Abstract
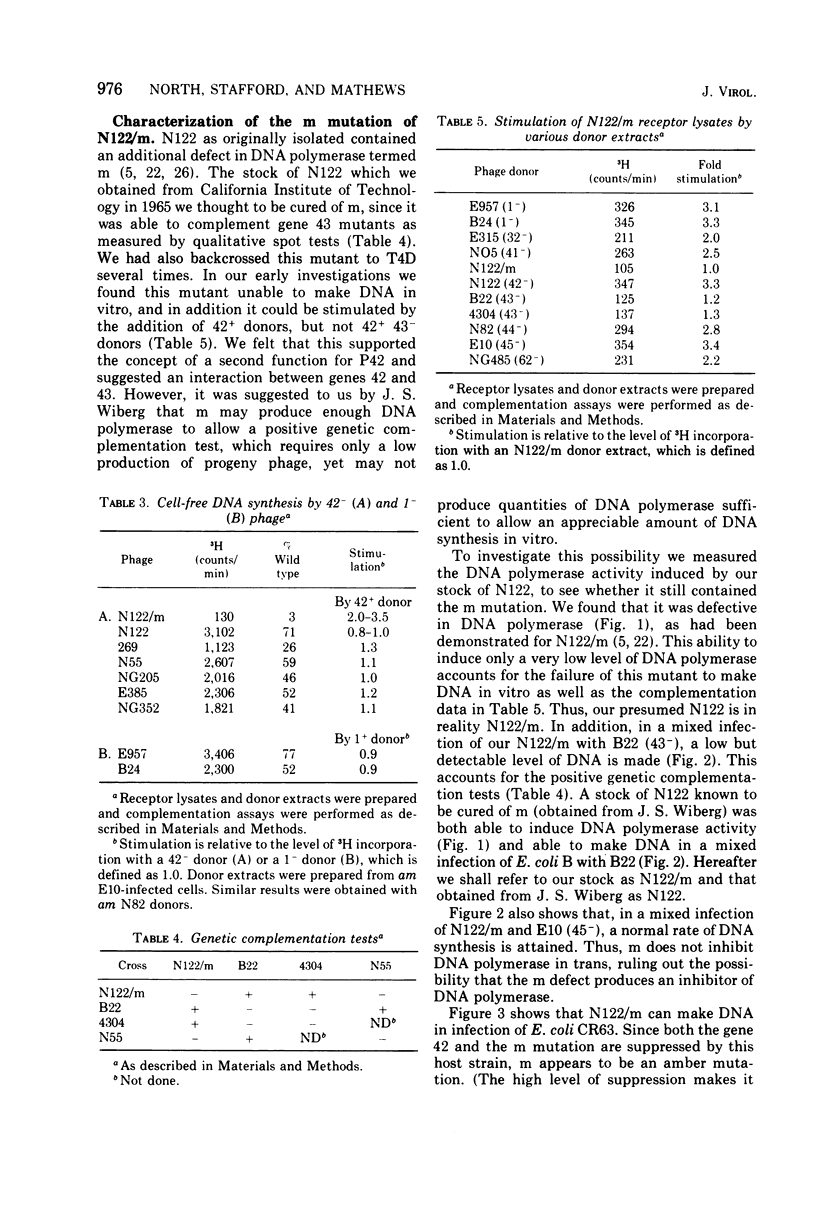
