov T. A., Filatov F. P., Bykovsky A. F. Isolation of a leukovirus from a continuous human cell line. Arch Gesamte Virusforsch. 1972;39(4):309–316. doi: 10.1007/BF01241009. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. M., Soloviev V. D., Bektemirov T. A., Ilyin K. V., Bykovsky A. F., Mazurenko N. P., Irlin I. S., Yershov F. I. Isolation of oncornaviruses from continuous human cell cultures. Intervirology. 1973;1(1):19–26. doi: 10.1159/000148828. [DOI] [PubMed] [Google Scholar]


