Abstract
Trichomoniasis, caused by the protozoan parasite Trichomonas vaginalis, is the most common, non-viral, sexually transmitted infection in the world, but only two closely related nitro drugs are approved for its treatment. New antimicrobials against trichomoniasis remain an urgent need. Several organic gold compounds were tested for activity against T. vaginalis thioredoxin reductase (TrxR) in cell-free systems as well as for activity against different trichomonads in vitro and in a murine infection model. The organic gold(I) compounds auranofin and chloro(diethylphenylphosphine)gold(I) inhibited TrxR in a concentration-dependent manner in assays with recombinant purified reductase and in cytoplasmic extracts of T. vaginalis transfected with a haemagglutinin epitope-tagged form of the reductase. Auranofin potently suppressed the growth of three independent clinical T. vaginalis isolates as well as several strains of another trichomonad (Tritrichomonas foetus) in a 24 h-assay, with 50% inhibitory concentrations of 0.7–2.5 µM and minimum lethal concentrations of 2–6 µM. The drug also compromised the ability of the parasite to overcome oxidant stress, supporting the notion that auranofin acts, in part, by inactivating TrxR-dependent antioxidant defences. Chloro(diethylphenylphosphine)gold(I) was 10-fold less effective against T. vaginalis in vitro than auranofin. Oral administration of auranofin for 4 days cleared the parasites in a murine model of vaginal T. foetus infection without displaying any apparent adverse effects. The approved human drug auranofin may be a promising agent as an alternative treatment of trichomoniasis in cases when standard nitro drug therapies have failed.
Keywords: Protozoa, Parasite, Antimicrobial therapy, Drug development, Gold compounds, Auranofin
1. Introduction
Trichomoniasis, caused by the protozoan parasite Trichomonas vaginalis, is the most common, non-viral, sexually transmitted infection in the world, with ca. 250 million new cases per year [1]. Symptomatic infections in women are characterised by vaginal discharge, foul odour and vaginal irritation, whereas infections in men are often asymptomatic. Trichomoniasis increases the risk of adverse pregnancy outcomes, human immunodeficiency virus (HIV) transmission, and the incidence and severity of cervical and prostate cancers [2,3]. Only two closely related drugs are currently approved by the US Food and Drug Administration (FDA) for the treatment of T. vaginalis infections, namely the nitroimidazoles metronidazole and tinidazole [4]. Oral administration of these dugs leads to clinical and microbiological cure in the majority of cases, but treatment failures occur in a significant fraction of patients, ranging from 1 to 17% depending on the sampled population [5,6]. Consequently, development of new antimicrobials against trichomoniasis remains an urgent need.
Trichomonas vaginalis is specialised for an anaerobic lifestyle. High concentrations of oxygen are detrimental to the parasite, as toxic reactive oxygen species (ROS) inactivate key enzymes in hydrogenosomes, unique organelles similar to mitochondria where oxidative carbohydrate metabolism takes place. Trichomonas vaginalis and other trichomonads lack the common antioxidant defence systems of glutathione reductase and catalase, but protect themselves from ROS using NADPH oxidase (which reduces oxygen to hydrogen peroxide) and thioredoxin (Trx)-dependent peroxidases [7,8]. Trx reductase (TrxR) uses NADPH to reduce Trx, which then catalytically reduces active cysteines of Trx peroxidase and thiol peroxidase. Following activation by Trx, these peroxidases act as a cellular protection system against oxidative damage by breaking down hydrogen peroxide. The importance of Trx in preventing oxidative damage in trichomonads is further supported by the upregulation of Trx and Trx peroxidase in T. vaginalis in response to oxidative stress [7], and the observation that TrxR is inactivated by nitroimidazole drugs [8]. These data suggest that Trx-dependent antioxidant defences may be attractive targets for drug development against trichomonads.
Studies in other parasites, including Schistosoma mansoni, Haemonchus contortus, Entamoeba histolytica and Giardia lamblia, have demonstrated that TrxR can be inhibited by the antirheumatic gold drug auranofin [9–12]. However, trichomonads are only distantly related to those microbes by phylogenetic standards, so here we explored whether auranofin or related organic gold compounds are effective inhibitors of T. vaginalis TrxR and have trichomonacidal activity in vitro and in vivo.
