Abstract
The hippocampus plays a critical role in verbal and spatial memory, thus any pathological damage to this formation may lead to cognitive impairment. It is suggested that right and left hippocampi are affected differentially in healthy or pathologic aging. The purpose of this study was to test the hypothesis that verbal episodic memory performance is associated with left hippocampal volume (HV) while spatial memory is associated with right HV. 115 non-demented adults over age 70 were drawn from the Einstein Aging Study. Verbal memory was measured using the free recall score from the Free and Cued Selective Reminding Test – immediate recall (FCSRT-IR), logical memory immediate and delayed subtests (LM-I and LM-II) from the Wechsler Memory Scale-Revised (WMS-R). Spatial Memory was measured using a computerized dot memory paradigm that has been validated for use in older adults. All participants underwent 3T MRI with subsequent automatized measurement of the volume of each hippocampus. The sample had a mean age of 78.7 years (SD=5.0); 57% were women, and 52% were white. Participants had a mean of 14.3 years (SD=3.5) of education. In regression models, two tests of verbal memory (FCSRT-IR free recall and LM-II) were positively associated with left HV, but not with right HV. Performance on the spatial memory task was associated with right HV, but not left HV. Our findings support the hypothesis that the left hippocampus plays a critical role in episodic verbal memory, while right hippocampus might be more important for spatial memory processing among non-demented older adults.
Keywords: Hippocampus, spatial memory, verbal episodic memory, older adults
1. Introduction
Hippocampus, a bilateral structure within the middle temporal lobe, is well known for being an essential part of neural network of learning and memory. Several studies have indicated that hippocampal volume (HV) can predict performance on a variety of memory tests in healthy controls (Convit, Wolf, Tarshish, & De Leon, 2003; Hackert, et al., 2002; Rosen, et al., 2003; C. Van Petten, 2004) and pathological diseases including schizophrenia(Seidman, et al., 2002), Alzheimer disease (AD) (Dubois, et al., 2014), and hippocampal sclerosis observed in temporal lobe epilepsy (Adda, Castro, e Silva, de Manreza, & Kashiara, 2008; Griffith, Pyzalski, Seidenberg, & Hermann, 2004).
Prior studies indicate that pathological factors may lead to brain asymmetry (Toga & Thompson, 2003). It has been suggested that right and left hippocampi are affected differentially in progressive phases of memory loss (Shi, Liu, Zhou, Yu, & Jiang, 2009). Though not always consistent, left HV’s association with different episodic memory tests is stronger than right HV association with the same tests in normal cognition, MCI, and AD groups (Shi, et al., 2009). Other studies have suggested specialized roles for structures in the right side of brain including dorsolateral prefrontal cortex, occipital cortex and hippocampus structures in spatial and navigational memory (Nemmi, Boccia, Piccardi, Galati, & Guariglia, 2013; Persson, et al., 2013).
In healthy populations, reports of the correlation between HV and its corresponding behavioral expression have been inconsistent. While some studies (Hackert, et al., 2002; Rosen, et al., 2003) have shown larger total HV is associated with better performance on episodic memory, other studies have found no significant correlation between these measures in healthy older adults (MacLullich, et al., 2002; Maguire, et al., 2000; Marquis, et al., 2002). A meta-analysis of studies in older adults have yielded a weak positive relationship between episodic memory and HV (Cyma Van Petten, 2004). Other studies have reported hippocampal atrophy in subjects with amnestic mild cognitive impairment (aMCI) (Jessen, et al., 2006; Muller, et al., 2005). In addition, HV has been suggested as a predictive measure of disease progression and worsening of memory function in a variety of neurodegenerative diseases including AD (den Heijer, et al., 2006; Dubois, et al., 2014) and Parkinson’s disease (Brück, Kurki, Kaasinen, Vahlberg, & Rinne, 2004). Overall, it seems that these relationships are most obvious in individuals with advanced stages of cognitive impairment or age-related neurodegeneration, which result in a reduction of HV.
