Abstract
This study explored anhedonia (lack of interest or pleasure in non-drug rewards) as a potentially modifiable individual difference associated with the effectiveness of Contingency Management (CM). It also tested the hypothesis that a dopaminergic drug, levodopa (L-DOPA), would improve the effectiveness of CM, particularly in individuals high in anhedonia. The study was a single-site, randomized, double-blind, parallel group, 12-week trial comparing L-DOPA with placebo, with both medication groups receiving voucher-based CM targeting cocaine-negative urines. Participants were N = 85 treatment-seeking adults with CUD. Anhedonia was measured at baseline using a validated self-report measure and a progressive ratio behavioral measure. Treatment Effectiveness Score (TES) was defined as the total number of cocaine-negative urines submitted. Analyses based on Frequentist general linear models were not significant, but Bayesian analyses indicated a high probability (92.6%) that self-reported anhedonia was associated with poor treatment outcomes (lower TES). L-DOPA did not significantly improve outcomes, nor was the effect of L-DOPA moderated by anhedonia. While the study failed to replicate positive findings from previous studies of L-DOPA in combination with CM, it does provide preliminary evidence that anhedonia may be a modifiable individual difference associated with poorer CM outcomes.
Keywords: Contingency management, Incentives, Cocaine use disorder, Anhedonia, Levodopa, Bayesian statistics
1. Introduction
Cocaine is the third most abused drug in the U.S. (SAMHSA, 2013b), and the drug most often involved in ER visits (SAMHSA, 2013a). Around one-fourth of cocaine users have cocaine use disorder (CUD) and these cases disproportionately account for the health costs associated with cocaine (Degenhardt et al., 2014; SAMHSA, 2013b). Thus, the development of effective interventions for CUD is a public health priority. One of the more effective behavioral interventions for CUD is contingency management (CM; Dutra et al., 2008; Farronato, Dursteler-Macfarland, Wiesbeck, & Petitjean, 2013), a reward-based approach in which individuals receive monetary incentives for objectively verified abstinence (Higgins, Heil, Rogers, & Chivers, 2008; Petry, 2000). CM has frequently been used to help individuals with CUD achieve initial abstinence, often prior to initiation of medication or other therapies (Carroll et al., 2016; Petry, Barry, Alessi, Rounsaville, & Carroll, 2012; Poling et al., 2006; Schmitz, Lindsay, Stotts, Green, & Moeller, 2010; Schmitz et al., 2008, 2014; Schottenfeld et al., 2005). However, studies using CM have found initial attainment of abstinence in anywhere from 20% to 60% of individuals with CUD (Dutra et al., 2008). Indeed, even very high-value, intensive CM results in initial abstinence in only about 40% of individuals with CUD (Schmitz et al., 2014). Thus, even this comparatively strong CUD intervention is variably effective.
Unmeasured individual differences may impact the effectiveness of all CUD treatments, including CM. There are many possible reasons someone may continue to use cocaine or relapse to cocaine, each with potentially different underlying risk factors and mechanisms. Indeed, such unmeasured diagnostic heterogeneity has been identified as a primary problem in the development of all psychiatric treatments (Hyman, 2007; Wong, Yocca, Smith, & Lee, 2010). Thus, one way to quickly increase CUD treatment effectiveness may be to identify individual differences that: 1. are associated with differential treatment effectiveness, and 2. have known mechanisms that are amenable to intervention, so that treatment outcomes can be rapidly improved.
Anhedonia is one individual difference that appears likely to impact CM outcomes. Anhedonia, defined here as lack of interest or pleasure in non-drug rewards, is common in CUD and other addictions (Franken, Rassin, & Muris, 2007; Garfield, Lubman, & Yücel, 2013; Leventhal et al., 2008, 2010). Anhedonia is distinct from negative mood (e.g. sadness, anxiety) and from clinical depression. Along with negative mood, it is one possible symptom of depression, but it is not necessary for a depression diagnosis (American Psychiatric Association, 2000). In fact, anhedonia has been hypothesized to constitute a distinct endophenotype related to, but not synonymous with, depression (Pizzagalli, 2014). Consistent with this, anhedonia is present at clinical levels in individuals without a depression diagnosis, including individuals with addictions (Franken et al., 2007). Anhedonia also appears particularly problematic in addiction treatment. For example, anhedonia predicts post-treatment relapse to smoking even after controlling for negative mood symptoms (Leventhal, Piper, Japuntich, Baker, & Cook, 2014). Similarly, in a prospective study of CUD, low positive moods predicted relapse to cocaine following treatment, while negative moods did not, suggesting anhedonia is a risk factor for a difficult clinical course in CUD (Hall, Havassy, & Wasserman, 1991). Anhedonia has high face validity as a moderator of CM treatment in particular, as lack of interest in non-drug rewards would naturally tend to work against the reward-based mechanism of CM; however, to our knowledge, this hypothesis has not previously been tested. Of note, studies examining the impact of depression on CM outcomes have produced mixed results (no association, worse outcomes, better outcomes), possibly due to the failure to distinguish anhedonia from negative mood symptoms of depression (Garcia-Fernandez, Secades-Villa, Garcia-Rodriguez, Pena-Suarez, & Sanchez-Hervas, 2013; Garcia-Fernandez et al., 2011; Gonzalez, Feingold, Oliveto, Gonsai, & Kosten, 2003; Milby et al., 2015). Thus, one objective of this study is to specifically test the relationship of anhedonia with CM outcomes.
Anhedonia is associated with relatively well-understood deficits in underlying neural circuitry, which may be addressable using pharmacological interventions. Recent reviews suggest that the clinical phenomenon of anhedonia may be caused by deficits in either the ability to experience pleasure, thought to be mediated by opioid “hotspots” in NAcc and ventral pallidum (VP), or the motivation to pursue pleasure, thought to be mediated by dopaminergic (DA) activity in the ventral tegmental area (VTA) and NAcc (Argyropoulos & Nutt, 2013; Treadway & Zald, 2011). Given its known neural pathology, CUD might impair either of these processes (Volkow, 2010). However, attention has largely focused on prominent DAergic impairments in CUD, including deficient striatal dopamine functioning and lowered neural responsiveness to non-drug rewards in DAergic brain regions (Goldstein, Alia-Klein, et al., 2007; Goldstein, Tomasi, et al., 2007; Goldstein et al., 2008; Martinez et al., 2004, 2009; Tomasi et al., 2010; Volkow et al., 2010). Importantly, deficient striatal DA functioning predicts failure to attain abstinence in CM (Martinez et al., 2011). Further, medications that enhance DA, such as levodopa (L-DOPA) increase CM success rates (Schmitz et al., 2008). Previous studies in non-CUD populations show that DA enhancing medications increase responsiveness to reward (Leyton et al., 2007; Wardle & de Wit, 2012; Wardle, Treadway, Mayo, Zald, & de Wit, 2011), suggesting that these medications may enhance CM by increasing responses to the incentives provided in CM. Putting this evidence together, we hypothesize that variations in anhedonia may help explain differences in CM outcomes, and that DAergic drugs may improve CM outcomes by improving anhedonia (Martinez et al., 2011).