2. Materials and methods
2.1. TrxR cloning, expression and purification
The TrxR gene TVAG_474980was amplified by PCR from genomic DNA of T. vaginalis G3 using the primers 5′-CAT ATG TCT GCT CAA GCA TTC GAT-3′ and 5′-GGT ACC GTC ACT GAG ATA TCT CTC AGC-3′ and 35 cycles of 30 s denaturation at 94 °C, 30 s annealing at 57 °C and 1 min extension at 72 °C. The PCR product was cloned into the Master-Neo-(HA)2 expression vector [13], was confirmed by sequencing and was transfected into T. vaginalis G3 as described previously [14]. Expression was analysed by indirect immunofluorescence with antibodies against the haemagglutinin (HA) tag or against hydrogenosomal HSP70 as a marker of hydrogenosomes [15]. For production of recombinant proteins, TrxR (TVAG_474980) and Trx (TVAG_125500) were first obtained by PCR from T. vaginalis G3 genomic DNA using the same amplification conditions described above but using the following primers: 5′-GGA TCC ATG TCT GCT CAA GCA TTC GAT C-3′ and 5′-CTG CAG TTA GTC ACT GAG ATA TCT CTC AG-3′ for TrxR; and 5′-GGA TCC ATG TCC GAT CCA ATT GTT CAC-3′ and 5′-GTC GAC TTA TTT GAA CTT TTC AAT ATC AGC-3′ for Trx. PCR products were purified, cloned into the pQE80L expression vector (QIAGEN, Valencia, CA) and confirmed by sequencing. Following vector transformation into Escherichia coli strain BL21, recombinant protein synthesis was induced by isopropyl β-d-1-thiogalactopyranoside (IPTG), and proteins were purified by Ni-NTA affinity chromatography (QIAGEN).
2.2. TrxR activity assays and immunoblots
TrxR activity was assayed in 100mMpotassium phosphate buffer (pH 7.0) containing the substrates 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) (1 mM) and NADPH (0.2 mM). DTNB conversion to 2-nitro-5-thiobenzoic acid was determined by absorbance measurements at 420 nm [7,12]. As controls, assays were performed with substrates but without TrxR, and with TrxR but without substrates. In both cases, activities were <5% of those observed in assays with TrxR and substrates (data not shown). Levels of TrxR were assayed by immunoblotting of extracts from HA-TrxR-expressing T. vaginalis G3 trophozoites using a commercial antibody against the HA epitope tag.
2.3. Trichomonad cultures and drug assays
Trichomonas vaginalis strains G3, F1623 and S1469 [16] [or kindly provided by Dr Evan Secor, US Center for Disease Control and Prevention (CDC), Atlanta, GA] and Tritrichomonas foetus strains T-21, D1, C1 and C3 [17,18] were grown at 37 °C in TYM Diamond’s medium supplemented with 180 µM ferrous ammonium sulphate [19]. Drug susceptibility assays were performed as described previously [16]. Briefly, stocks of the test compounds were diluted in phosphate-buffered saline to 75 µM, and 1:3 serial dilutions were made. Trophozoites (3 × 103/well) were added to the wells in 96-well plates and cultures were grown for 24 h at 37 °C under anaerobic conditions (AnaeroPack™-Anaero System; Remel, Lenexa, KS). Growth and viability were determined with an ATP assay by adding BacTiter-Glo™ Microbial Cell Viability Assay reagent (Promega, San Luis Obispo, CA) and measuring ATP-dependent luminescence in a microplate reader. The 50% inhibitory concentration (IC50) was derived from the concentration–response curves using BioAssay software (CambridgeSoft Software, Cambridge, MA). For assays of the minimal lethal concentration (MLC), 96-well plates were set up and were incubated as detailed above, but plates were subsequently examined by phase-contrast microscopy for surviving cells by searching for motile trophozoites of typical size and contrast appearance [20]. The lowest compound concentration that yielded no viable cells was taken as the MLC.
2.4. Murine trichomonad infection model
Young adult (4–6-week-old) female C57BL/6 mice were infected with a single vaginal inoculum of 106 T. foetus D1 trophozoites as described previously [17]. Mice were not pre-treated for these studies with immunosuppressant drugs or oestrogen and were not vaginally pre-colonised with conditioning bacteria such as lactobacilli. For time course studies, groups of four to six mice were examined for live trophozoites in vaginal washes every other day. For drug treatment studies, groups of six to seven mice were given daily auranofin (5 mg/kg) or solvent control by oral gavage for 4 days beginning 2 days after infection. On Day 6, live trophozoites were enumerated in vaginal washes [17].
