Abstract
From the outset, it was apparent that developing new therapies with mesenchymal stem/stromal cells (MSCs) was not a simple or easy task. Among the earliest experiments was administration of MSCs from normal mice to transgenic mice that developed brittle bones because they expressed a mutated gene for type 1 collagen isolated from a patient with osteogenesis imperfecta. The results prompted a clinical trial of MSCs in patients with severe osteogenesis imperfecta. Subsequent work by large numbers of scientists and clinicians has established that, with minor exceptions, MSCs do not engraft or differentiate to a large extent in vivo. Instead the cells produce beneficial effects in a large number of animal models and some clinical trials by secreting paracrine factors and extracellular vesicles in a “hit and run” scenario. The field faces a number of challenges, but the results indicate that we are on the way to effective therapies for millions of patients who suffer from devastating diseases. This report was presented by the Author on receipt of the Career Achievement Award in Cell Therapy from the International Society for Cellular Therapy. Singapore, May 25, 2016.
Keywords: MSCs, clinical trials, future prospects
I was doing research for many years before I realized a simple truth: no matter how hard I tried, there were only a small group of scientists in the world who understood whether what I had done was a small step or a big step in advancing the field. Therefore, this award from my peers in the field of cell therapy has special meaning to me.
By way of background, I might mention that I came into the field of cell therapy by an unusual path. I had gone to medical school and had one year of hospital training. I then went to the US National Institutes of Health under a program that surprisingly allowed me to do research instead of serving in the military. While at NIH, I was able to complete a Ph. D. degree for research on collagen biosynthesis. What followed was 30 happy years doing research on much the same topic. They were happy years because with experimental tools that seem childishly simple today, we and others in the field were able to unravel the complex pathway by which cells assembled the precursor molecule procollagen and then processed it by seven different enzymes to collagen fibers [1]. While defining the pathway, we searched for drugs that might inhibit the excess deposition of collagen in scars. One series of the drugs, unexpectedly, inhibited degradation of hypoxia induced factor (HIF) and are currently being developed for the therapy of anemias [2]. They were also happy years because I was privileged to work with a group of exciting graduate students and postdoctoral fellows, many of whom went on to outstanding scientific careers and thereby became the most important products of my laboratory. Then some unexpected developments. My laboratory isolated the first genes for human collagens [1]. They had fascinating structures but after “cloning and moaning” for several years, we began to wonder what else we could do with them. Fortuitously, we and our competitors at the time discovered that the collagen genes harbored mutations that caused a large family of genetic diseases of bone and cartilage. And after we had identified well over fifty of such mutations, we were faced with the next question: what could we do for children such as those who had collagen mutations causing severely brittle bones, the genetic disease of osteogenesis imperfect (OI)? Or what we could do for their parents who were devastated by experiences such as breaking an arm or leg when lifting their child from a crib as carefully as they could. One of the few answers seemed to be therapy with cells that could differentiate into bone, cells like mesenchymal stem/stromal cells (MSCs).
I mention all this to illustrate that I came into the field of cell therapy with some strengths as a scientist but also some glaring weaknesses. Therefore I needed to learn, and to keep learning, from members of this Society and others who knew and still know far more than I about hematology, organ transplants, cell biology, immunology and many other topics.
When we and others began working with potential therapies with MSCs, we knew the journey would not be a short or easy one. Also, we knew that hard facts would be much more difficult to extract than in research on the biosynthesis of collagen or mutations in collagen genes. However, we were reassured by the history of most new therapies in medicine. As is well recognized, the process was rarely a linear one (Fig. 1). The basic research that first suggested the idea was rarely as convincing as one would like. The initial trials in animal models left much to be desired. With MSCs, the experiments were particularly challenging since they were designed to reduce injury and improve repair of tissues, but rodents that provided the most accessible models healed injured tissues much more readily than patients. In the end, data from carefully designed clinical trials were essential to return to and improve the basic research and the animal models so as to provide new therapies that were both effective and safe for patients. The history of such non-linear developments of medical therapies is a long one. It includes an example very familiar to members of this Society: the fifty-year long dialogue between basic science and clinical trials [3] that now provides successful therapies with hematopoietic stem cells for thousands of patients each year. But in testing the therapeutic potentials of MSCs, we were targeting not one organ or tissue but many. Therefore we were entering a vast new field of cell therapy whose limits were still unknown.