This study is the first to test the possible role of anhedonia in CM outcomes, and the role of anhedonia in enhancement of CM with DAergic medication. Our hypotheses were: 1. greater anhedonia at baseline, as measured using both a self-report and a behavioral task, would be associated with poorer outcomes in a treatment involving CM, 2. L-DOPA would improve outcomes in a treatment involving CM, replicating previous findings, and 3. individuals higher in anhedonia would benefit more from L-DOPA enhancement of CM, as this drug would target the deficits in DAergic circuitry and corresponding low reward motivation that interfere with the ability of these individuals to succeed in CM.
2. Methods
2.1. Study design
The study was a single-site, randomized, double-blind, parallel group, 12-week trial comparing levodopa/carbidopa with placebo. Medication was administered along with behavioral therapy with a prominent CM component.
Participants providing written informed consent underwent a one-week screening and pretreatment assessment to determine eligibility, including medical history and physical examination, laboratory tests, thrice-weekly on-site urinalysis, and cardiac evaluation (i.e., 12-lead electrocardiogram). Self-report and behavioral measures of anhedonia were also taken at this time. Eligible participants were then urn randomized to medication group based on severity of cocaine dependence (>15 days cocaine use in past 30 days vs. ≤15 days), baseline level of cognitive functioning as determined by the MicroCog (global cognitive functioning score >80 vs. <80; Aharonovich et al., 2006), attentional bias (baseline cocaine-Stroop >46 ms vs. <46 ms, measure not further reported on here; Liu et al., 2011), and the behavioral measure of anhedonia (baseline performance in progressive ratio task >66%, see Measures; Lane, Cherek, Pietras, & Steinberg, 2005). The behavioral anhedonia measure was selected for use as a stratification variable based on the study’s original conceptual model linking this task with motivational anhedonia, and thus the dopaminergic basis of L-DOPA treatment (see Measures).
Following randomization to a medication group, participants entered treatment, which began with a 2-day dose escalation run-up, followed by 12 weeks of behavioral treatment. Behavioral treatment consisted of abstinence-based CM thrice weekly, with cocaine levels in urine samples used to determine rewards (see Treatment). Participants also received one 50-minute manual-driven cognitive behavioral therapy (CBT) session weekly (see Treatment). The 12 treatment weeks were followed by a 2-day dose reduction run-down. Throughout treatment, participants made thrice-weekly clinic visits (Monday, Wednesday, and Friday). At each visit vital signs and adverse events were assessed, study medication was dispensed, and urine samples obtained for on-site testing of cocaine use (see Measures).
2.2. Participants
Participants were treatment-seeking adults (18–60 years old) with cocaine dependence per the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Ed. (SCID-IV; First, Spitzer, Gibbon, & Williams, 1996). In addition to a diagnosis of cocaine dependence, other required inclusion criteria were: 1. self-reported cocaine use in the past 30 days; 2. at least one cocaine positive urine during the screening/pre-treatment period; 3. no medical contraindications to L-DOPA, including use of medications known to have significant interactions with L-DOPA; 4. no current dependence on any psychoactive substances aside from cocaine, nicotine, and marijuana; 5. no other current neurological or psychiatric disorders that would make participation difficult (e.g. bipolar disorder, psychosis, major depression); 6. no other current or recent (within the last 3 months) treatment for substance use or another psychiatric disorder; 7. no conditions of probation or parole requiring reports of drug use to officers of the court; 8. no impending incarceration; 9. no pregnant or nursing females.
All participants were recruited from the greater Houston and surrounding areas via news media advertisements, public service announcements, community flyers, and various informational outreach projects. The study took place at the outpatient Center for Neurobehavioral Research on Addiction (CNRA), part of the University of Texas Health Science Center Department of Psychiatry and Behavioral Sciences. There were 129 participants screened for eligibility with N = 85 enrolled and randomly allocated to placebo (N = 40) or L-DOPA (N = 45). See study CONSORT diagram (Fig. 1).
Fig. 1.
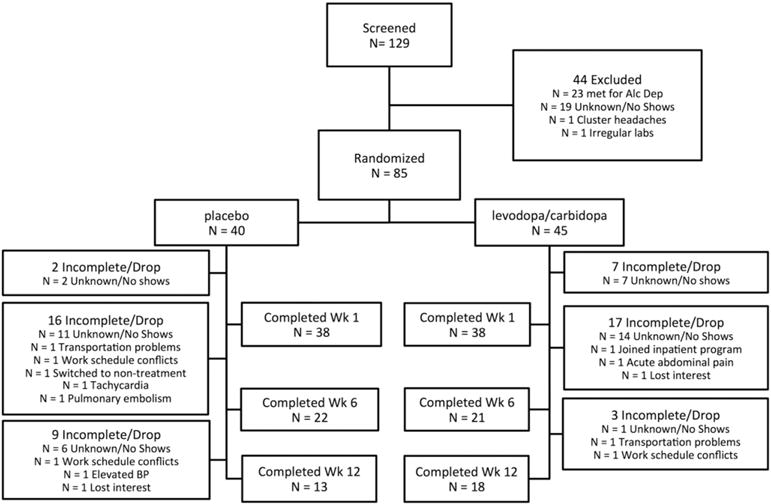
CONSORT diagram.
2.3. Treatments
2.3.1. Contingency management (CM)
The study used a very similar abstinence-based CM procedure to that which demonstrated efficacy when combined with L-DOPA in a previous study (Schmitz et al., 2010). Participants earned vouchers according to the reward schedule recommended by Budney and Higgins (1998), beginning at $2.50 for the first cocaine-negative urine. For each consecutive cocaine-negative urine, voucher values increased by $1.25 with a $10 bonus given for provision of three consecutive cocaine-negative urines within a week. A cocaine-positive urine or failure to provide a scheduled urine sample resulted in a reset of the schedule to the initial value of $2.50. After provision of five negative urines, the voucher returned to the value prior to the reset. Participants were able to redeem their vouchers for small amounts of cash (≤$25) or gift cards for goods and services.