Identifying specific function of right and left hippocampi and their effect on cognitive tests may facilitate efforts toward earlier detection of pathological changes that lead to cognitive decline and AD. The purpose of the current study was to characterize associations between left or right HV and performance on episodic verbal and spatial memory tests in a population of non-demented older adults. We hypothesized that performance on verbal memory tests would be associated with left HV and performance on a spatial memory task would be associated with right HV.
2. Methods
2.1. Participants
This cross-sectional study was conducted on 115 non-demented adults over the age of 70 enrolled in the Einstein Aging Study (EAS). The study design and methods of the EAS have been described in detail previously (Katz, et al., 2012). Briefly, potential participants were recruited through systematic sampling from Medicare and voter registration lists for Bronx County, New York. Eligible participants were aged 70 and older, Bronx residents, non-institutionalized, and English-speaking. Exclusion criteria included severe visual or auditory impairments that precluded neuropsychological testing, active psychiatric symptomatology that interfered with the ability to complete assessments, and non-ambulatory status. Participants received annual in-person assessments including medical history, neuropsychological testing and general and neurologic examinations.
Participants who met diagnostic criteria for dementia or did not meet standard MRI eligibility criteria – safety contraindications or metallic implants that would create image artifacts – were excluded from this study. Dementia diagnosis was based on the Diagnostic and Statistical Manual, Fourth Edition (DSM-IV) (American Psychiatric Association. & American Psychiatric Association. Task Force on DSM-IV., 2000) and was assigned at consensus case conferences attended by the study clinicians and licensed neuropsychologist, and included a comprehensive review of neuropsychological test results, relevant neurological signs and symptoms, and functional status (Katz, et al., 2012).
All studies were approved by the Institutional Review Board of the Albert Einstein College of Medicine.
2.2. Verbal memory assessment
Details of the EAS neuropsychological test battery has been previously described (Katz, et al., 2012). The Free and Cued Selective Reminding Test – Immediate Recall (FCSRT-IR) (Buschke, 1984; Grober, Buschke, Crystal, Bang, & Dresner, 1988) is an episodic memory test, which requires learning of 16 pictures by naming each picture and identifying the category to which it belongs. It also consists of three trials of immediate free recall (range 0-48), each of which is followed by cued recall in which a category cue is given to the subject to facilitate recall of the items not freely recalled. Total recall is the sum of free and cued recall together. Since total recall demonstrates ceiling effects in our community-based sample (Zimmerman, et al., 2015), in this analysis we only used the free recall score.
Logical Memory I (LM-I) from the Wechsler Memory Scale- Revised (WMS-R) (Wechsler, 1987) is an immediate declarative memory test (range 0–50), in which two different stories are read to the participant, and after each story the participant immediately recalls it from memory. Scores are given on the accuracy of the retelling of the story. In Logical Memory II (LM-II), recall is probed 20 min after the immediate condition.
2.3. Spatial memory assessment
Spatial memory was assessed using a computerized dot memory task. The dot memory task consisted of 3 phases: encoding, distraction, and retrieval. During the encoding phase, participants were shown and asked to remember the location of 3 red dots on a 5×5 grid. Participants were allowed to study the grid for a 3 second period. A distraction phase began after the grid was removed in which participants were presented with the letters F and E, and were required to locate and touch the Fs among the array of Es. This distraction phase lasted 8 seconds. In the retrieval phase, the 5×5 grid reappeared empty and the participants’ task was to recall the locations of the 3 dots that had been presented in the encoding phase. Participants completed 11 trials (encoding, distractors, and retrieval). Scores were based on errors, with credit being based on the deviation from the correct positions. If all dots were recalled in their correct location participants were given a score of 0. If there were 1 or more retrieval errors, the Euclidean distance of the location of the incorrect dot to the correct grid location was calculated, with lower scores indicating more accurate placement and better performance (Siedlecki, 2007). In order to simplify interpretation of results, the reversed score (spatial error mean x -1) was used to report spatial memory function so that higher scores indicated better performance.
