Abstract
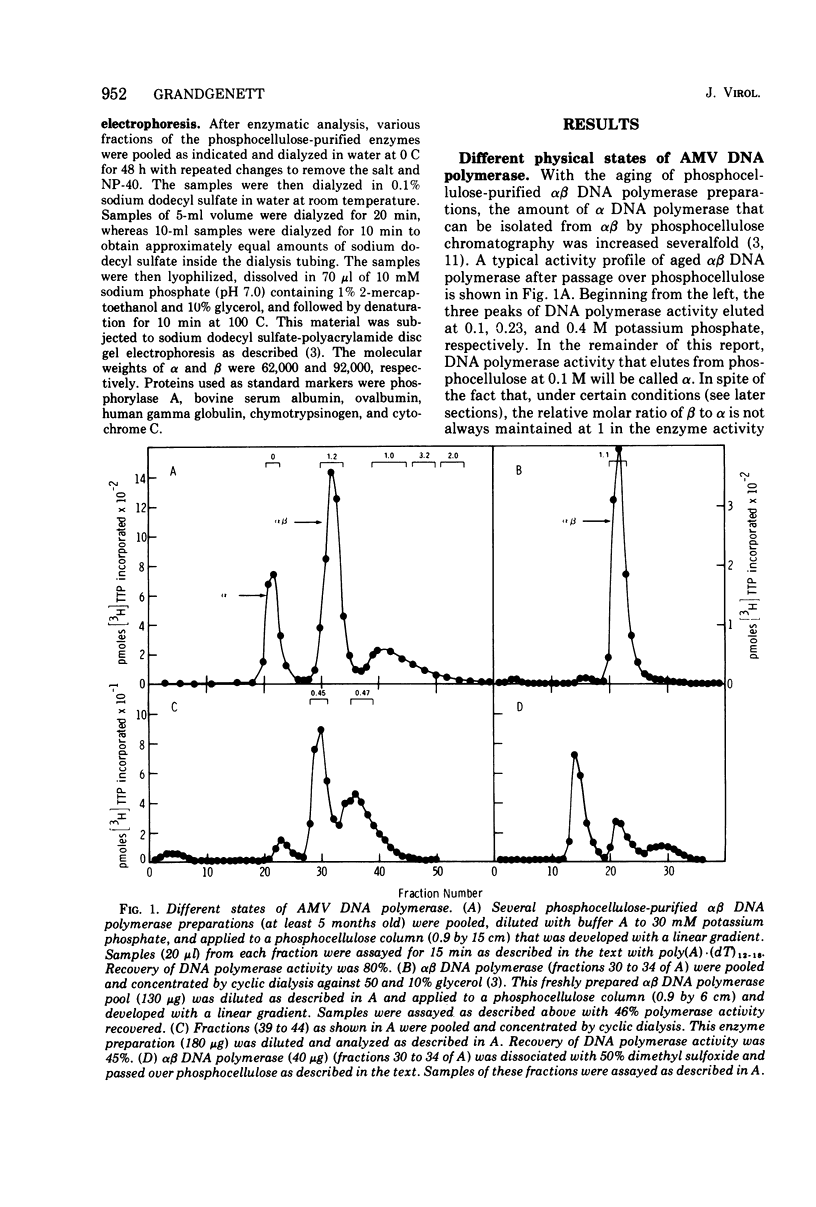
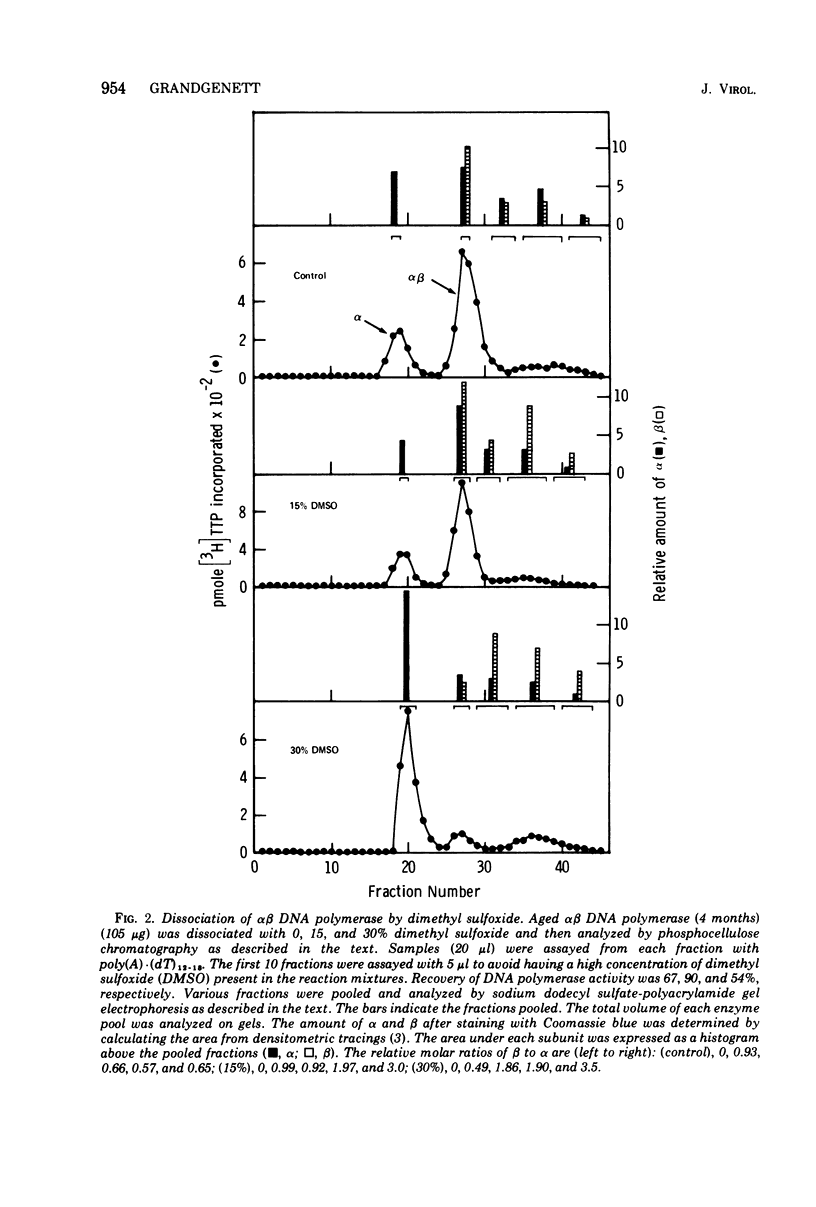
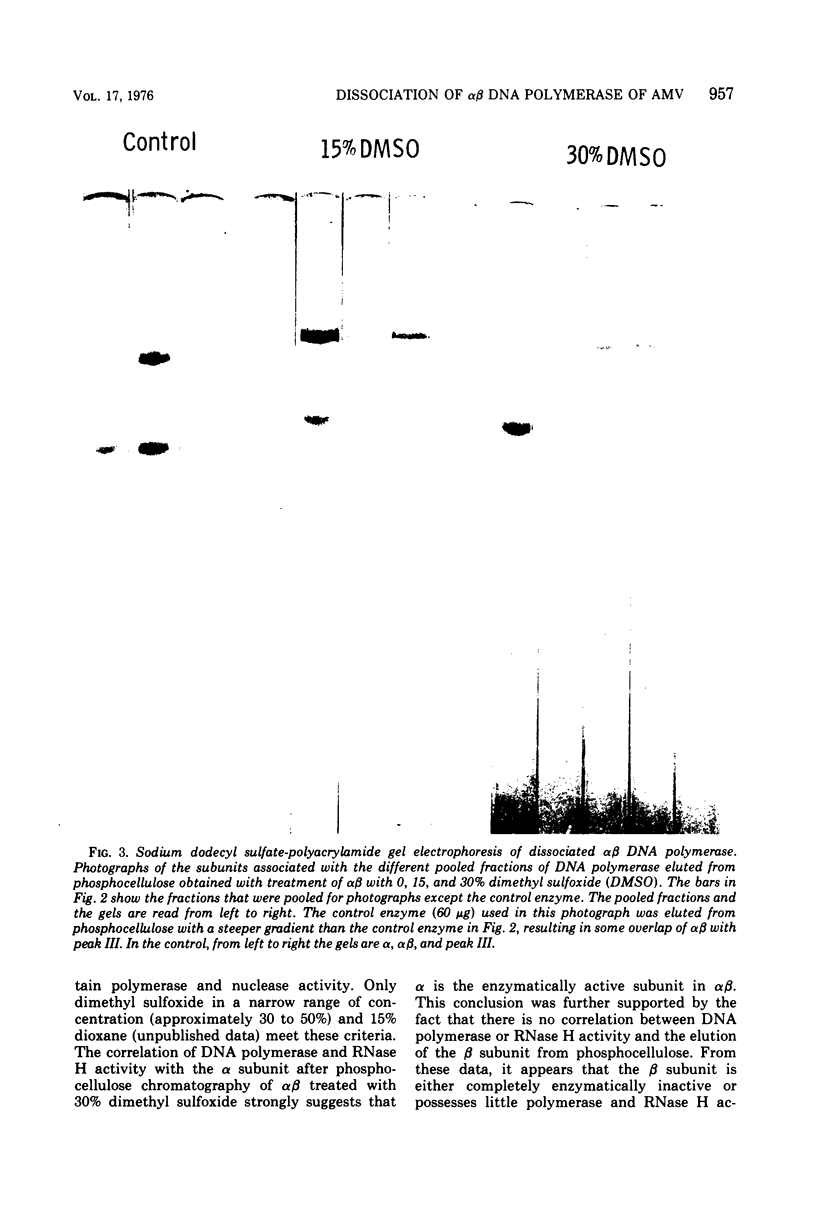
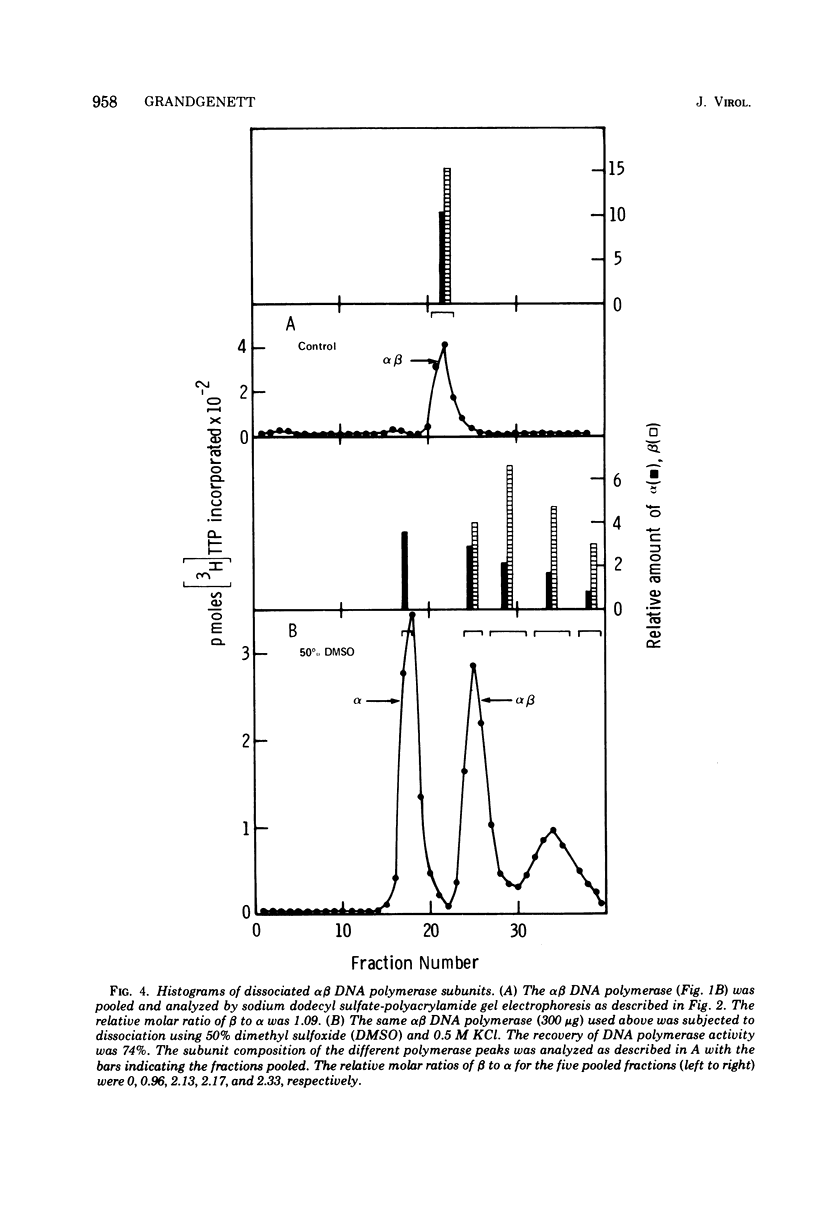
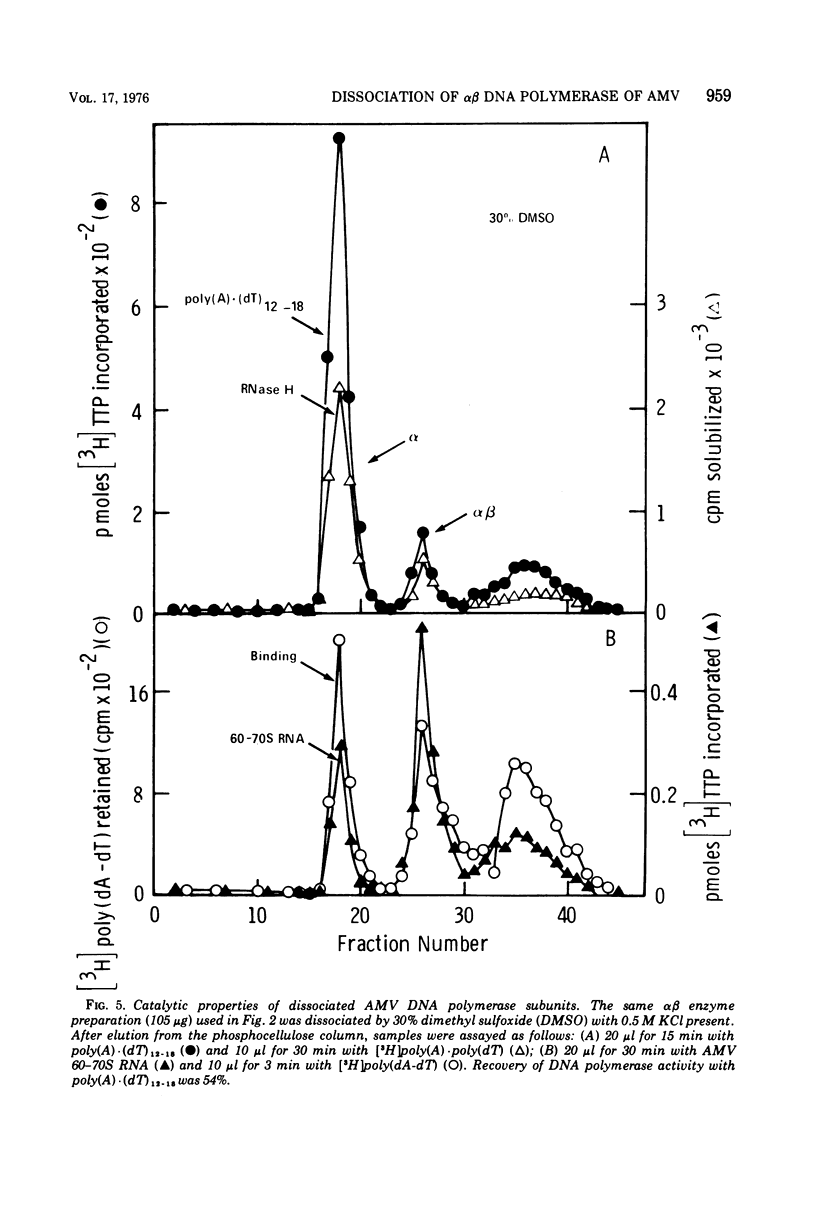
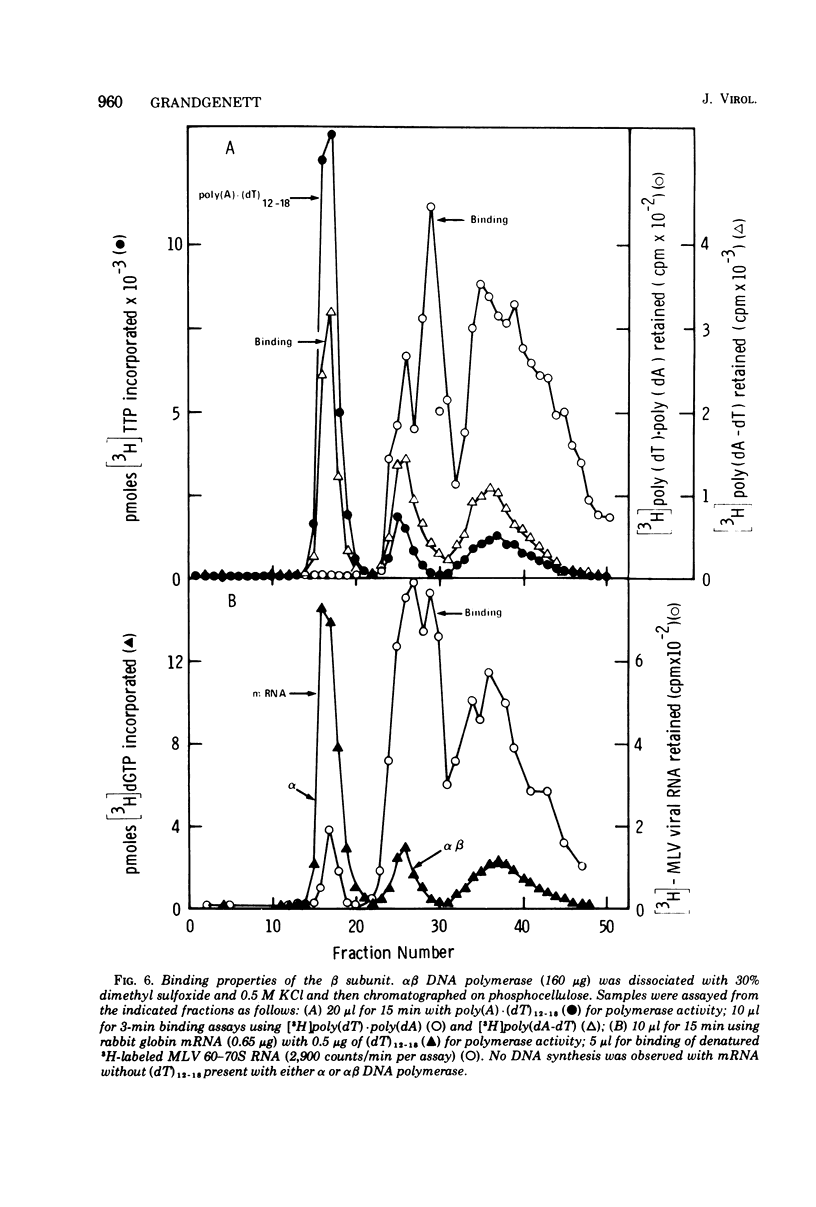
The alpha beta DNA polymerase of avian myeloblastosis virus was treated with dimethyl sulfoxide to dissociate the enzyme subunits. The dimethyl sulfoxide treated enzymes were passed over phosphocellulose to purify and characterize the dissociated subunits as well as to remove the dimethyl sulfoxide. RNA-directed DNA polymerase, RNase H, and nucleic acid-binding activity were monitored, as well as the subunit structure (on sodium dodecyl sulfate-polyacrylamide gels) of the various enzyme species