2.3.2. Cognitive behavioral therapy
Participants received individual CBT in 50-minute weekly sessions. These sessions were manual-driven and based on the relapse prevention model proposed by Marlatt and Gordon (1985). Trained master’s level therapists, under the supervision of senior therapists and the principal investigator, worked with participants to teach them how to recognize and cope with risky situations that could influence their cocaine use through self-monitoring of situational craving and drug-use stimuli, coping skills training, and lifestyle modifications (Schmitz et al., 2008).
2.3.3. Pharmacotherapy
Participants randomized to L-DOPA received a sustained-release combination of levodopa and carbidopa (Sinemet CR) starting with a 2-day dose escalation run-up period (400 mg L-DOPA/100 mg Carbidopa per day) that was titrated to the maximum study dose (400/100 mg twice daily) maintained throughout the study until the 2-day dose reduction run-down at week 12 (400/100 mg per day). Participants in the placebo group received inactive medication (cornstarch) on an identical schedule, including a “run up” and “run down”, and in capsules identical to those in the L-DOPA group in order to maintain the blind. Additionally, all capsules contained 50 mg riboflavin as a medication compliance measure. All research staff, except study pharmacists who had no participant contact, were blind to medication assignment.
2.4. Measures
2.4.1. Efficacy, compliance and safety
The primary outcome was cocaine use, assessed in urine samples by means of the Readitest 6 Cassette urine drug screen (Redwood Toxicology Laboratory, Santa Rosa, CA) that detects benzoylecgonine (BE) at a concentration of 300 ng/ml. Medication compliance was primarily assessed by nurse observation of doses taken at thrice-weekly scheduled clinic visits, and by self-reported number of pills taken each day at home, and entered as “none”, “some”, or “all”, using a written Timeline Followback (TLFB) method. Weekly urine samples were shipped offsite for fluorescence testing of riboflavin levels; however unexpected problems in the timely collection and transport of samples raised questions about the accuracy and suitability of this marker as a primary or sole measure of medication compliance. Thus it is presented only as a supplemental measure to the TLFB self-reports of medication use described above. A weekly side effects questionnaire was composed of 38 symptoms (headache, dizziness, etc.) and asked participants to rate the intensity of each symptom over the past week as “none”, “mild”, “moderate”, or “severe”. An adverse event log was maintained by the study nurse with all serious adverse events reported to the institution’s IRB immediately.
2.4.2. Anhedonia
We used two measures of anhedonia, a self-report scale and a behavioral measure.
The self-report scale was the Snaith-Hamilton Pleasure Scale (SHAPS), which measures hedonic capacity by assessing participants’ ability to enjoy non-drug rewards (Snaith et al., 1995). The SHAPS is a 14-item, self-report scale that asks participants to indicate on a scale from 0 = “strongly agree” to 3 = “strongly disagree” their ability to enjoy 14 normally pleasurable events (“I would enjoy my favorite television or radio program” or “I would get pleasure from helping others”). Scores range from 0 to 42 with higher scores indicating greater anhedonia. This measure was selected because it is one of the most widely used and validated self-reports of anhedonia in humans, with the best psychometric characteristics in head-to-head evaluations (Leventhal, Chasson, Tapia, Miller, & Pettit, 2006), and previous validation in a substance dependent population (Franken et al., 2007). Of note, the SHAPS is hypothesized to tap aspects of anhedonia related to ability to experience pleasure (Der-Avakian & Markou, 2012), but no specific studies of the relationship between this scale and the hypothesized opioidergic neural basis of pleasure have been conducted in humans.
The progressive ratio (PR) procedure was used as a behavioral measure of anhedonia. This task was adapted from PR tasks used in animals, with money substituted as the reward. The task consisted of a series of trials in which the participant chose between: 1.) choice “A”, which required work to earn rewards on a progressive ratio (PR) schedule, such that the first choice A earned $0.01 in exchange for 10 key-presses on the keyboard, and for each subsequent choice A, the amount of key presses required to earn the reward increased by ten percent of the previous value, rounded up to the nearest whole number (e.g. from 10 to 11 presses, then to 12, 13, 14, 15, 17, 19 and so on, with no maximum) while the reward also increased by $0.01; and 2.) choice “C”, which required simply waiting for a reward on a fixed time (FT) schedule. The reward for choice C was always identical to the last choice A reward received, and the amount of time the participant had to wait was equal to either the time required to complete the last choice A or 120 s, whichever was longer. All participants were informed that the task would last 20 minutes regardless of their choices. Thus, greater selections of choice A resulted in greater rewards in exchange for greater effort, while selections of choice C resulted in less reward, but required less effort. The number of times the participant key pressed in choice A was the dependent variable, with fewer responses indicating motivational anhedonia. This task was selected for translational validity, and has previously been used to study motivation during drug administration, and in groups with substance dependence (e.g. Cherek, Lane, & Dougherty, 2002; Lane et al., 2005; Sigmon, Tidey, Badger, & Higgins, 2003; Stoops, Lile, Glaser, Hays, & Rush, 2010; Strickland, Lile, Rush, & Stoops, 2014). This task was hypothesized to tap motivational aspects of anhedonia (Der-Avakian & Markou, 2012), and consistent with this, similar measures have been shown to be sensitive to dopaminergic functioning in humans (Treadway et al., 2012; Wardle et al., 2011). Previous studies indicate this type of behavioral measure is not strongly related to the SHAPS (Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009).
2.5. Data analytic plan
Treatment effectiveness score (TES; Ling et al., 1997), defined as the number of cocaine-negative urines collected out of the total scheduled urines for the 12-week trial (i.e., 36), was examined as the primary outcome of interest in the sample of participants who were randomized to a medication treatment. This measure was chosen because it affords several advantages over both longitudinal analyses and other summary metrics like longest duration of sustained abstinence. First, use of a summary measure provides greater power in this initial study, and avoids difficulties with extensive missing data that can hamper longitudinal analysis in clinical trials for addiction (Ling et al., 1997). Regarding missing data, while participants were actively enrolled in the study (i.e. prior to drop-out, defined here as failing to attend any further sessions) 24% of regularly scheduled urines were missed (22% in L-DOPA and 26% in placebo). In contrast to other summary measures, such as percent of negative urines among only those urines submitted, the TES acknowledges that both treatment acceptability (which can contribute to missing urines and drop out) and effects on drug use, are both key elements of treatment effectiveness (Ling et al., 1997). TES was significantly skewed, so all analyses used a negative binomial distribution to model TES. This outcome distribution was chosen by model comparison using a penalty-based criterion (the Bayesian information criterion, or BIC) from four competing distributions frequently used to model count data: Poisson, zero-inflated Poisson, negative binomial, and zero-inflated negative binomial. The negative binomial model provided the best fit (lowest BIC value), and thus was chosen here. Our primary independent variables were medication group, anhedonia measured using the SHAPS and anhedonia measured using the PR. The anhedonia variables were analyzed in two separate analyses because they were not significantly correlated in our sample.