2.4. MRI Acquisition and Processing
Imaging was performed using a 3.0 T MRI scanner (Achieva Quasar TX; Philips Medical Systems, Best, the Netherlands) and 32-channel head coil (Sense Head Coil; Philips Medical Systems, Best, the Netherlands). T1-weighted whole-head structural imaging was performed using sagittal three-dimensional magnetization-prepared rapid acquisition gradient echo (MP-RAGE) with TR/TE 9.9/4.6ms; 240 mm2 FOV; 240×240 matrix; partition thickness, 1 mm; and parallel acceleration factor 2.0. In addition, a 3D T2-weighted fluid-attenuated inversion recovery (T2W-FLAIR) acquisition was obtained with the following pulse sequence parameters: TR/TE/TI 11000/120/2800ms; 240 × 240 mm FOV; 240 × 240 matrix; 1mm partition thickness and parallel acceleration factor 2.0.
2.5. Image processing
We processed all MRIs automatically using the FreeSurfer software package (version 5.2, available at http://surfer.nmr.mgh.harvard.edu/). Image processing methods in the EAS have been previously described in detail (Ezzati, Katz, Lipton, Zimmerman, & Lipton, 2015; Ezzati, et al., 2014). T1 and T2-FLAIR images were used to segment the cortical and subcortical volumes including whole hippocampal formation by FreeSurfer’s standard segmentation procedure using a probabilistic brain atlas (Fischl, et al., 2002). Additionally, for each subject the estimated intracranial volume (TICV) was calculated by the procedure described by Buckner et al (Buckner, et al., 2004).
2.6. Statistical Analyses
All statistical analyses were conducted using SPSS, version 20 (Chicago, IL: SPSS Inc.). We examined the associations between demographic variables, memory tests, and volumetric MRI measures using the Pearson product-moment correlation coefficient (r) for continuous variables, and independent t-test for categorical variables.
A series of multivariate linear regression analyses were performed to examine the association between each memory test and hippocampal volumetric measures accounting for the influence of covariates. Initially we looked at the association between HVs and memory tests in unadjusted models. Sidak correction factor (Šidák, 1967) with an adjusted p value of 0.0125 for total volumetric analysis—and separately for left HV and right HV —(α=0.05, four comparisons for each memory test: FCSRT-IR free recall, LM-I, LM-II, and Spatial memory) was used to correct for type I error. Only the tests that were significantly associated with at least one of the hippocampal volumes in the unadjusted preliminary models were entered in more complex models. Subsequently, we investigated the effect of total HV on each of the memory tests while controlling for age, gender, education, and TICV as covariates. We then examined the association between each of the memory tests and left or right HV in a series of linear regressions to find out if there are lateral HV associations with verbal and/or spatial memory. We also investigated whether a lateralized association between left HV and memory performances is independent from right HV, and vice versa, by evaluating the joint effects of left and right HVs in the models. In our models we thus tested the association between each of the memory tests and left HV (model 1), right HV (model 2), and both left and right HVs simultaneously (model 3). We adjusted for demographics in each of these models.
3. Results
In correlational analyses including the entire sample (Table 1), older age was associated with poorer spatial memory performance (r=−0.25, p=0.008), but not with FCSRT-IR free recall (r=−0.17, p=0.07), LM1 (r=−0.06, p=0.50) LM2 (r=−0.10, p=0.24) tests. Women had smaller HVs (left HV: t= −2.55, p=0.013; right HV: t= −2.46, p=0.016) and performed significantly worse than men on the spatial memory paradigm (t=−4.20, p<0.001), however there was no gender difference on verbal episodic memory tests (FCSRT-IR free recall: t=1.76, p=0.08; LM1: t=0.65, p=0.51; LM2: t=0.20, p=0.83). Years of education did not show any significant correlations with volumetric measures or memory tests. There were no significant associations between spatial memory performance and FCSRT-IR free recall or LM memory tests. Sample characteristics are summarized in Table 1.
Table 1.