Fig. 1.
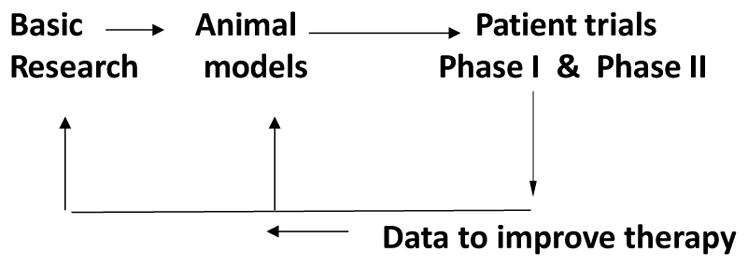
Schematic to illustrate that development of a new therapy is frequently non-linear. The basic research that generates the idea is rarely entirely convincing. The tests of the therapy in animal models leave much to be desired, particularly since rodents repair tissues better than patients. Then carefully designed clinical trials are required to return to and improve the research so as to produce an effective and safe therapy.
At the time we entered the field in the mid-1990s, there was already a rich literature on MSCs. Beginning on the late 1960’s and early 1970’s, Fiedenstein and others called attention to the cells from bone marrow that adhered to hydrophilic tissue culture surfaces and were spindle-shaped like the cells that provided the stromal support for hematopoietic cells in marrow [4–6]. Over the next 20 or so years, Friedenstein and a large number of other investigators demonstrated that the cells had several attractive features, including rapid expansion in culture, an ability to generate single-cell derived colonies, and ready differentiation to mineralized cells, chondrocytes, and adipocytes both in culture and in capsules in vivo. Most importantly, research on MSCs also demonstrated that the cells served as excellent feeder layers for cultures of hematopoietic cells [7]. This literature was the basis of an experiment we carried out with a line of transgenic mice [8] we had prepared in the course of our research on OI. The mice over-expressed an internally deleted gene for type 1 collagen that we had isolated from fibroblasts of a patient who died from a lethal form of OI. As expected, the mice developed brittle bones and multiple fractures. The experiment consisted of infusion of MSCs from normal mice of the same strain into the transgenic mice. The results were not dramatic, but the recipient mice demonstrated significant improvements in mechanical tests for bone strength, increases in bone collagen and increases in bone calcium. Also, crude assays we developed before more refined technologies were available suggested that the donor MSCs had engrafted into multiple tissues.
The results we obtained with MSCs in the transgenic mice persuaded Malcolm Brenner to ask Ed Horwitz to join his research group and carry out a trial of MSCs in patients with severe OI [9]. The trial was complex in that the patients first underwent marrow ablation and a bone marrow transplant from a matched sibling or matched potential donor so that they acquired the immune system of the donor. Four or so years later they received MSCs from the same donors. Four of 5 children in the initial cohort began to grow whereas they had stopped growing before the treatment. Also, they were able to sit up and stand with support for the first time. Most importantly, there were no adverse effects, a result consistent with an earlier trial in which autologous MSCs were infused into cancer patients in remission [10]. However, the beneficial effects in the children persisted for only a few months.
The trial in the patients with OI was based on the hypothesis that the donor’s normal MSCs might engraft into sites of recent fractures, differentiate into osteoblasts, and produce an adequate amount of normal type 1 collagen to improve the strength of the patient’s bones. The results in the transgenic mice, the patients with OI, and other observations that we and others were making [11–17] returned attention to an even bolder earlier hypothesis [6]: perhaps MSCs could engraft into any injured tissue of the body and differentiate to replace damaged cells.