2.5. Data analysis and statistics
For TrxR enzyme assays, at least three independent experiments were performed and the data were used to calculate the mean and standard error of the mean (S.E.M.). Results were expressed either as percentage of activity in solvent controls or as absolute activity in nmol/min DTNB conversion. Differences between groups were analysed for statistical significance (P < 0.05) by t-test. For in vitro drug sensitivity testing of trophozoites, experiments were repeated three times and the mean ± S.E.M. of the IC50 values were calculated. Statistical significance (P < 0.05) was evaluated by t-test. For in vivo drug efficacy assays, groups of four to seven animals were analysed for trophozoite load per animal. Data are shown as mean ± S.E.M. or as individual data points along with the geometric mean for each group. Differences between groups were analysed for significance (P < 0.01) by Wilcoxon rank-sum test.
3. Results
3.1. Inhibition of recombinant Trichomonas vaginalis TrxR by organic gold compounds
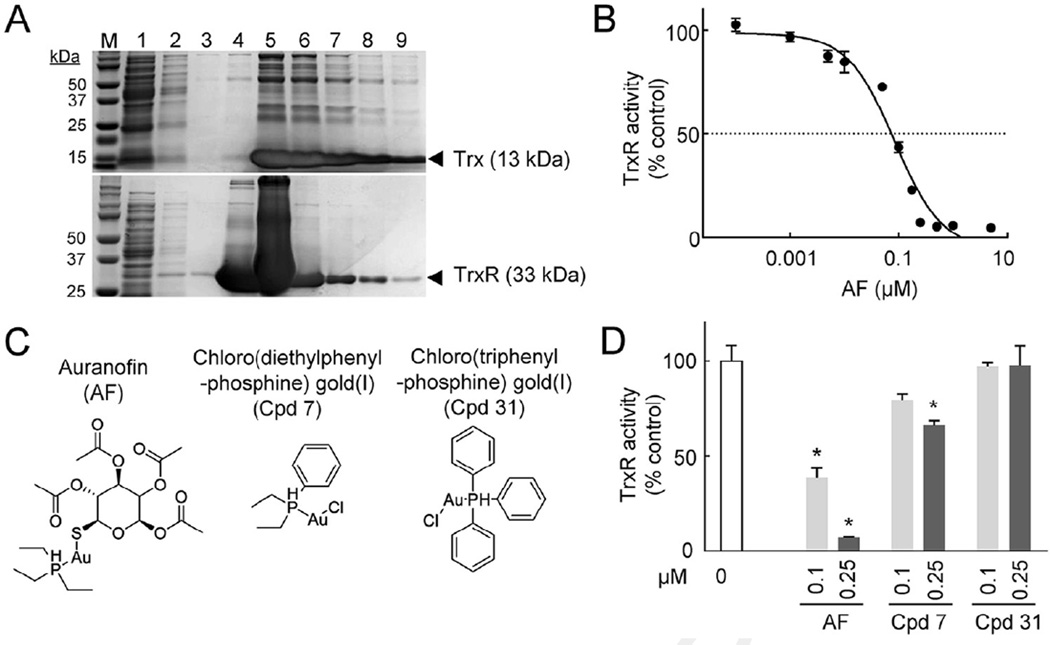
Characterisation of TrxR activity in T. vaginalis led to identification of the TrxR gene TVAG_474980 [7,8]. To test whether this gene product can be targeted with auranofin, recombinant forms of TrxR (TVAG_474980) and its electron donor Trx (TVAG_125500) were synthesised in E. coli and they were purified from the bacterial extracts by Ni-NTA affinity chromatography (Fig. 1A). The recombinant proteins were used in a reductase assay with DTNB and NADPH as substrates [8]. Auranofin inhibited reductase activity very effectively, with an IC50 of 0.085 µM (Fig. 1B). Trx alone showed no activity in this assay, nor did the substrates alone without added proteins.
Fig. 1.
Auranofin inactivates purified recombinant Trichomonas vaginalis TrxR. (A) Recombinant TrxR (TVAG_474980) and Trx (TVAG_125500) were expressed in Escherichia coli, purified and analysed by polyacrylamide gel electrophoresis (PAGE) and Coomassie straining. Lane M, markers; lane 1, flow through; lane 2, first wash; lane 3, second wash; lanes 4–9, elution fractions. (B,D) TrxR activity was tested in a cell-free system using final concentrations of 80 nM TrxR and 640 nM Trx, and 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) and NADPH as substrates. Auranofin (AF) and the organic gold(I) compounds 7 and 31 [21], whose structures are shown in (C), were tested at the indicated concentrations. Data (mean ± standard error of the mean; n = 3–8) are expressed as percentage of activity in solvent controls. * P < 0.05 (t-test) vs. controls without test compounds.