Sample demographics and correlation with memory performance and MRI measures.
| total sample (N=115) |
FCSRT-IR free recall |
Spatial memory performance |
Left HV | Right HV |
|
|---|---|---|---|---|---|
|
| |||||
| N or Mean (SD) |
r/ta | r/t | r/t | r/t | |
| Men/Women | 50/65 | 1.76 | 4.20*** | 2.55* | 2.46* |
| White/Other | 60/55 | −0.06 | −0.42 | 0.25 | 0.15 |
| Age, years | 78.74(5.02) | −0.17 | −0.25** | - | −0.44*** |
| 0.35*** | |||||
| Education, years | 14.39(3.55) | 0.08 | 0.11 | −0.13 | −0.06 |
|
Spatial Memory
performance |
−2.60(0.77) | 0.07 | - | 0.27** | 0.34*** |
| FCSRT-IR free recall | 31.52(6.55) | - | 0.07 | 0.30*** | 0.17 |
| FCSRT-IR total recall | 47.47(1.27) | 0.53*** | 0.09 | 0.32*** | 0.24* |
| LM 1 | 22.04(7.40) | 0.41*** | 0.08 | 0.08 | 0.08 |
| LM 2 | 10.40(3.58) | 0.50*** | 0.05 | 0.09 | 0.08 |
| Left HV | 3.24(0.43) | 0.30*** | 0.27** | - | 0.72*** |
| Right HV | 3.30(0.47) | 0.17 | 0.33*** | 0.72*** | - |
| TBV | 988(109) | −0.03 | 0.38*** | 0.46*** | 0.41*** |
| TICV | 1368(220) | −0.19* | 0.27** | 0.17 | 0.17 |
P < 0.05,
P < 0.01,
P < 0.001. A p-value of less than 0.05 was considered statistically significant and the corresponding results are shown in bold. MRI volumetric data are given in cubic centimeters.
Using Pearson’s correlation (r) for continuous variables, and t-test for categorical variables. FCSRT-IR = Buschke and Grober Free and Cued Selective Reminding Test–Immediate Recall; HV = hippocampal volume; TBV = total brain volume; TICV = total intracranial volume; LM-I= logical memory immediate subtest, and LM-=logical memory delayed subtest.
In unadjusted regression models (e-table 1) and after correction for multiple comparisons, the association between HVs and LM tests were not significant and therefore, they were not entered in further adjusted models. Table 2 shows a series of models assessing the relationship of total HV to FCSRT-IR free recall and spatial memory. After adjusting for age, sex, years of education, and TICV, smaller total HV was associated with poorer performance on FCSRT-IR free recall (β = 0.32, p = 0.002), and spatial memory (β = 0.20, p = 0.040).
Table 2.
Adjusted Regression models for the effect of total HV on memory tests
| FCSRT-IR free recall | Spatial memory a | |||
|---|---|---|---|---|
|
| ||||
| Predictor | β | p | β | p |
| Age | −0.04 | 0.685 | −0.14 | 0.141 |
| Gender | −0.14 | 0.175 | 0.25 | 0.015 |
| Years of education | 0.16 | 0.070 | 0.12 | 0.161 |
| TICV | −0.20 | 0.076 | 0.07 | 0.500 |
| Total HV | 0.32 | 0.002 | 0.20 | 0.040 |
β = standardized regression coefficient; THV= total hippocampal volume; TICV=total intracranial volume.
Spatial memory scores were reversed so that higher numbers indicate better performance.
Table 3 shows the separate and joint effect of the left and right HVs on each of memory tests. After adjusting for age, sex, years of education, and TICV, smaller left HV was associated with poor performance on FCSRT-IR free recall (β = 0.38, p < 0.001) , but there was no association with performance on the dot memory test (Table 3, model 1). Smaller right HV was associated with poorer performance on dot memory test (β = 0.21, p = 0.03), however there was no association with the FCSRT-IR free recall (Table 3, model 2)). In the final model (Table 2, model 3), the association between left HV and poor performance on the free recall score from the FCSRT-IR remained significant (β = 0.44, p < 0.001) even when right HV was also included in the models. However the effect of right HV on spatial memory as attenuated and lost its significance in models that also included left HV.
Table 3.