At the time, there was still considerable uncertainty about the interplay between stem cells and their early progeny, a category that seemed to define MSCs. For example, spleen cells that were devoid of stem cells were known to rescue marrow ablated mice, but a hypothesis to explain the observation [18] was not recognized in the hematological literature until many years later when the role of the stem cell niche was more fully defined [19, 20]. Experiments with MSCs continued to show beneficial effects in animal models for a variety of diseases, including parkinsonism, multiple sclerosis, spinal cord injury, and skin transplants (see 16, 21 for reviews). It seemed logical to assume therefore that the MSCs had in fact engrafted and differentiated into multiple tissues. At the time, however, there were few techniques available for definitively assaying engraftment of MSCs, particularly if they acquired new phenotypes after engraftment. As the technologies for assaying engraftment of MSCs improved, the answer became clear: the assumption was wrong [16, 21]. Except for a few unusual situations, systemically administered MSCs did not engraft in significant numbers. They did not survive for long periods of time in vivo, and they showed limited tendencies to differentiate in vivo. But the cells had startling effects. Literally hundreds of reports continued to demonstrate that MSCs produced dramatic therapeutic benefits in multiple animal models for human diseases and in a few patients [16, 21] even though they disappeared with half-lives as short as 24 hours [22]. Hence the current hypothesis that has been thoroughly established: MSCs act by secreting paracrine factors in a “hit-and-run” scenario. The hypothesis has been broaden to include secretion not only of cytokines and other soluble factors but also extracellular vesicles that can contain cargos that include peptides, proteins, metabolites, microRNAs, and even mitochondria [23–27]. But in spite of the best efforts of thousands of scientists, we are still at a loss to explain most of the therapeutic benefits of MSCs. In effect, we have to work backward from the positive results obtained in vivo to defining their mechanisms of action at the cellular and molecular level. In the meantime, clinicians treating desperately ill patients for whom they have no other therapy are using the impressive results in animal models to test MSCs in clinical trials, some of which show promising results but others have encountered considerable variability [28–31].
What is needed for the field to move forward?
One of the critical steps in developing a therapy is to define as rigorously as possible the reagent or reagents that are used. Here MSCs fall outside the boundaries of all the criteria that have been used previously in medicine. The heart of the problem is the chimeric nature of the cells: they change dramatically in culture depending on a large number of conditions that include plating density, culture medium, population doublings, and exposure to oxygen [16, 32–37]. They also vary with or without expansion in culture by tissue source, the donor of the tissue, and even samples taken from the same donor and the same tissue at the same session. We have advocated use of early passage and low density cultures because these are enriched for small, rapidly self-renewing MSCs that are highly plastic in differentiation and highly clonogenic [36, 37]. However, therapeutic effects have been observed with high density cultures of MSCs that require less medium, fewer manipulations, and therefore are less costly. Apparently for these reasons, high density cultures have been used in most clinical trials. Recently, there has been a renewed interest in the properties of low density cultures of MSCs [38–40] and a current large NIH-sponsored clinical trial in patients with ARDS is employing MSCs expanded at low densities [41]. The issue of how best to isolate and expand MSCs in culture is still unresolved. The best one can say is that beneficial effects have been observed with MSCs that have been prepared with different protocols and that have measureable differences in morphology, transcriptomes and other properties. In spite of these differences, they serve in many situations as “guardians” [42] excessive responses of the inflammatory and immune systems that underlie many disease processes.
At the same time, in order to develop therapies that a standard part of medical care, there clearly is a need to define more completely the characteristics of the MSCs used in experiments and in clinical trials. The criteria to define MSCs originally proposed by a committee of this Society is a first step [43], but has well-recognized limitations. The epitopes to define MSCs, although still among the best available, are not specific. In addition, the assays for differentiation of the cells are difficult to standardize among different laboratories.