We assessed for potential confounds using the criteria proposed for clinical trials. Specifically, to be considered a possible confound, a variable had to predict both the independent and dependent variable under consideration (Assmann, Pocock, Enos, & Kasten, 2000; Pocock, Assmann, Enos, & Kasten, 2002). We evaluated all of the demographic (sex, age, race etc.), and drug use (positive baseline UDS, years using cocaine etc.) variables presented in Table 1 as possible confounds based on these criteria. None of these variables significantly related to both a predictor (medication group, SHAPS score or PR score) and to TES score. Thus none met the definition of a confounding variable, and no covariates were included in our models.
Table 1.
Participant demographics and relationships to variables of interest.
| Placebo group M (SD) or N (%) (n = 40) | L-DOPA group M (SD) or N (%) (n = 45) | Difference between groups | |
|---|---|---|---|
| Sex | |||
| Female | 5 (13%) | 10 (22%) | χ2 = 1.38 |
| Race/Ethnicity | |||
| White | 8 (2%) | 7 (16%) | Fisher Exact = 0.0222 |
| Black | 26 (65%) | 33 (73%) | |
| Hispanic | 5 (13%) | 5 (11%) | |
| Other | 1 (3%) | 0 (0%) | |
| Baseline UDS | |||
| Positive | 34 (85%) | 38 (84%) | χ2 = 0.01 |
| Age | 45 (9.2) | 46 (7.9) | χ2 = 0.21 |
| Education | 12 (1.6) | 13 (1.5) | χ2 = 3.85* |
| Days using alcohol this month | 10 (9.7) | 7 (8.8) | χ2 = 0.39 |
| Years using alcohol | 16 (10.7) | 15 (15.2) | χ2 = 0.92 |
| Days using cocaine this month | 16 (9.0) | 15 (9.1) | χ2 = 0.62 |
| Years using cocaine | 15 (7.1) | 15 (8.4) | χ2 = 0.08 |
Note: UDS = urine drug screen; L-DOPA = levodopa.
p < 0.05.
The primary hypotheses of interest were: 1. the effect of anhedonia (measured by the SHAPS and PR) on TES, 2. the effect of medication group on TES, and 3. the interaction between anhedonia and medication group on TES. Analyses were performed using a traditional Frequentist general linear modeling testing of the null hypothesis with a significance level α=0.05, along with a Bayesian modeling approach for quantifying the probability of each hypothesis (null and alternative) given observed data. Prior distributions in Bayesian analysis allow incorporation of a-priori hypotheses into analyses, so here we used vague and neutral priors (~Normal [μ = 0, σ2 = 1 × 106] for coefficients in the log-form), to indicate minimal assumptions. We considered a Bayesian probability of 80% or greater that an effect exists to merit further consideration. Together, these two complementary approaches provide a more accurate evaluation of hypotheses when the sample size is small (Green et al., 2009). In particular, although conventional Frequentist approaches to statistical testing permit rejection of the null hypothesis given the observed data or data more extreme, Bayesian analysis permits conclusions regarding the alternative hypothesis: the probability that a subgroup effect of a specified magnitude exists (Simon, 2002; Wijeysundera, Austin, Hux, Beattie, & Laupacis, 2009).
3. Results
3.1. Sample characteristics
Demographics and substance use characteristics of randomized participants are presented in Table 1. The sample was primarily male (82%) and African American (69%), with a mean education level of 13 years (SD = 1.6). Mean cocaine use was 16 days of the past 30 (SD = 9.1), and 15 years lifetime (SD = 7.7). Medication groups were not significantly different on any of these sample characteristics, with the exception of education, where the L-DOPA group had more education than the placebo group. But education was not significantly related to TES (r = 0.12, p = 0.31), and therefore did not meet criteria to be included as a covariate (as described in Data Analytic Plan).
3.2. Retention in treatment
Medication groups did not differ in retention: log-rank χ2(1)=0.0440, p=0.8338. Overall, 36% of participants remained in treatment at week 12. Overall median number of days in treatment was 39 (95% C.I. 35–50).
3.3. Side effects, adverse events, compliance
Participants reported taking “all” of their medication 50% of the time (SD = 39.8) and “some” of their medication an additional 2% of the time (SD = 6.6). Self-reports of medication compliance were not significantly different between the placebo (“all” M = 59%, SD = 43.2, “some” M = 1%, SD = 4.7) and L-DOPA (“all” M = 41%, SD = 40.0, “some” M = 2%, SD = 7.9) medication groups (t[83] = 1.72, p = 0.09). Medication compliance based on urinary riboflavin results was slightly lower, 45% (SD = 36.2) but also was not significantly different between placebo (M = 46%, SD = 36.2) and L-DOPA (M = 43%, SD = 39.9) medication groups (t[83] = 0.38, p = .71). The side effects most commonly endorsed by participants during the study were increased energy (80%), low energy or weakness (62%), feeling down or sad (62%) and changes in sleep patterns (61%). Chi-Square tests showed no significant differences between the placebo and L-DOPA groups on rate of these side effects. Eight serious adverse events (SAEs) occurred during this study, 6 in the L-DOPA and 3 in the placebo condition, none of which were judged to be related to study treatment. All adverse events were reviewed by the institutional review board and data safety monitoring board.
3.4. Anhedonia and treatment effects
For self-reported anhedonia, as measured using the SHAPS, the average score was 11 (SD = 8.7). SHAPS scores were not different for the L-DOPA (M = 11, SD = 9.7) and placebo (M = 12, SD = 7.4) groups. 30% of our participants met a cut point for clinically significant anhedonia established by the original authors of the SHAPS (Snaith et al., 1995). This proportion is similar to results from other studies using this measure in substance abusing populations (Franken et al., 2007). The relationship between the SHAPS and TES did not reach significance, based on Frequentist results (χ2(1)=2.41, p=0.12); however, corresponding Bayesian results indicated a 92.6% probability that a negative relationship between SHAPS and TES existed. In other words, given the current data, it appears highly probable that there is a decrease in treatment effectiveness as self-reported anhedonia increases (see Fig. 2).