To determine whether analogues of auranofin may have improved activity against TrxR, two additional biologically active gold(I) compounds were tested (Fig. 1C) [21]. Chloro(triphenylphosphine)gold(I) had no significant inhibitory effects, while chloro(diethylphenylphosphine)gold(I) showed significant activity at the higher concentration (0.25 µM), although inhibition was less effective than with auranofin (Fig. 1D). These data demonstrate that auranofin is a strong inhibitor of T. vaginalis TrxR in the submicromolar range in vitro, while the other tested gold(I) compounds are less effective or are ineffective.
3.2. Auranofin targets TrxR in live trophozoites
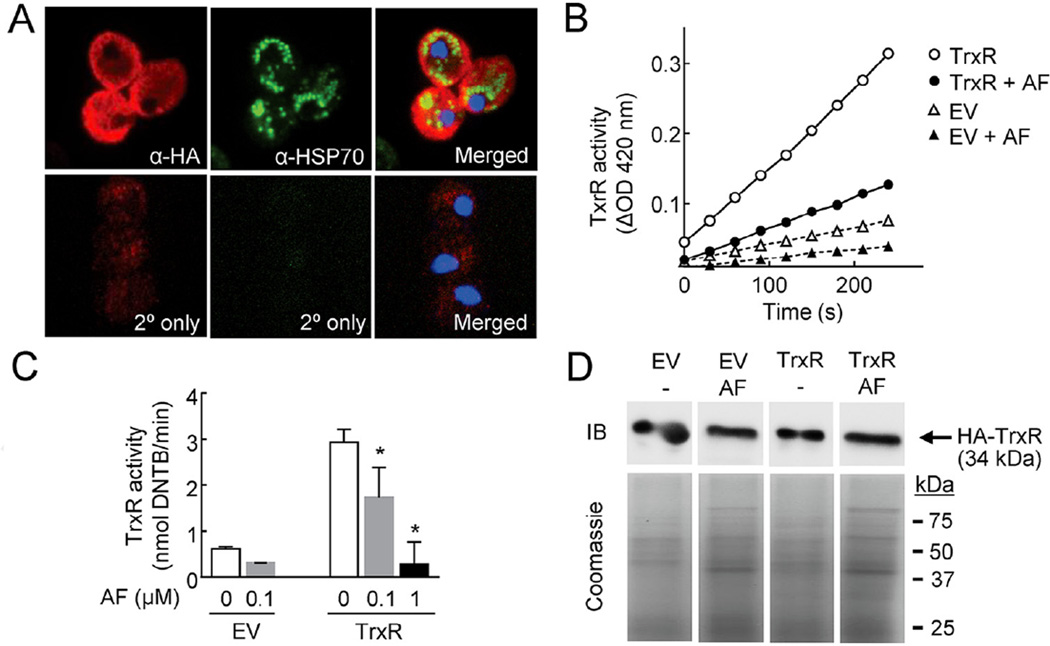
To further explore whether TrxR can be targeted for drug development when expressed natively in trophozoites, an HA-tagged version of TrxR (TVAG_474980) was constructed and expressed constitutively in T. vaginalis strain G3. Immunostaining with anti-HA antibodies showed strong expression in the cytoplasm, and the staining pattern for TrxR was different from hydrogenosomal HSP70 (Fig. 2A), suggesting a primary cytoplasmic location of TrxR, which is consistent with prior immunoblot data that had found TrxR in the soluble cytosolic fraction [7]. However, some overlap between the staining signals of TrxR and HSP70 was also observed (Fig. 2A, merged image), so we cannot rule out the possibility that a fraction of TrxR may also be present in hydrogenosomes. Subsequently, the soluble fraction of cells expressing HA-TrxR and control cells [T. vaginalis strain G3 transfected with an empty vector (EV)] were tested for reductase activity using DTNB and NADPH as substrates. Lysates from HA-TrxR-expressing cells had significantly more activity than control EV lysates (Fig. 2B), and this activity could be inhibited by auranofin in a concentration-dependent manner (Fig. 2B,C). Furthermore, treatment of live trophozoite with auranofin did not lead to TrxR degradation, suggesting that the drug inhibited the activity of the enzyme but not its expression (Fig. 2D). Together, these results indicate that TrxR (TVAG_474980) encodes a functional reductase in T. vaginalis trophozoites that is targetable with auranofin.
Fig. 2.