obtained. With 30% dimethyl sulfoxide, the majority of DNA polymerase and RNase H activities as well as the alpha subunit were displaced from the alpha beta DNA polymerase position on phosphocellulose (0.23 M potassium phosphate) to the alpha DNA polymerase position (0.1 M). The association of DNA polymerase and RNase H activities with the alpha subunit suggests that alpha is the enzymatically active subunit in alpha beta. In addition to alpha DNA polymerase, a minor polymerase species eluted from phosphocellulose at 0.4 M potassium phosphate. The dissociated beta subunit eluted from phosphocellulose at a wide range of salt concentrations (0.28 to 0.5 M potassium phosphate). The dissociated beta subunit bound 3H-labeled murine leukemia virus RNA and [3H]poly(dT)-poly(dA) approximately 20-fold more avidly than alpha DNA polymerase alone. In contrast to the results with the alpha subunit, there was no correlation between DNA polymerase and RNase H activity profiles and the elution profile of the beta subunit from phosphocellulose. These observations suggest the beta subunit is either enzymatically inactive or possesses limited DNA polymerase and RNase H activity when compared with the alpha subunit.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duesberg P. H., Robinson W. S. Nucleic acid and proteins isolated from the Rauscher mouse leukemia virus (MLV). Proc Natl Acad Sci U S A. 1966 Jan;55(1):219–227. doi: 10.1073/pnas.55.