Fig. 2.
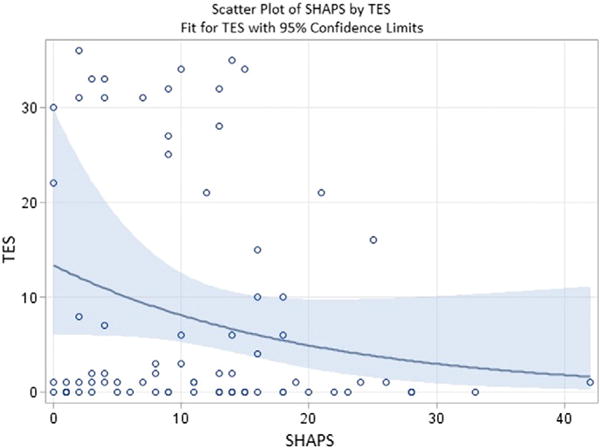
Scatter plot of Treatment Effectiveness Score (TES) by Snaith-Hamilton Pleasure Scale (SHAPS) scores, including a line depicting the negative binomial fit for TES with 95% confidence limits, showing that higher self-reported anhedonia, as indicated by higher scores on the SHAPS, related to lower effectiveness for contingency management treatment, as indicated by lower TES. This graph depicts scores for all participants regardless of medication condition, as all participants received contingency management. Of note, we selected a negative binomial model for the fitted curve to address significant skew in the raw data.
For behavioral anhedonia, the average score on the PR task was 3689 (SD = 2922.0) presses before the participants stopped pressing for the larger reward and settled on the low work option. This was not different for the L-DOPA (M = 3564, SD = 2792.3) and placebo (M = 3825, SD = 2792.3) groups. Mean earnings on the task were $6.54 (SD = $3.70). There was not a significant association between PR and TES scores based on Frequentist results (χ2(1)=0.00, p=0.99). Bayesian results similarly failed to suggest a reliable effect of behavioral anhedonia on TES, indicating only a 48% chance that a negative relationship between behavioral anhedonia and TES existed, given the current data.
Next, evaluation of TES as a function of medication group failed to identify statistically reliable effects, using both Frequentist (χ2(1)=0.42, p=0.51) and Bayesian analyses (73% probability of L-DOPA conferring benefit, i.e., increasing TES, given the current data). On average, the total number of cocaine-negative urines submitted was 9 (SD = 12.6) for the whole sample, and not different for the L-DOPA (M = 7, SD = 11.7) and placebo (M = 9, SD = 12.6) group.
Last, we examined possible interactions between medication group and anhedonia, to test whether individuals high in anhedonia at baseline received greater benefit from L-DOPA treatment. Frequentist analyses suggested no interaction between baseline SHAPS scores and L-DOPA treatment (χ2(1)=0.01, p=0.93), or between baseline PR scores and L-DOPA treatment (χ2(1)=0.19, p=0.66). Bayesian analysis similarly suggested no reliable interactions between baseline SHAPS scores and L-DOPA treatment (55% probability of an interaction) or PR scores and L-DOPA treatment (65% probability of an interaction).
4. Discussion
In summary, we found some support for our first hypothesis that greater anhedonia at baseline would be associated with poorer outcomes in a treatment involving CM. Bayesian analyses suggested a 92.6% probability that higher self-reported anhedonia was associated with worse treatment effectiveness scores. Visual inspection of the results suggested that SHAPS scores of 15 and above in particular were associated with few good outcomes, while individuals with the best outcomes had SHAPS scores of 15 or less. However, the clinical relevance of this possible cut point will need to be empirically investigated in further research. In contrast, our behavioral measure of anhedonia was not associated with treatment effectiveness. We also did not find support for our second hypothesis, as we failed to replicate previous findings that the DAergic drug L-DOPA improves outcomes overall in a treatment involving CM. Finally, we did not find support for our third hypothesis, as baseline anhedonia levels did not appear to moderate the effect of L-DOPA treatment.
It should be noted that Bayesian, but not Frequentist, analyses supported the hypothesis that anhedonia was associated with outcomes in CM treatment. The combined results mean that while we cannot reject the null hypothesis of no difference, there is relatively strong evidence favoring the alternative hypothesis over no effect. As West (2016) writes in an editorial encouraging adoption of Bayesian methods in addiction science, “…this is more informative than simply concluding that there is no statistically significant difference…” We believe such Bayesian results are particularly useful in this nascent phase of personalized medicine for CUD. A clinical trial that is well-powered to examine a contrast between medication groups may be underpowered for examining moderators of that treatment effect within a Frequentist framework (Brookes, Whitley, Peters, Mulheran, & Egger, 2001; Green et al., 2009). The Bayesian approach used here can thus usefully supplement classical Frequentist hypothesis testing by using smaller studies, such as this one, to produce “good bets” for key individual characteristics to be tested in larger and longer studies. Our results indicate anhedonia should be considered at least a “good bet”, worthy of investigation in a definitive study.
The probability that self-reported anhedonia is associated with the treatment effectiveness of abstinence-based CM suggests several specific avenues for following research. First, we and others have hypothesized that the SHAPS may reflect “experience” of pleasure, while PR tasks measure “motivation” for pleasure (Der-Avakian & Markou, 2012). However, these tasks differ from each other in many ways, and the current study included no measures of the putative neural basis of these functions. Further, there are several tasks that putatively tap experience of pleasure and motivation for pleasure (Der-Avakian & Markou, 2012), and little is known about the convergent or divergent validity of these various tasks. Thus, different findings might have been obtained with different specific measures. Therefore, a more comprehensive assessment of the proposed subtypes of anhedonia in CUD, their relationship to the neural deficits seen in CUD, and their role in CUD symptoms and treatment outcomes seems warranted. Second, although these tentative findings are not sufficient to recommend treatment matching, the findings here do suggest that individuals with greater ability to enjoy non-drug rewards may benefit most from CM. It remains an open question whether anhedonia is particularly detrimental to CM, in which case individuals high in anhedonia may benefit more from other treatments, or whether it is a generalized poor sign for behavioral treatment. The inclusion of a CBT component in the current study suggests it may be the latter, but this will also be an area requiring further study.