Inhibition of TrxR in Trichomonas vaginalis by auranofin. (A) Haemagglutinin (HA)-tagged TrxR (TVAG_474980) was transfected into T. vaginalis G3 and expression was analysed by indirect immunostaining against the HA tag (red; left and right panels). Hydrogenosomes were detected by immunostaining of hydrogenosomal HSP70 (green; middle and right panels). Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole) (blue; right panels). As controls, primary antibody was omitted (2° only). The right panels show the merged images. (B,C) Cytoplasmic extracts were prepared from HA-TrxR-expressing T. vaginalis G3 (TrxR) or cells transfected with empty vector (EV), and 100 mg of total protein was tested for TrxR activity by following reduction of 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) at 420 nm. Auranofin (AF) was added at 0.1 µM or the indicated concentrations 30 min before NADPH addition. Data in (B) are from one of two representative experiments, while the results in (C) represent mean ± standard error of the mean of four experiments. * P < 0.05 vs. controls with auranofin by t-test. (D) HA-TrxR-expressing T. vaginalis G3 (TrxR) or cells transfected with empty vector (EV) were treated for 2 h with 0.5 µM AF or were left untreated (−), and cell extracts were analysed for levels of HA-TrxR by immunoblotting (IB) or for total protein by Coomassie Blue staining.
3.3. Auranofin inhibits trichomonad growth and viability in culture
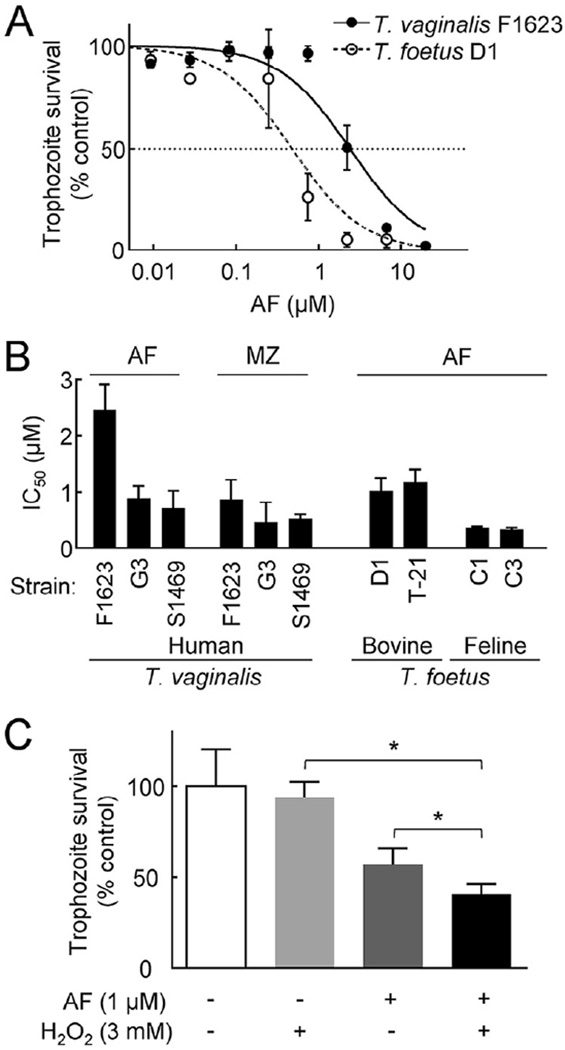
Encouraged by the marked TrxR-inhibitory activity of auranofin, we next tested its ability to kill T. vaginalis trophozoites in vitro. The drug potently inhibited parasite growth and survival in a 24-h assay, with IC50 values of 0.7–2.5 µM (Fig. 3A,B) and MLCs of 2–6 µM. In comparison, metronidazole had IC50 values of 0.8–3.5 µM under the same culture conditions, showing that auranofin had similar in vitro potency. The other gold compound with significant TrxR-inhibitory activity [chloro(diethylphenylphosphine)gold(I)] was four- to five-fold less potent in killing T. vaginalis F1623 (IC50 = 10 µM), which was consistent with its lower in vitro TrxR-inhibitory activity compared with auranofin (see Fig. 1D).
Fig. 3.
Activity of auranofin (AF) against trichomonads in vitro. The activity of AF, with and without added H2O2, against the indicated strains of Trichomonas vaginalis and Tritrichomonas foetus was evaluated in a 24-h growth and survival assay using ATP as a read-out. Results in (A) and (C) are expressed as percentage of surviving cells compared with untreated controls, while 50% inhibitory concentration (IC50) values in (B) were calculated by graphic extrapolation from concentration–response curves. Metronidazole (MZ) IC50 values against T. vaginalis are shown in (B) for comparison. In all three panels, data are the mean ± standard error of the mean (n = 3). * P < 0.05 vs. untreated cells (t-test).