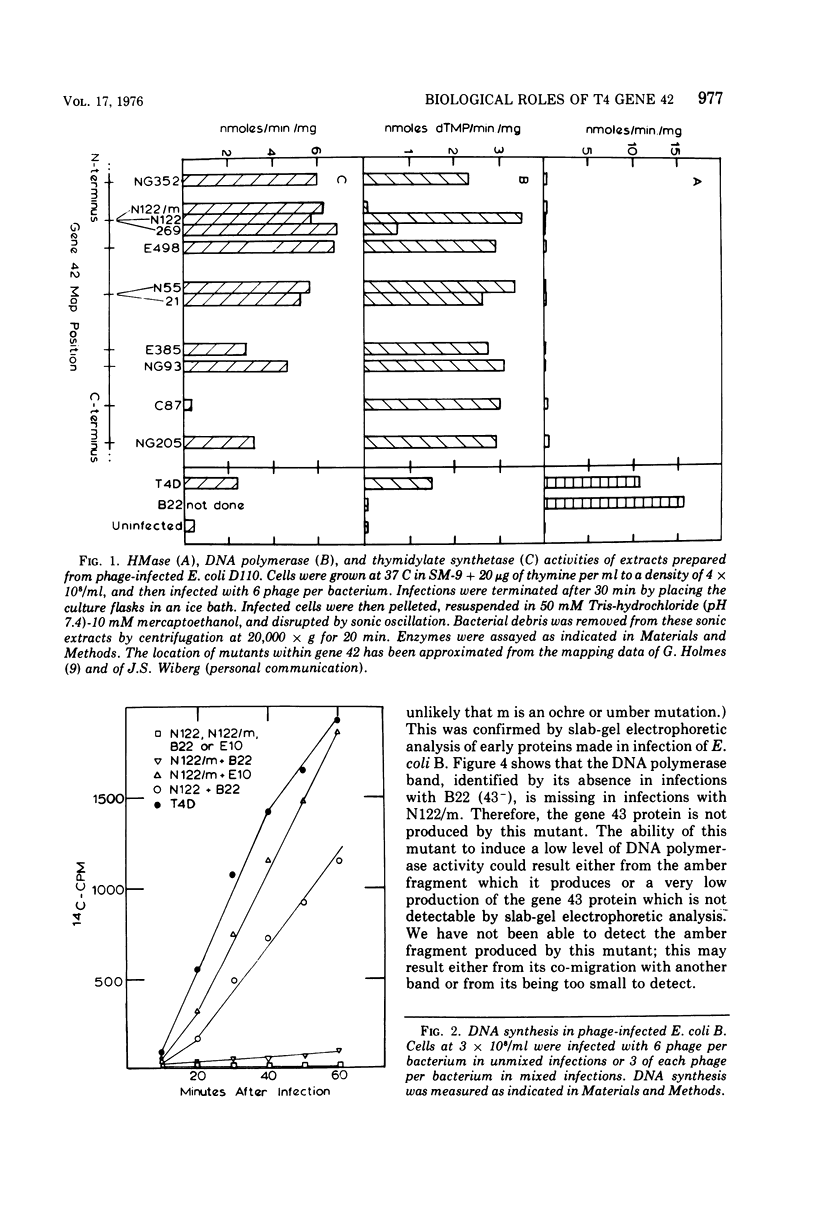
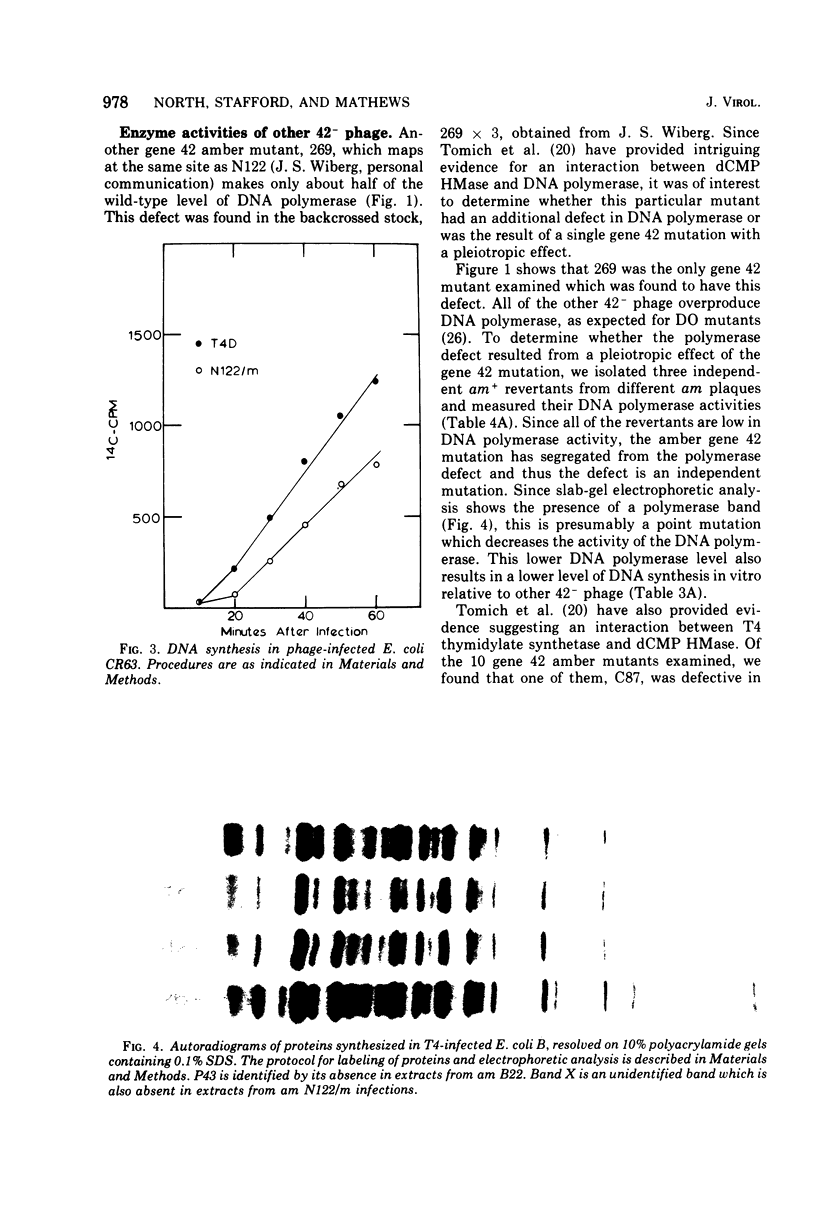