Equally important, we failed to replicate a treatment effect for L-DOPA, or to find evidence specifically supporting L-DOPA enhancement of CM for individuals high in anhedonia. These negative results for L-DOPA may have been due in part to factors such as a somewhat higher rate of attrition and medication non-compliance observed here compared to previous studies with L-DOPA (Schmitz et al., 2008, 2010, 2014). Also, the behavioral therapy platform used here was closely related, but not identical, to the original study. In Schmitz et al., 2008, the CM procedure was primarily cash-based, compared to the gift-card payments used here, which may have influenced the desirability of the rewards. Weekly nurse-delivered clinical management sessions were also included in the previous study, but not here, which may have added support to the behavioral platform. Finally, outcome variables differed slightly, with previous studies using proportion of cocaine negative urines (a closely related, but not identical, measure), or two weeks of consecutive abstinence as their primary outcomes. However, overall, studies in our clinic and elsewhere (Liu et al., 2014; Mooney, Schmitz, Moeller, & Grabowski, 2007; Schmitz et al., 2008, 2010, 2014; Shoptaw et al., 2005) have produced mixed findings regarding the efficacy of L-DOPA for CUD. Like many other CUD medications, L-DOPA effects tend to be less than robust and dependent on other variables, including baseline cocaine-use level (Schmitz et al., 2014). Some have argued that the effect of L-DOPA is somewhat subtle, and that “stronger” interventions such as d-amphetamine or other stimulant drugs will be required to ameliorate the dopaminergic reward deficits seen in CUD and consistently enhance CM outcomes (Herin, Rush, & Grabowski, 2010; Mooney et al., 2009). Our findings add to this concern.
The current study had several limitations. First, as mentioned, lower than expected rates of retention and medication compliance likely reduced the statistical power of the study for estimating treatment effects of the medication intervention. Compliance and side-effect profiles were similar across medication conditions, so side-effects do not appear to explain this issue. Second, participants were urn-randomized based on the behavioral, but not the self-report, measure of anhedonia even though ultimately only the self-report measure predicted outcomes. Future studies of CM should consider randomizing based on self-reported anhedonia, to assure even group distributions on this variable. Third, although we utilized two measures of anhedonia, multi-modal measurements using several measures to tap each theoretical aspect of anhedonia would be preferable. Fourth, although we have presented a strong hypothesis about the link between DAergic functioning, anhedonia and CM outcomes, this study did not measure DAergic functioning directly. Indeed, no single study to date has investigated all of the key neural, behavioral and clinical links of this hypothesis simultaneously. Finally, although our primary interest was in a theory-based relationship between anhedonia and CM, our intervention also included elements of CBT. Although this is fairly typical for studies using CM, it makes it difficult to determine whether anhedonia is influencing the effectiveness of CM, CBT, or both.
In conclusion, this study provides initial information suggesting that baseline anhedonia may be associated with the success of CM for CUD. Although this initial finding will require replication, it suggests potentially promising avenues for treatment research, as there are both plausible medication (e.g. stimulant drugs) and behavioral (e.g. behavioral activation) treatments that target anhedonia. Therefore, anhedonia may be a good candidate for investigation as a modifiable individual difference that could be addressed to quickly improve treatment outcomes in CM for CUD.
Acknowledgments
This study was funded by the National Institute on Drug Abuse through grant P50 DA009262. The funders had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug and Alcohol Dependence. 2006;81(3):313–322. doi: 10.1016/j.drugalcdep.2005.08.003. http://dx.doi.org/10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, D.C.: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Argyropoulos SV, Nutt DJ. Anhedonia revisited: Is there a role for dopamine-targeting drugs for depression? Journal of Psychopharmacology. 2013;27(10):869–877. doi: 10.1177/0269881113494104. http://dx.doi.org/10.1177/0269881113494104. [DOI] [PubMed] [Google Scholar]
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)-uses of baseline data in clinical trials. The Lancet. 2000;355(9209):1064–1069. doi: 10.1016/S0140-6736(00)02039-0. http://dx.doi.org/10.1016/S0140–6736(00)02039–0. [DOI] [PubMed] [Google Scholar]
- Brookes S, Whitley E, Peters T, Mulheran P, Egger M. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technology Assessment. 2001;5(33):56. doi: 10.3310/hta5330. http://dx.doi.org/10.3310/hta5330. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST. A community reinforcement plus vouchers approach: Treating cocaine addiction. Vol. 2. United States: National Institute on Drug Abuse; 1998. [Google Scholar]
- Carroll KM, Nich C, Petry NM, Eagan DA, Shi JM, Ball SA. A randomized factorial trial of disulfiram and contingency management to enhance cognitive behavioral therapy for cocaine dependence. Drug and Alcohol Dependence. 2016;160:135–142. doi: 10.1016/j.drugalcdep.2015.12.036. http://dx.doi.org/10.1016/j.drugalcdep.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherek DR, Lane SD, Dougherty DM. Possible amotivational effects following marijuana smoking under laboratory conditions. Experimental and Clinical Psychopharmacology. 2002;10(1):26–38. doi: 10.1037//1064-1297.10.1.26. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Baxter AJ, Lee YY, Hall W, Sara GE, Johns N, Vos T. The global epidemiology and burden of psychostimulant dependence: Findings from the Global Burden of Disease Study 2010. Drug and Alcohol Dependence. 2014;137(0):36–47. doi: 10.1016/j.drugalcdep.2013.12.025. http://dx.doi.org/10.1016/j.drugalcdep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends in Neurosciences. 2012;35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165(2):179–187. doi: 10.1176/appi.ajp.2007.06111851. http://dx.doi.org/10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Farronato NS, Dursteler-Macfarland KM, Wiesbeck GA, Petitjean SA. A systematic review comparing cognitive-behavioral therapy and contingency management for cocaine dependence. Journal of Addictive Diseases. 2013;32(3):274–287. doi: 10.1080/10550887.2013.824328. http://dx.doi.org/10.1080/10550887.2013.824328. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Strutured clinical interview for DSM-IV axis I disorders. New York: Biometrics Research Department; 1996. [Google Scholar]