Because auranofin showed significant in vitro activity against T. vaginalis trophozoites, we sought to determine whether it was also effective against other trichomonads. Tritrichomonas foetus causes genital trichomoniasis in bovines and intestinal tritrichomoniasis in cats. The drug effectively inhibited growth and viability of two bovine isolates and two feline isolates of T. foetus, displaying IC50 values of 0.4–1.2 µM (Fig. 3A,B). Together, these findings indicate that auranofin is a potent antimicrobial drug against a range of trichomonads in vitro.
3.4. Inhibition of TrxR by auranofin leads to increased oxidative stress in trophozoites
Although auranofin effectively inhibited T. vaginalis TrxR in cell-free systems, these observations do not directly prove that this effect occurs in live trophozoites or is responsible for the trichomonacidal activity of the drug. TrxR plays a major role in combating oxidative stress, so its inhibition would predictably compromise the ability of the parasite to overcome such stress. To test this prediction, we determined the impact of auranofin on trophozoite survival in the presence of different concentrations of the oxidant H2O2. Auranofin significantly reduced the ability of trophozoites to survive a modest H2O2 stress at a concentration that, by itself, was not toxic to the cells (Fig. 3C). These data support the notion that auranofin may act, in part, by inactivating TrxR-dependent antioxidant defences in T. vaginalis.
3.5. Auranofin is effective against vaginal trichomonad infection in vivo
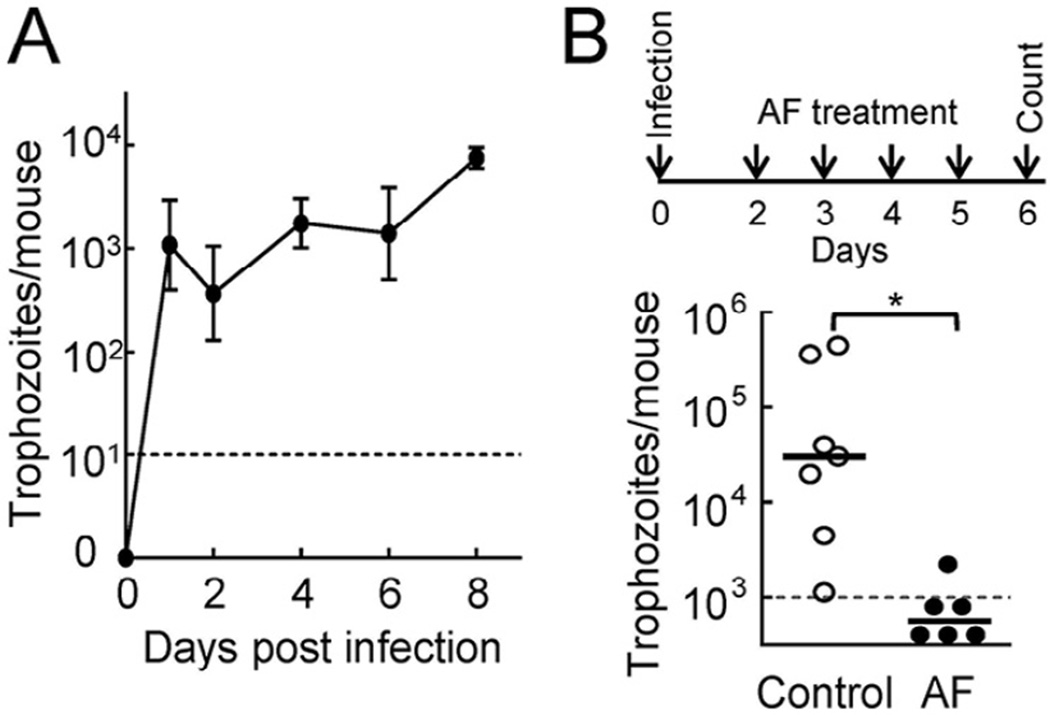
Next, to evaluate whether auranofin is efficacious against trichomonads in vivo, we utilised a murine model of vaginal infection with T. foetus [17]. Following intravaginal inoculation of T. foetus D1 into young adult C57BL/6 mice, the mice remained colonised for ≥8 days (Fig. 4A), which provided an adequate time window for efficacy testing. Within this window, we began treating mice 2 days after inoculation with a single daily oral dose of 5 mg/kg auranofin and continued treatment for 4 days. Trophozoite numbers were determined 1 day after the completion of treatment (on Day 6). The drug cleared infection in five of six infected animals, while none of the mock-treated animals showed clearance (Fig. 4B). None of the animals showed any apparent adverse effects during treatment. These results demonstrate that auranofin is efficacious and safe against trichomonad infection in vivo.