Adjusted regression models for the effect of HV in each hemisphere on memory tests.
| Outcome | Predictor | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| β | p | β | p | β | p | ||
| FCSRT-IR free recall | |||||||
| Left HV | 0.38 | <0.001 | 0.44 | <0.001 | |||
| Right HV | 0.20 | 0.07 | −0.10 | 0.43 | |||
| Spatial memory | |||||||
| Left HV | 0.15 | 0.11 | 0.03 | 0.84 | |||
| Right HV | 0.21 | 0.03 | 0.18 | 0.15 | |||
Model 1: Model includes demographics and left HV as predictors; Model 2: Model includes demographics and right HV as predictors; Model 3: Model includes demographics and both left and right HV as predictors
Note. Demographics = Age, sex, and years of education, and total intracranial volume
• A p-value of less than 0.05 was considered statistically significant.
β = standardized regression coefficient; HV=Hippocampal volume.
4. Discussion
In this study, we examined the association of HV with performance on a set of verbal and spatial memory tasks. In agreement with our initial hypothesis, spatial memory performance was associated with right HV, while performance on verbal episodic memory test of FCSRT-IR free recall was associated with left HV. In addition the effect of right HV on spatial memory diminished in models that included left HV. However the association between left HV and FCSRT-IR free recall remained significant in models including both hippocampi.
Although some studies have shown a direct correlation between the total HV and episodic memory in healthy older adults (Hackert, et al., 2002; Rosen, et al., 2003), there are many studies reporting no significant correlation between these measures in this population (MacLullich, et al., 2002; Maguire, et al., 2000; Marquis, et al., 2002). Interestingly, a meta-analysis of studies of elderly subjects shows a weak positive relationship between hippocampal size and episodic memory ability in older adults (Cyma Van Petten, 2004). In the present study, although total HV was correlated with performance on episodic memory free recall, the association of the volume of the left hippocampus with this measure was stronger and was the only significant association between the two hippocampi. One explanation for such discrepant results might be the sensitivity of specific tests used in each of these studies. Our findings support the hypothesis that these cognitive tests are specifically associated with hippocampal laterality, and using the volume of the hippocampi in aggregate, might lead to weakening of the associations.
Our results indicated that THV and left HV were only associated with the FCSRT-IR free recall and not LM. Some previous studies also found no association between LM and HV (Marquis, et al., 2002; Rodrigue & Raz, 2004). Other studies have suggested that list learning tests and paragraph recall differentially load on various MTL structures, e.g. hippocampal volume was more strongly associated with paragraph recall but list learning was primarily associated with-entorhinal in AD (De Toledo-Morrel, Goncharova, Dickerson, Wilson, & Bennett, 2000; Wilson, Sullivan, deToledo-Morrell, Stebbins, & Bennett, 1996). This differential association might be due to difference in the cognitive functions assessed by these tests and their underlying structural correlates. Interpreting differences among studies is made more difficult by differences in sample characteristics and volumetric procedures. If the automated procedures we used do not separate MTL structures with the precision of manual tracing, that could contribute to discrepancies with studies using manual tracing.
In our study, we showed that only left HV is associated with FCSRT-IR free recall, which is a classic test of episodic verbal memory. Studies of medial temporal lobe resection specimens support an important role for left hippocampus in verbal abilities (Sass, et al., 1992). In accordance with our findings, prior studies have also demonstrated a left-lateralized association between hippocampus and verbal memory in different populations (Shi, et al., 2009; Travis, et al., 2014; Wolf, et al., 2001). Travis et al (Travis, et al., 2014) showed an association between left HV and immediate verbal memory performances in healthy adults. Wolf et al. (Wolf, et al., 2001) reported significant left-less-than-right hippocampal asymmetry in controls and MCI patients. A meta-analysis of 14 studies including control, MCI, and AD patients revealed asymmetric pattern in association of hippocampal volume with episodic memory, with left hippocampus showing stronger associations (Shi, et al., 2009).