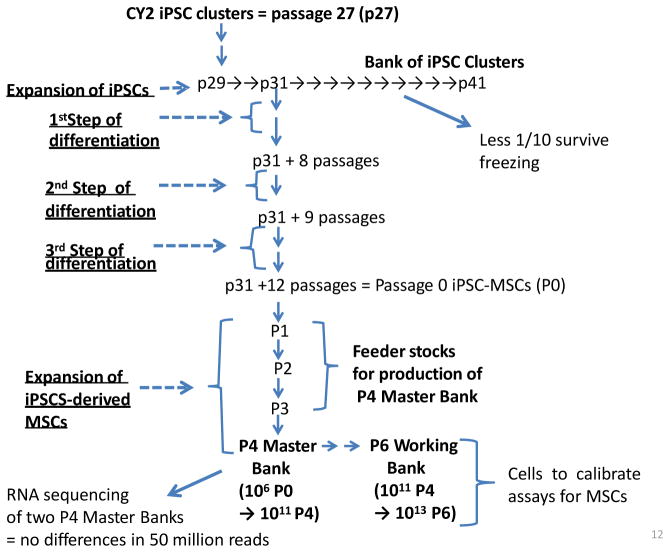
One suggestion for a better definition of MSCs has been to create repositories of standardized MSCs that can be shared with scientists and clinicians, and that can be used to calibrate in vitro assays for MSC preparations [44]. As one response to this suggestion, we have prepared iPSC-derived MSCs [45] and then developed a strategy of using feeder stocks of the cells that might serve this purpose [ Prockop et al., in prep.]. The feeder stocks (Fig. 2) have the advantage that they can be used to prepare Master Banks that are indistinguishable by standard in vitro assays for MSCs (not shown) and by deep RNA sequencing. Therefore, whenever the need arises, they can be used to generate 1013 Working Bank cells per million feeder stock cells. Accordingly, adequate numbers of standardized cells can be prepared for distribution to other laboratories as calibration standards. The use of iPSC-derived MSCs has an advantage over bone marrow-derived MSCs in that the cells propagate through more population doublings before reaching growth arrest in senescence [45]. They have the advantage over immortalized MSCs in that they are less subject to genetic drift.
Fig. 2.
Schematic illustrating how induced pluripotent stem cells (iPSC) were used to produce MSCs [45] and the MSCs used to produce feeder stocks that can be expanded as needed into Master Banks and Working Banks. As indicated, iPSC must be expanded as clusters of the cells but the banks of iPSC clusters (p29 to p41) do not efficiently survive freezing and thawing, and show phenotypic variability. One of the frozen clusters (p31) was expanded with an efficient protocol [45] to provide cells that met the classical criteria for MSCs (p31 + 12 passages = Passage 0 iPSC-MSCs or P0 MSCs). The P0 MSCs were then expanded to provide feeder stocks for a P4 Master Bank and a P6 Working Bank. Deep RNA sequencing of two P4 Master Banks prepared independently by two operators did not show any significant differences in their transcriptomes. Under the conditions employed, 1 million P0 MSCs can provide over 1011 P4 Master Bank cells and 1013 P6 Working Bank cells.
A major step forward with research on MSCs will be to provide biomarkers that can predict the in vivo efficacy of the cells. As one approach to this problem, Ryang Hwa Lee and others in our Institute for Regenerative Medicine have focused on the expression in MSCs of TSG-6, a natural modulator of inflammation [46]. They first demonstrated large differences in the mRNA levels for TSG-6 in different preparations of bone-marrow derived MSCs. They then demonstrated that preparations that expressed high levels were very effective in reducing sterile inflammation of the cornea whereas preparations that expressed low levels were ineffective. There was a strong correlation between the levels of TSG-6 mRNA assayed in the cultured MSCs and their effectiveness in suppressing induced inflammation of the cornea in vivo as assayed by the myeloperoxidase content of cornea, a surrogate marker for activated neutrophils (Fig. 3). They obtained similar results in two other models for sterile inflammation in mice: zymogen-induced peritonitis and bleomycin-induced lung injury. Therefore assays of MSCs by RT-PCR for expression of TSG-6 provided a biomarker for their effectiveness in suppressing inflammation in vivo. It will obviously be very helpful to identify other biomarkers for other therapeutic effects of MSCs such as immune suppression and anti-cancer activity.
Fig. 3.