Fig. 4.
Auranofin (AF) is efficacious against trichomonads in vivo. (A) Adult female C57BL/6 mice were infected intravaginally with 106 Tritrichomonas foetus D1 trophozoites and the infectious load was determined in vaginal washes at the indicated times. (B) Two days after infection, a subset of mice was given daily AF (5 mg/kg) or solvent control by oral gavage for 4 days (top scheme). On the next day, live trophozoites were enumerated in vaginal washes. Data in (A) represent the mean ± standard error of the mean (n = 5–6 animals), while each data point in (B) represents an individual animal (horizontal bars are geometric means). * P < 0.01 by Wilcoxon rank-sum test. The dashed lines indicate the detection limit of the assay.
4. Discussion
These studies demonstrate that the antirheumatic agent auranofin is an effective inhibitor in the submicromolar range of TrxR in T. vaginalis in vitro, extending related findings in the parasitic worms S. mansoni [1] and H. contortus [10] as well as the intestinal protozoal parasites E. histolytica [11] and G. lamblia [12]. The mechanism by which auranofin inhibits TrxR in T. vaginalis remains to be established, but the tested TrxR isoform has the active-site redox centre sequence Cys–Ala–Thr–Cys [7], whose thiols have been proposed as binding sites for auranofin in TrxR of E. histolytica [11]. Furthermore, crystallographic studies with a related reductase (thioredoxin glutathione reductase) from S. mansoni have identified two additional sites that bind to auranofin, one close to an FAD-binding site and another near a NADPH-binding site [9]. The presence of an active site C-A-X-C sequence and NAD(P)-binding domains in other, poorly characterised, TrxR isoforms of T. vaginalis suggests that auranofin is likely to target several, if not all, TrxR isoforms at one or both of these sites.
Auranofin effectively killed trichomonads in vitro with IC50 values of 0.4–2.5 µM, which are similar to the findings in E. histolytica [11] but are significantly lower than those observed in G. lamblia [12]. Oral dosing of auranofin in the treatment of rheumatoid arthritis produces steady-state blood plasma levels of 1–2 µM in humans [22], which are within the range of IC50 values observed for T. vaginalis in vitro. Consistent with this, oral auranofin proved to be efficacious in eradicating vaginal trichomonad infection in mice.
In conclusion, the FDA-approved human drug auranofin may be a promising agent as an alternative treatment of trichomoniasis in cases when standard nitro drug therapies have failed.
Acknowledgments
The authors thank Elaine Hanson and Lucia Hall for expert technical assistance and Amy Barrios (University of Utah, Salt Lake City, UT) for providing two of the gold compounds.
Funding: This work was supported by the National Institutes of Health [grant AI119459].
Footnotes
Competing interests: None declared.
Ethical approval: All animal studies were approved by the Institutional Animal Care and Use Committee of University of California, San Diego (La Jolla, CA).