We also showed that smaller right HV is associated with poorer spatial memory performance. Despite numerous studies examining the associations of spatial memory and hippocampus, few studies have examined the link between regional HV and spatial memory or navigational skills. One neuroimaging study of normal elderly using a maze learning task showed an association between spatial memory and right hippocampal tail volume (Chen, Chuah, Sim, & Chee, 2010). In a series of MRI volumetric studies on London taxi drivers, number of years of professional taxi driving was positively correlated to the drivers' right and posterior HVs, while this association was not seen in those without a profession requiring intense spatial representations (Maguire, et al., 2000; Woollett & Maguire, 2011). Surgical interventions in epilepsy patients, with selective damage to middle temporal structures have also confirmed the role of the right hippocampus and right parahippocampal cortex in visuospatial memory tasks (Bohbot, et al., 1998). Functional MRI studies have also suggested positive correlation between right-lateralized activation during the navigation phase with greater spatial memory task performance (Persson, et al., 2013). These studies are largely in accordance with our finding that spatial memory is primarily associated with right HV. Although due to the cross sectional nature of this study it is not possible to interpret causation, the findings from this study suggest that hippocampal pathology in each hemisphere may contributes more to decline in performance on specific memory tests (spatial vs verbal in our case).
Models comprising both left and right HV help to investigate whether the observed lateralized association between hemispheric HV and memory performances is independent from the HV in the other hemisphere. Based on our findings, we suggest that smaller left HV after adjustment for TICV, as well as age, sex, education, and right HV, is associated lower performance on verbal memory as measured by FCSRT-IR free recall. This might be indicative of critical and independent role of left hippocampus in verbal episodic memory processes. The association between right HV and spatial memory performance was significantly diminished by inclusion of left HV in the models. This might suggest that both left and right hippocampi contribute in formation or retrieval of spatial memory.
Differential function of left and right hippocampus has been also reported in MCI and early AD populations (Nedelska, et al., 2012). Hippocampal atrophy has been widely suggested as a strong predictor of cognitive decline (Devanand, et al., 2008; Jack, et al., 2000), while it has been suggest that that left and right HV might have different predictive values in models predicting risk of progression to AD (Eckerström, et al., 2008; Risacher, et al., 2009). Our results, together with prior studies, might suggest that left and right HVs should be used separately in the predictive models for AD progression.
While our findings are promising, a few limitations should be noted. First, performance on various memory paradigms is dependent on complex neural networks, not just the hippocampus. The coordinated involvement of other cortical and subcortical regions as well as their interconnecting white matter tracts needs to be clarified. Although we looked at the inter-hemispheric associations with memory tests, we did not look at hippocampal subfields. Other neuroimaging studies have shown differential functional specialization of hippocampal subfields in different memory paradigms (Travis, et al., 2014). Finally, the study population is only comprised of adults older than 70 years, therefore the findings might not be generalizable to the whole population.
In conclusion, our study provides further evidence of divergent functional specialization of the right and left hippocampus. Right hippocampus plays a critical role in spatial memory in older adults, while the role of left hippocampus in verbal memory is more prominent. Together, the results can serve as a basis for future research to ascertain the ability of spatial memory testing to identify patients in the preclinical stage of AD, where hippocampal deficits are among the primary symptoms.
Supplementary Material
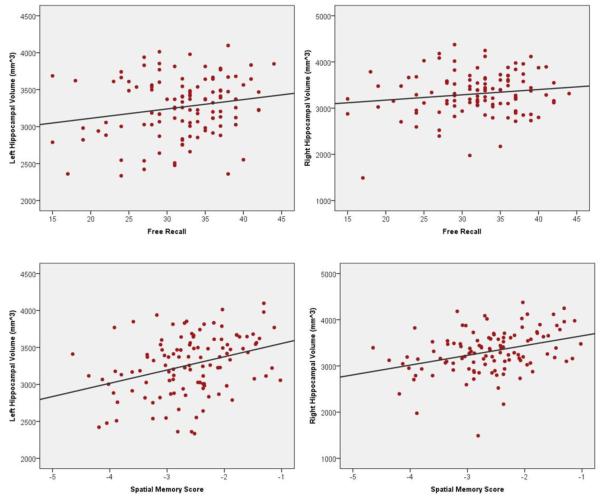
Figure 1.