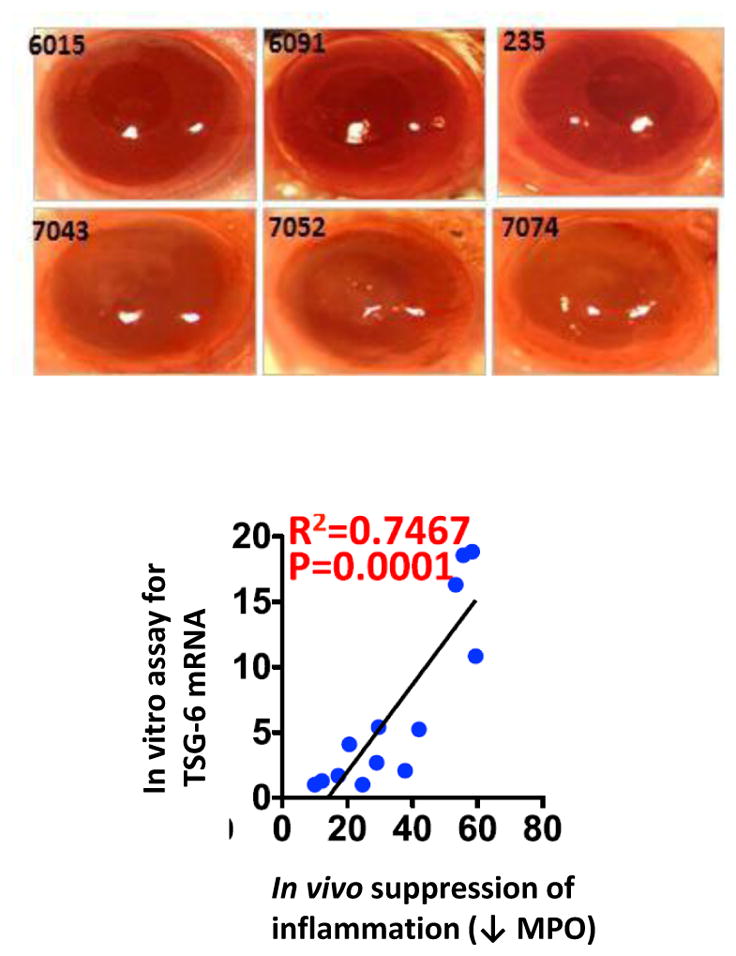
Correlation between in vitro assay for expression of mRNA for TSG-6 in different preparations of human bone marrow-derived MSCs and in vivo anti-inflammatory activity of the same MSCs. Top frames: Photos of pupils of mice in which sterile inflammation was induced by brief exposure to ethanol followed by scrapping off of the epithelium. Human marrow-derived MSCs (1 million) were infused intravenously shortly thereafter and photographs of pupils taken 7 days later. Pupils of mice that received MSCs expressing high levels of TSG-6 (# 6015, 6091 and 235) are transparent: pupils are round and red retina is visible through them. Pupils of mice that received MSCs expressing low levels of TSG-6 (#7043, 7052 and 7074) are opaque. Bottom graph: Correlation between in vitro assays for TSG-6 mRNA in different preparations of bone marrow-derived MSCs and in vivo suppression of inflammation in the cornea model as assayed by myeloperoxidase levels. Modified from Lee et al. [46].
To further define MSCs and obtain useful biomarkers, we may look forward to the application of some of the dramatic new technologies of RNA sequencing, proteomics, and metabolics that provide unprecedented amounts of information about cells at a modest cost. These new technologies are moving us beyond defining cells by the presence or absence of a handful of transcripts or protein epitopes. Instead they are beginning to define different networks of genes driven by transcription factors that respond to different signals, to the same signal as a function of dose and time, or to combinations of different signals. In the case of macrophages where the technologies have been recently applied, the cells are being defined not by the M1 and M2 phenotypes of classically and alternatively activated cells, but as a “color wheel” of phenotypes [47], some of which are readily interchangeable depending on the environment of the cell [48, 49]. There is an attempt to use the “big data” now available on cells to define the factors that control expression of networks of genes in response to specific signals but the research is still in the early stages [49]. Accordingly, as with most emerging areas of research, the benefits are not yet certain, but acquiring the vast amounts of data that can now be accessible about MSCs may well provide the important tools for selecting preparations of MSCs most likely to be beneficial for patients. Also, the activity of MSCs may have to be related to specific phenotypes of macrophages from patients, since recent reports suggest that a major target of MSCs are macrophages [26, 50]. One way forward would be to follow the example being followed by research on macrophages [48]: establish one or more websites that collect and make available complete data on the transcriptomes and other features of the MSCs being used for experimentation and clinical trials.