- Franken IHA, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: Further validation of the Snaith–Hamilton Pleasure Scale (SHAPS) Journal of Affective Disorders. 2007;99(1–3):83–89. doi: 10.1016/j.jad.2006.08.020. http://dx.doi.org/10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez G, Secades-Villa R, Garcia-Rodriguez O, Alvarez-Lopez H, Sanchez-Hervas E, Fernandez-Hermida JR, Fernandez-Artamendi S. Individual characteristics and response to contingency management treatment for cocaine addiction. Psicothema. 2011;23(1):114–118. [PubMed] [Google Scholar]
- Garcia-Fernandez G, Secades-Villa R, Garcia-Rodriguez O, Pena-Suarez E, Sanchez-Hervas E. Contingency management improves outcomes in cocaine-dependent outpatients with depressive symptoms. Experimental and Clinical Psychopharmacology. 2013;21(6):482–489. doi: 10.1037/a0033995. http://dx.doi.org/10.1037/a0033995. [DOI] [PubMed] [Google Scholar]
- Garfield JBB, Lubman DI, Yücel M. Anhedonia in substance use disorders: A systematic review of its nature, course and clinical correlates. Australian and New Zealand Journal of Psychiatry. 2013;48:36–51. doi: 10.1177/0004867413508455. http://dx.doi.org/10.1177/0004867413508455. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? The American Journal of Psychiatry. 2007a;164(1):43–51. doi: 10.1176/appi.ajp.164.1.43. http://dx.doi.org/10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Parvaz MA, Maloney T, Alia-Klein N, Woicik PA, Telang F, Volkow ND. Compromised sensitivity to monetary reward in current cocaine users: An ERP study. Psychophysiology. 2008;45(5):705–713. doi: 10.1111/j.1469-8986.2008.00670.x. http://dx.doi.org/10. 1111/j.1469–8986.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, Volkow ND. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug and Alcohol Dependence. 2007b;87(2–3):233–240. doi: 10.1016/j.drugalcdep.2006.08.022. http://dx.doi.org/10.1016/j.drugalcdep.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, Feingold A, Oliveto A, Gonsai K, Kosten TR. Comorbid major depressive disorder as a prognostic factor in cocaine-abusing buprenorphine-maintained patients treated with desipramine and contingency management. American Journal of Drug and Alcohol Abuse. 2003;29(3):497–514. doi: 10.1081/ada-120023455. http://dx.doi.org/10.1081/ADA-120023455. [DOI] [PubMed] [Google Scholar]
- Green CE, Moeller FG, Schmitz JM, Lucke JF, Lane SD, Swann AC, Carbonari JP. Evaluation of heterogeneity in pharmacotherapy trials for drug dependence: A Bayesian approach. The American Journal of Drug and Alcohol Abuse. 2009;35(2):95–102. doi: 10.1080/00952990802647503. http://dx.doi.org/10.1080/00952990802647503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, Wasserman DA. Effects of commitment to abstinence, positive moods, stress, and coping on relapse to cocaine use. Journal of Consulting and Clinical Psychology. 1991;59(4):526–532. doi: 10.1037//0022-006x.59.4.526. http://dx.doi.org/10.1037/0022-006X.59.4.526. [DOI] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Annals of the New York Academy of Sciences. 2010;1187(1):76–100. doi: 10.1111/j.1749-6632.2009.05145.x. http://dx.doi.org/10.1111/j.1749–6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Rogers RE, Chivers L. Cocaine. In: Higgins ST, Sliverman K, Heil SH, editors. Contingency management in substance abuse treatment. New York: Guildford Press; 2008. pp. 19–41. [Google Scholar]
- Hyman SE. Can neuroscience be integrated into the DSM-V? Nature Reviews Neuroscience. 2007;8(9):725–732. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Pietras CJ, Steinberg JL. Performance of heavy marijuana-smoking adolescents on a laboratory measure of motivation. Addictive Behaviors. 2005;30(4):815–828. doi: 10.1016/j.addbeh.2004.08.026. http://dx.doi.org/10.1016/j.addbeh.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Brightman M, Ameringer KJ, Greenberg J, Mickens L, Ray LA, Sussman S. Anhedonia associated with stimulant use and dependence in a population-based sample of American adults. Experimental and Clinical Psychopharmacology. 2010;18(6):562–569. doi: 10.1037/a0021964. http://dx.doi.org/10.1037/a0021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: A psychometric analysis of three anhedonia scales. Journal of Clinical Psychology. 2006;62(12):1545–1558. doi: 10.1002/jclp.20327. http://dx.doi.org/10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I, Zimmerman M. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. American Journal on Addictions. 2008;17(3):218–223. doi: 10.1080/10550490802019774. http://dx.doi.org/10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Piper ME, Japuntich SJ, Baker TB, Cook JW. Anhedonia, depressed mood, and smoking cessation outcome. Journal of Consulting and Clinical Psychology. 2014;82(1):122–129. doi: 10.1037/a0035046. http://dx.doi.org/10.1037/a0035046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, aan het Rot M, Booij L, Baker GB, Young SN, Benkelfat C. Mood-elevating effects of d-amphetamine and incentive salience: The effect of acute dopamine precursor depletion. Journal of Psychiatry and Neuroscience. 2007;32(2):129–136. [PMC free article] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Wesson D, Rawson RA, Compton M, Klett CJ. Treatment effectiveness score as an outcome measure in clinical trials. NIDA Research Monograph. 1997;175:208–220. [PubMed] [Google Scholar]
- Liu S, Green CE, Lane SD, Kosten TR, Moeller FG, Nielsen DA, Schmitz JM. The influence of dopamine β-hydroxylase gene polymorphism rs1611115 on levodopa/carbidopa treatment for cocaine dependence: a preliminary study. Pharmacogenetics and Genomics. 2014;24(7):370–373. doi: 10.1097/FPC.0000000000000055. http://dx.doi.org/10.1097/fpc.0000000000000055. [DOI] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. The American Journal of Drug and Alcohol Abuse. 2011;37(2):117–122. doi: 10.3109/00952990.2010.543204. http://dx.doi.org/10.3109/00952990.2010.543204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. Guilford Press; 1985. [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang D-R, Huang Y, Laruelle M. Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: Relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29(6):1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Nunes E. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. American Journal of Psychiatry. 2011;168(6):634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Kleber H. Lower level of endogenous dopamine in patients with cocaine dependence: Findings from PET imaging of D2/D3 receptors following acute dopamine depletion. American Journal of Psychiatry. 2009;166(10):1170–1177. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milby JB, Conti K, Wallace D, Mennemeyer S, Mrug S, Schumacher JE. Comorbidity effects on cocaine dependence treatment and examination of reciprocal relationships between abstinence and depression. Journal of Consulting and Clinical Psychology. 