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Verma I. M. Studies on the reverse transcriptase of RNA tumor viruses. Structural relatedness of two subunits of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4991–4994. doi: 10.1073/pnas.71.12.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Green M. Different mode of action of ribonuclease H in purified alpha and alpha beta ribonucleic acid-directed deoxyribonucleic acid polymerase from avian myeloblastosis virus. J Biol Chem. 1974 Aug 25;249(16):5148–5152. [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Panet A., Baltimore D., Hanafusa T. Quantitation of avian RNA tumor virus reverse transcriptase by radioimmunoassay. J Virol. 1975 Jul;16(1):146–152. doi: 10.1128/jvi.16.1.146-152.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Verma I. M., Baltimore D. Role of the subunits of the avian RNA tumor virus reverse transcriptase. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):919–923. doi: 10.1101/sqb.1974.039.01.107. [DOI] [PubMed] [Google Scholar]
- Rho H. M., Grandgenett D. P., Green M. Sequence relatedness between the subunits of avian myeloblastosis virus reverse transcriptase. J Biol Chem. 1975 Jul 10;250(13):5278–5280. [PubMed] [Google Scholar]
- Riggin C. H., Bondurant M. C., Mitchell W. M. Differences between murine leukemia virus and murine sarcoma virus: effects of virion age and multiplicity of infection on viral RNA. Intervirology. 1974;2(4):209–221. doi: 10.1159/000149426. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Mason W. S., Drost S. D., Baltimore D. DNA polymerase activity from two temperature-sensitive mutants of Rous sarcoma virus is thermolabile. Nature. 1974 Sep 6;251(5470):27–31. doi: 10.1038/251027a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M. Studies on reverse transcriptase of RNA tumor viruses. I. Localization of thermolabile DNA polymerase and RNase H activities on one polypeptide. J Virol. 1975 Jan;15(1):121–126. doi: 10.1128/jvi.15.1.121-126.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D., Flügel R. M., Larson J. E., Schendel P. F., Sweet R. W. Comparison of some reactions catalyzed by deoxyribonucleic acid polymerase from avian myeloblastosis virus, Escherichia coli, and Micrococcus luteus. Biochemistry. 1972 Feb 15;11(4):621–629. doi: 10.1021/bi00754a025. [DOI] [PubMed] [Google Scholar]