Simple scatter plots of the associations between each hemisphere’s hippocampal volume and memory tests. Note: Spatial memory scores are reversed so that higher numbers indicate better performance.
Highlights.
Verbal episodic memory performance is associated with left hippocampal volume
Spatial memory performance is primarily associated with right hippocampal volume
Right and left hippocampus show divergent specialization for different memory tasks
Acknowledgments
The Authors would like to thank the Einstein Aging Study staff for assistance with recruitment, and clinical and neuropsychological assessments. In addition, we appreciate all of the study participants who generously gave their time in support of this research.
Funding/Support: This research was supported in part by National Institutes of Health grants NIA 2 P01 AG03949, NIA 1R01AG039409-01, NIA R03 AG045474, CTSA 1UL1TR001073 from the National Center for Advancing Translational Sciences (NCATS), the Leonard and Sylvia Marx Foundation, and the Czap Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None reported.
References
- Adda CC, Castro LH, e Silva LCA-M, de Manreza ML, Kashiara R. Prospective memory and mesial temporal epilepsy associated with hippocampal sclerosis. Neuropsychologia. 2008;46:1954–1964. doi: 10.1016/j.neuropsychologia.2008.01.016. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. American Psychiatric Association. Task Force on DSM-IV . Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th ed American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36:1217–1238. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Brück A, Kurki T, Kaasinen V, Vahlberg T, Rinne J. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75:1467–1469. doi: 10.1136/jnnp.2003.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buschke H. Cued recall in amnesia. J Clin Neuropsychol. 1984;6:433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- Chen KH, Chuah LY, Sim SK, Chee MW. Hippocampal region-specific contributions to memory performance in normal elderly. Brain Cogn. 2010;72:400–407. doi: 10.1016/j.bandc.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, Tarshish C, De Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proceedings of the National Academy of Sciences. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Toledo-Morrel L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer's disease: in vivo detection of entorhinal cortex atrophy. Annals of the New York Academy of Sciences. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry. 2006;63:57–62. doi: 10.1001/archpsyc.63.1.57. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer's disease. Biological psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. The Lancet Neurology. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Eckerström C, Olsson E, Borga M, Ekholm S, Ribbelin S, Rolstad S, Starck G, Edman Å, Wallin A, Malmgren H. Small baseline volume of left hippocampus is associated with subsequent conversion of MCI into dementia: the Göteborg MCI study. Journal of the neurological sciences. 2008;272:48–59. doi: 10.1016/j.jns.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Ezzati A, Katz MJ, Lipton ML, Zimmerman ME, Lipton RB. Hippocampal volume and cingulum bundle fractional anisotropy are independently associated with verbal memory in older adults. Brain Imaging Behav. 2015 doi: 10.1007/s11682-015-9452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A, Zimmerman ME, Katz MJ, Sundermann EE, Smith JL, Lipton ML, Lipton RB. Hippocampal subfields differentially correlate with chronic pain in older adults. Brain Res. 2014;1573:54–62. doi: 10.1016/j.brainres.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Griffith HR, Pyzalski RW, Seidenberg M, Hermann BP. Memory relationships between MRI volumes and resting PET metabolism of medial temporal lobe structures. Epilepsy & Behavior. 2004;5:669–676. doi: 10.1016/j.yebeh.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Hackert V, Den Heijer T, Oudkerk M, Koudstaal P, Hofman A, Breteler M. Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage. 2002;17:1365–1372. doi: 10.1006/nimg.2002.1248. [DOI] [PubMed] [Google Scholar]