Another need in the field has received less attention than I think it should: the need for animal models of human diseases in which quantitative assays can be made of the efficacy of MSCs. The emphasis here should be on assays that are quantitative instead of simply qualitative. Without quantitative assays, we cannot define the classical parameters for any therapy such as effective dose and therapeutic index. As indicated (Fig. 3), the myeloperoxidase assay was the key to model for inflammation of the cornea. Without the myeloperoxidase assay, we could not have made quantitative comparisons of a series of MSC preparations that differed in their levels of expression TSG-6. More recently [51], we assayed the anti-inflammatory activity of exosomes secreted by MSCs in a second model: injecting them intravenously into mice together with low doses of the bacterial extract LPS. RT-PCR assays of pro-inflammatory cytokines expressed in spleen 3 hours later provided a quantitative assay for the efficacy of the exosomes. The effective exosomes were subsequently shown to suppress neuroinflammation 12 hours after traumatic brain injury in mice and rescue behavioral deficits that were present over a month later (Fig. 3). Neither of the assays (Figs. 3 and 51) is ideal. The assay on inflammation of the cornea requires some surgical skill. Both assays required a 15 or more mice and have a limited linear range. But both assays provide quantitative data which, like the first assays for insulin based on induction of hypoglycemic seizures in rabbits [52], are a very useful first step. Also, the results suggest levels of mRNAs for TSG-6 in MSCs may in themselves be useful (Fig. 3). The field would certainly benefit from improvements in these assays and similar assays in animal models that provided quantitative assays of other therapeutic effects of MSCs.
Finally it is obvious that progress in the field would be greatly accelerated if systems were established to correlate benefits from MSCs therapies in patients with the defined characteristics of the MSCs in culture and their efficacy in disease models. The barriers here are large. Trials with MSCs are being conducted in many diseases in which controls are difficult to employ, placebo effects are large, underlying pathologies are heterogeneous, and the costs of the trials are very large. Again, a first step might be to establish one or more websites that collect and make available complete data on the transciptomes and other features of the MSCs that are administered to patients. Similar websites for the clinical data would be equally important. All in all, a major undertaking. But we may be encouraged by fact that similar problems were encountered in the early efforts of bone marrow transplants. They were overcome by the combined efforts of members of this Society and hematologists around the world.
In the end, our attempts at cell therapies with MSCs have taken us into uncharted fields of cell biology and medicine. To some extent, they have added to the progress with all cell therapies. Two aspects of what we have found are most striking. One is that few if any well-documented adverse events have been encountered in the thousands of patients who have been treated with MSCs. The few exceptions have come with cells of unusual or totally undocumented histories. The absence or low incidence of adverse events is particularly striking since we learned, after many of the trials were initiated, that most intravenously administered MSCs are trapped in the lung as micro-emboli [22]. The other striking aspect is that, even though we are still struggling with the explanations, MSCs have produced dramatic effects in a large series of animal models and in some patients with devastating diseases. So we know we are on the track of some amazing treatments, and perhaps cures, for diseases that plague millions of patients.
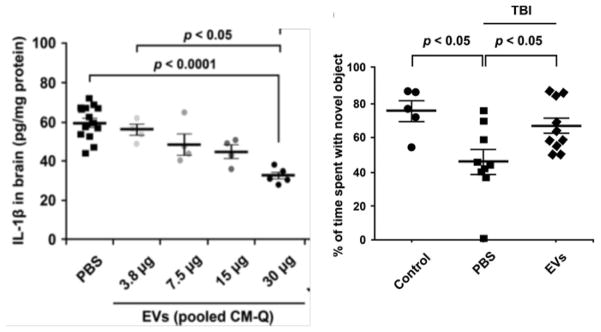
Fig. 4.