2015;83(1):45–55. doi: 10.1037/a0037960. http://dx.doi.org/10.1037/a0037960. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: A randomized, double-blind, placebo-controlled trial. Drug and Alcohol Dependence. 2009;101(1–2):34–41. doi: 10.1016/j.drugalcdep.2008.10.016. http://dx.doi.org/10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Schmitz JM, Moeller FG, Grabowski J. Safety, tolerability and efficacy of levodopa–carbidopa treatment for cocaine dependence: Two double-blind, randomized, clinical trials. Drug and Alcohol Dependence. 2007;88(2–3):214–223. doi: 10.1016/j.drugalcdep.2006.10.011. http://dx.doi.org/10.1016/j.drugalcdep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug and Alcohol Dependence. 2000;58(1–2):9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. Journal of Consulting and Clinical Psychology. 2012;80(2):276. doi: 10.1037/a0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annual Review of Clinical Psychology. 2014;10(1):393–423. doi: 10.1146/annurev-clinpsy-050212-185606. http://dx.doi.org/10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: Current practice and problems. Statistics in Medicine. 2002;21(19):2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry NM, Sofuoglu M, Kishorchandra G, Gonzalez G, Kosten TR. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Archives of General Psychiatry. 2006;63(2):219–228. doi: 10.1001/archpsyc.63.2.219. http://dx.doi.org/10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- SAMHSA. HHS publication no (SMA) 13–4760, DAWN series D-39. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013a. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. [PubMed] [Google Scholar]
- SAMHSA. NSDUH Series H-46, HHS Publication No (SMA) 13–4795. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013b. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- Schmitz JM, Green CE, Stotts AL, Lindsay JA, Rathnayaka NS, Grabowski J, Moeller FG. A two-phased screening paradigm for evaluating candidate medications for cocaine cessation or relapse prevention: Modafinil, levodopa–carbidopa, naltrexone. Drug and Alcohol Dependence. 2014;136(0):100–107. doi: 10.1016/j.drugalcdep.2013.12.015. http://dx.doi.org/10.1016/j.drugalcdep.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Lindsay JA, Stotts AL, Green CE, Moeller FG. Contingency management and levodopacarbidopa for cocaine treatment: A comparison of three behavioral targets. Experimental and Clinical Psychopharmacology. 2010;18(3):238. doi: 10.1037/a0019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Mooney ME, Moeller FG, Stotts AL, Green CE, Grabowski J. Levodopa pharmacotherapy for cocaine dependence: Choosing the optimal behavioral therapy platform. Drug and Alcohol Dependence. 2008;94(1–3):142–150. doi: 10.1016/j.drugalcdep.2007.11.004. http://dx.doi.org/10.1016/j.drugalcdep.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR. Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. American Journal of Psychiatry. 2005;162(2):340–349. doi: 10.1176/appi.ajp.162.2.340. http://dx.doi.org/10.1176/appi.ajp.162.2.340. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Watson DW, Reiber C, Rawson RA, Montgomery MA, Majewska MD, Ling W. Randomized controlled pilot trial of cabergoline, hydergine and levodopa/carbidopa: Los Angeles Cocaine Rapid Efficacy Screening Trial (CREST) Addiction. 2005;100:78–90. doi: 10.1111/j.1360-0443.2005.00991.x. http://dx.doi.org/10.1111/j.1360–0443.2005.00991.x. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Tidey JW, Badger GJ, Higgins ST. Acute effects of D-amphetamine on progressive-ratio performance maintained by cigarette smoking and money. Psychopharmacology. 2003;167(4):393–402. doi: 10.1007/s00213-003-1416-z. [DOI] [PubMed] [Google Scholar]
- Simon R. Bayesian subset analysis: Application to studying treatment-by-gender interactions. Statistics in Medicine. 2002;21(19):2909–2916. doi: 10.1002/sim.1295. http://dx.doi.org/10.1002/sim.1295. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PEA, Hays LR, Rush CR. Intranasal cocaine functions as reinforcer on a progressive ratio schedule in humans. European Journal of Pharmacology. 2010;644(1–3):101–105. doi: 10.1016/j.ejphar.2010.06.055. http://dx.doi.org/10.1016/j.ejphar.2010.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Lile JA, Rush CR, Stoops WW. Relationship between intranasal cocaine self-administration and subject-rated effects: Predictors of cocaine taking on progressive-ratio schedules. Human Psychopharmacology: Clinical and Experimental. 2014;29(4):342–350. doi: 10.1002/hup.2409. http://dx.doi.org/10.1002/hup.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Goldstein RZ. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5(5):e10815. doi: 10.1371/journal.pone.0010815. http://dx.doi.org/10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. The Journal of Neuroscience. 2012;32(18):6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. http://dx.doi.org/10.1523/jneurosci.6459–11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the “EEfRT”? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598. http://dx.doi.org/10.1371/journal.pone.0006598.t004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND. Opioid–dopamine interactions: Implications for substance use disorders and their treatment. Biological Psychiatry. 2010;68(8):685–686. doi: 10.1016/j.biopsych.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: Decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32(9):748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, de Wit H. Effects of amphetamine on reactivity to emotional stimuli. Psychopharmacology. 2012;220(1):143–153. doi: 10.1007/s00213-011-2498-7. http://dx.doi.org/10.1007/s00213–011–2498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: Effects of d-amphetamine on human effort-based decision-making. The Journal of Neuroscience. 2011;31(46):16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. http://dx.doi.org/10.1523/jneurosci.4387–11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R. Using Bayesian analysis for hypothesis testing in addiction science. Addiction. 2016;111(1):3–4. doi: 10.1111/add.13053. http://dx.doi.org/10.1111/add.13053. [DOI] [PubMed] [Google Scholar]
- Wijeysundera DN, Austin PC, Hux JE, Beattie WS, Laupacis A. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. Journal of Clinical Epidemiology. 2009;62(1):13–21. doi: 10.1016/j.jclinepi.2008.07.006. e15. http://dx.doi.org/10.1016/j.jclinepi.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Wong EH, Yocca F, Smith MA, Lee C-M. Challenges and opportunities for drug discovery in psychiatric disorders: The drug hunters’ perspective. The International Journal of Neuropsychopharmacology. 2010;13(09):1269–1284. doi: 10.1017/S1461145710000866. [DOI] [PubMed] [Google Scholar]