References
- 1.Poole DN, McClelland RS. Global epidemiology of Trichomonas vaginalis. Sex Transm Infect. 2013;89:418–422. doi: 10.1136/sextrans-2013-051075. [DOI] [PubMed] [Google Scholar]
- 2.McClelland RS, Sangare L, Hassan WM, Lavreys L, Mandaliya K, Kiarie J, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 3.Stark JR, Judson G, Alderete JF, Mundodi V, Kucknoor AS, Giovannucci EL, et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians’ Health Study. J Natl Cancer Inst. 2009;101:1406–1411. doi: 10.1093/jnci/djp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kissinger P. Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infect Dis. 2015;15:307. doi: 10.1186/s12879-015-1055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das S, Huengsberg M, Shahmanesh M. Treatment failure of vaginal trichomoniasis in clinical practice. Int J STD AIDS. 2005;16:284–286. doi: 10.1258/0956462053654258. [DOI] [PubMed] [Google Scholar]
- 6.Upcroft JA, Dunn LA, Wal T, Tabrizi S, Delgadillo-Correa MG, Johnson PJ, et al. Metronidazole resistance in Trichomonas vaginalis from highland women in Papua New Guinea. Sex Health. 2009;6:334–338. doi: 10.1071/SH09011. [DOI] [PubMed] [Google Scholar]
- 7.Coombs GH, Westrop GD, Suchan P, Puzova G, Hirt RP, Embley TM, et al. The amitochondriate eukaryote Trichomonas vaginalis contains a divergent thioredoxin-linked peroxiredoxin antioxidant system. J Biol Chem. 2004;279:5249–5256. doi: 10.1074/jbc.M304359200. [DOI] [PubMed] [Google Scholar]
- 8.Leitsch D, Kolarich D, Binder M, Stadlmann J, Altmann F, Duchene M. Trichomonas vaginalis: metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system. Implications for nitroimidazole toxicity and resistance. Mol Microbiol. 2009;72:518–536. doi: 10.1111/j.1365-2958.2009.06675.x. [DOI] [PubMed] [Google Scholar]
- 9.Angelucci F, Sayed AA, Williams DL, Boumis G, Brunori M, Dimastrogiovanni D, et al. Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: structural and kinetic aspects. J Biol Chem. 2009;284:28977–28985. doi: 10.1074/jbc.M109.020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudson AL, Sotirchos IM, Davey MW. Substrate specificity of the mitochondrial thioredoxin reductase of the parasitic nematode Haemonchus contortus. Parasitol Res. 2010;107:487–493. doi: 10.1007/s00436-010-1916-9. [DOI] [PubMed] [Google Scholar]
- 11.Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, et al. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med. 2012;18:956–960. doi: 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tejman-Yarden N, Miyamoto Y, Leitsch D, Santini J, Debnath A, Gut J, et al. A reprofiled drug, auranofin, is effective against metronidazole-resistant Giardia lamblia. Antimicrob Agents Chemother. 2013;57:2029–2035. doi: 10.1128/AAC.01675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riestra AM, Gandhi S, Sweredoski MJ, Moradian A, Hess S, Urban S, et al. A Trichomonas vaginalis rhomboid protease and its substrate modulate parasite attachment and cytolysis of host cells. PLoS Pathog. 2015;11:e1005294. doi: 10.1371/journal.ppat.1005294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delgadillo MG, Liston DR, Niazi K, Johnson PJ. Transient and selectable transformation of the parasitic protist Trichomonas vaginalis. Proc Natl Acad Sci USA. 1997;94:4716–4720. doi: 10.1073/pnas.94.9.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee M, Sievers SA, Brown MT, Johnson PJ. Identification and biochemical characterization of serine hydroxymethyl transferase in the hydrogenosome of Trichomonas vaginalis. Eukaryot Cell. 2006;5:2072–2078. doi: 10.1128/EC.00249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto Y, Kalisiak J, Korthals K, Lauwaet T, Cheung DY, Lozano R, et al. Expanded therapeutic potential in activity space of next-generation 5-nitroimidazole antimicrobials with broad structural diversity. Proc Natl Acad Sci USA. 2013;110:17564–17569. doi: 10.1073/pnas.1302664110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cobo ER, Eckmann L, Corbeil LB. Murine models of vaginal trichomonad infections. Am J Trop Med Hyg. 2011;85:667–673. doi: 10.4269/ajtmh.2011.11-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nisha KK, Bhargava G, Land KM, Chang KH, Arora R, Sen S, et al. N-propargylated isatin-Mannich mono- and bis-adducts: synthesis and preliminary analysis of in vitro activity against Tritrichomonas foetus. Eur J Med Chem. 2014;74:657–663. doi: 10.1016/j.ejmech.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002;15:329–341. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar K, Liu N, Yang D, Na D, Thompson J, Wrischnik LA, et al. Synthesis and antiprotozoal activity of mono- and bis-uracil isatin conjugates against the human pathogen Trichomonas vaginalis. Bioorg Med Chem. 2015;23:5190–5197. doi: 10.1016/j.bmc.2015.04.075. [DOI] [PubMed] [Google Scholar]
- 21.Karver MR, Krishnamurthy D, Kulkarni RA, Bottini N, Barrios AM. Identifying potent, selective protein tyrosine phosphatase inhibitors from a library of Au(I) complexes. J Med Chem. 2009;52:6912–6918. doi: 10.1021/jm901220m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blocka KL, Paulus HE, Furst DE. Clinical pharmacokinetics of oral and injectable gold compounds. Clin Pharmacokinet. 1986;11:133–143. doi: 10.2165/00003088-198611020-00003. [DOI] [PubMed] [Google Scholar]