Exosomes from MSCs reduce neuroinflammation and improve long-term behavioral deficit after traumatic brain injury (TBI) in mice. Left Panel: Dose-dependent decreases in IL-1β after intravenous administration of PBS or exosomes [EVs (pooled CM-Q)] after TBI. Amounts of EVs varied from 3.5 to 30μg protein or 1.8 to 15.3 × 109 EVs. PBS or EVs were administered 1 hour after TBI and assays were by ELISA for IL-1β on homogenates of ipsilateral brain isolated 12 hours after TBI. Right Panel: Improved pattern separation function after TBI and intravenous exosomes (EVs). Pattern separation test was performed 35 days after TBI and administration of EVs. Treated mice performed better than TBI control mice. Reproduced from Kim et al. [51].
Acknowledgments
The work described in the paper was supported in part by an NIH grant (P40OD011050).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–34. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 2.Chan MC, Holt-Martyn JP, Schofield CJ, Ratcliffe PJ. Pharmacological targeting of the HIF hydroxylases--A new field in medicine development. Mol Aspects Med. 2016 Feb-Mar;47–48:54–75. doi: 10.1016/j.mam.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Prockop DJ, Prockop SE, Bertoncello I. Are clinical trials with mesenchymal stem/progenitor cells too far ahead of the science? Lessons from experimental hematology. Stem Cells. 2014 Dec;32(12):3055–61. doi: 10.1002/stem.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966 Dec;16(3):381–90. [PubMed] [Google Scholar]
- 5.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 6.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997 Apr 4;276(5309):71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 7.Dexter TM, Spooncer E, Schofield R, Lord BI, Simmons P. Haemopoietic stem cells and the problem of self-renewal. Blood Cells. 1984;10(2–3):315–39. [PubMed] [Google Scholar]
- 8.Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998 Feb 3;95( 3):1142–7. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999 Mar;5(3):309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995 Oct;16(4):557–64. [PubMed] [Google Scholar]
- 11.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999 Sep 14;96(19):10711–6. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000 Jan;18(2):307–16. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 13.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2397–402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pochampally RR, Neville BT, Schwarz EJ, Li MM, Prockop DJ. Rat adult stem cells (marrow stromal cells) engraft and differentiate in chick embryos without evidence of cell fusion. Proc Natl Acad Sci U S A. 2004 Jun 22;101(25):9282–5. doi: 10.1073/pnas.0401558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 16.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010 Sep;14(9):2190–9. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci U S A. 2003 Sep 30;100( Suppl 1):11917–23. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- 19.Xie T, Spradling AC. Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998 Jul 24;94(2):251–60. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 20.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008 Feb 22;132(4):631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012 Jun 14;10(6):709–16. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009 Jul 2;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proceedings of the National Academy of Sciences of the United States of America; 2006. pp. 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islam MN, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature medicine. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallabhaneni KC, et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6(7):4953–4967. doi: 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phinney DG, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nature communications. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 2016 Jan 5;113(1):170–5. doi: 10.1073/pnas.1522297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014 May;54(5):1418–37. doi: 10.1111/trf.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015 Jul 2;17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Rizk M, Monaghan M, Shorr R, Kekre N, Bredeson CN, Allan DS. Heterogeneity in Studies of Mesenchymal Stromal Cells to Treat or Prevent Graft-versus-Host Disease: A Scoping Review of the Evidence. Biol Blood Marrow Transplant. 2016 Apr 27; doi: 10.1016/j.bbmt.2016.04.010. pii: S1083-8791(16)30029–5. [DOI] [PubMed] [Google Scholar]