- Jack C, Petersen RC, Xu Y, O’brien P, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–490. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, Schild HH, Scheef L. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27:1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, Dickson DW, Derby CA. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLullich AM, Ferguson KJ, Deary IJ, Seckl JR, Starr JM, Wardlaw JM. Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurology. 2002;59:169–174. doi: 10.1212/wnl.59.2.169. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis S, Moore MM, Howieson DB, Sexton G, Payami H, Kaye JA, Camicioli R. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- Muller MJ, Greverus D, Dellani PR, Weibrich C, Wille PR, Scheurich A, Stoeter P, Fellgiebel A. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. Neuroimage. 2005;28:1033–1042. doi: 10.1016/j.neuroimage.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Nedelska Z, Andel R, Laczó J, Vlcek K, Horinek D, Lisy J, Sheardova K, Bureš J, Hort J. Spatial navigation impairment is proportional to right hippocampal volume. Proceedings of the National Academy of Sciences. 2012;109:2590–2594. doi: 10.1073/pnas.1121588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmi F, Boccia M, Piccardi L, Galati G, Guariglia C. Segregation of neural circuits involved in spatial learning in reaching and navigational space. Neuropsychologia. 2013;51:1561–1570. doi: 10.1016/j.neuropsychologia.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Persson J, Herlitz A, Engman J, Morell A, Sjolie D, Wikstrom J, Soderlund H. Remembering our origin: gender differences in spatial memory are reflected in gender differences in hippocampal lateralization. Behav Brain Res. 2013;256:219–228. doi: 10.1016/j.bbr.2013.07.050. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Saykin AJ, Wes JD, Shen L, Firpi HA, McDonald BC. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Current Alzheimer Research. 2009;6:347–361. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. The Journal of neuroscience. 2004;24:956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, Gabrieli JD, Stoub T, O'Hara R, Friedman L, Yesavage JA, deToledo-Morrell L. Differential associations between entorhinal and hippocampal volumes and memory performance in older adults. Behavioral neuroscience. 2003;117:1150. doi: 10.1037/0735-7044.117.6.1150. [DOI] [PubMed] [Google Scholar]
- Sass KJ, Sass A, Westerveld M, Lencz T, Novelly RA, Kim JH, Spencer DD. Specificity in the correlation of verbal memory and hippocampal neuron loss: dissociation of memory, language, and verbal intellectual ability. J Clin Exp Neuropsychol. 1992;14:662–672. doi: 10.1080/01688639208402854. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N, Toomey R, Kennedy D, Caviness VS, Tsuang MT. Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Archives of General Psychiatry. 2002;59:839–849. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus. 2009;19:1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Šidák Z. Rectangular confidence regions for the means of multivariate normal distributions. Journal of the American Statistical Association. 1967;62:626–633. [Google Scholar]
- Siedlecki KL. Investigating the structure and age invariance of episodic memory across the adult lifespan. Psychology and Aging. 2007;22:251. doi: 10.1037/0882-7974.22.2.251. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Travis SG, Huang Y, Fujiwara E, Radomski A, Olsen F, Carter R, Seres P, Malykhin NV. High field structural MRI reveals specific episodic memory correlates in the subfields of the hippocampus. Neuropsychologia. 2014;53:233–245. doi: 10.1016/j.neuropsychologia.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WMS-R : Wechsler Memory Scale--Revised : manual. Psychological Corp. : Harcourt Brace Jovanovich; San Antonio: 1987. [Google Scholar]
- Wilson RS, Sullivan M, deToledo-Morrell L, Stebbins GT, Bennett DA. Association of memory and cognition in Alzheimer's disease with volumetric estimates of temporal lobe structures. Neuropsychology. 1996;10:459. [Google Scholar]
- Wolf H, Grunwald M, Kruggel F, Riedel-Heller SG, Angerhofer S, Hojjatoleslami A, Hensel A, Arendt T, Gertz H. Hippocampal volume discriminates between normal cognition; questionable and mild dementia in the elderly. Neurobiol Aging. 2001;22:177–186. doi: 10.1016/s0197-4580(00)00238-4. [DOI] [PubMed] [Google Scholar]
- Woollett K, Maguire EA. Acquiring "the Knowledge" of London's layout drives structural brain changes. Curr Biol. 2011;21:2109–2114. doi: 10.1016/j.cub.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Katz MJ, Wang C, Burns LC, Berman RM, Derby CA, L'Italien G, Budd D, Lipton RB. Comparison of “Word” vs.“Picture” version of the Free and Cued Selective Reminding Test (FCSRT) in older adults. Alzheimer's & dementia: diagnosis, assessment & disease monitoring. 2015;1:94–100. doi: 10.1016/j.dadm.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.