- 31.Schepers K, Fibbe WE. Unraveling mechanisms of mesenchymal stromal cell-mediated immunomodulation through patient monitoring and product characterization. Ann N Y Acad Sci. 2016 Apr;1370(1):15–23. doi: 10.1111/nyas.12984. [DOI] [PubMed] [Google Scholar]
- 32.Phinney DG, Kopen G, Righter W, Webster S, Tremain N, Prockop DJ. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999 Dec 1;75(3):424–36. [PubMed] [Google Scholar]
- 33.Fraser J, Wulur I, Alfonso Z, Zhu M, Wheeler E. Differences in stem and progenitor cell yield in different subcutaneous adipose tissue depots. Cytotherapy. 2007;9(5):459–67. doi: 10.1080/14653240701358460. [DOI] [PubMed] [Google Scholar]
- 34.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20(6):530–41. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 35.Reger RL, Prockop DJ. Should publications on mesenchymal stem/progenitor cells include in-process data on the preparation of the cells? Stem Cells Transl Med. 2014 May;3(5):632–5. doi: 10.5966/sctm.2013-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ylöstalo J, Bazhanov N, Prockop DJ. Reversible commitment to differentiation by human multipotent stromal cells in single-cell-derived colonies. Exp Hematol. 2008 Oct;36(10):1390–402. doi: 10.1016/j.exphem.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001 Jul 3;98(14):7841–5. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balint R, Richardson SM, Cartmell SH. Low-density subculture: a technical note on the importance of avoiding cell-to-cell contact during mesenchymal stromal cell expansion. J Tissue Eng Regen Med. 2015 Oct;9(10):1200–3. doi: 10.1002/term.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim DS, Lee MW, Yoo KH, Lee TH, Kim HJ, Jang IK, Chun YH, Kim HJ, Park SJ, Lee SH, Son MH, Jung HL, Sung KW, Koo HH. Gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PLoS One. 2014 Jan 6;9(1):e83363. doi: 10.1371/journal.pone.0083363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mareschi K, Rustichelli D, Calabrese R, Gunetti M, Sanavio F, Castiglia S, Risso A, Ferrero I, Tarella C, Fagioli F. Multipotent mesenchymal stromal stem cell expansion by plating whole bone marrow at a low cellular density: a more advantageous method for clinical use. Stem Cells Int. 2012;2012:920581. doi: 10.1155/2012/920581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015 Jan;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012 Jan;20(1):14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 44.Viswanathan S, Keating A, Deans R, Hematti P, Prockop D, Stroncek DF, Stacey G, Weiss DJ, Mason C, Rao MS. Soliciting strategies for developing cell-based reference materials to advance mesenchymal stromal cell research and clinical translation. Stem Cells Dev. 2014 Jun 1;23(11):1157–67. doi: 10.1089/scd.2013.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Q, Gregory CA, Lee RH, Reger RL, Qin L, Hai B, Park MS, Yoon N, Clough B, McNeill E, Prockop DJ, Liu F. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):530–5. doi: 10.1073/pnas.1423008112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee RH, Yu JM, Foskett AM, Peltier G, Reneau JC, Bazhanov N, Oh JY, Prockop DJ. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci U S A. 2014 Nov 25;111(47):16766–71. doi: 10.1073/pnas.1416121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008 Dec;8(12):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hume DA. The Many Alternative Faces of Macrophage Activation. Front Immunol. 2015 Jul 22;6:370. doi: 10.3389/fimmu.2015.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glass CK, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol. 2016 Jan;17(1):26–33. doi: 10.1038/ni.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko JH, Lee HJ, Jeong HJ, Kim MK, Wee WR, Yoon SO, Choi H, Prockop DJ, Oh JY. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc Natl Acad Sci U S A. 2016 Jan 5;113(1):158–63. doi: 10.1073/pnas.1522905113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 2016 Jan 5;113(1):170–5. doi: 10.1073/pnas.1522297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trethewey J. Bio-assays for the analysis of insulin. J Pharm Biomed Anal. 1989;7(2):189–97. doi: 10.1016/0731-7085(89)80083-4. [DOI] [PubMed] [Google Scholar]