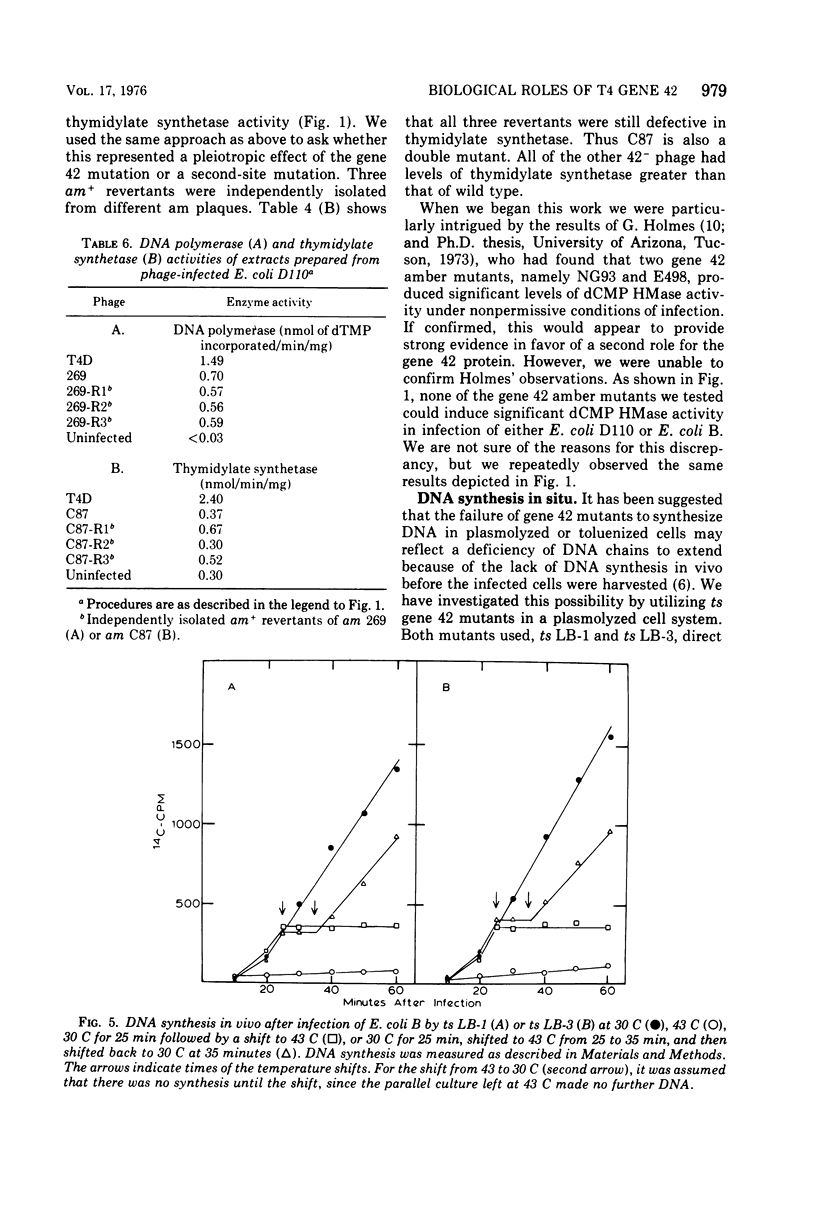
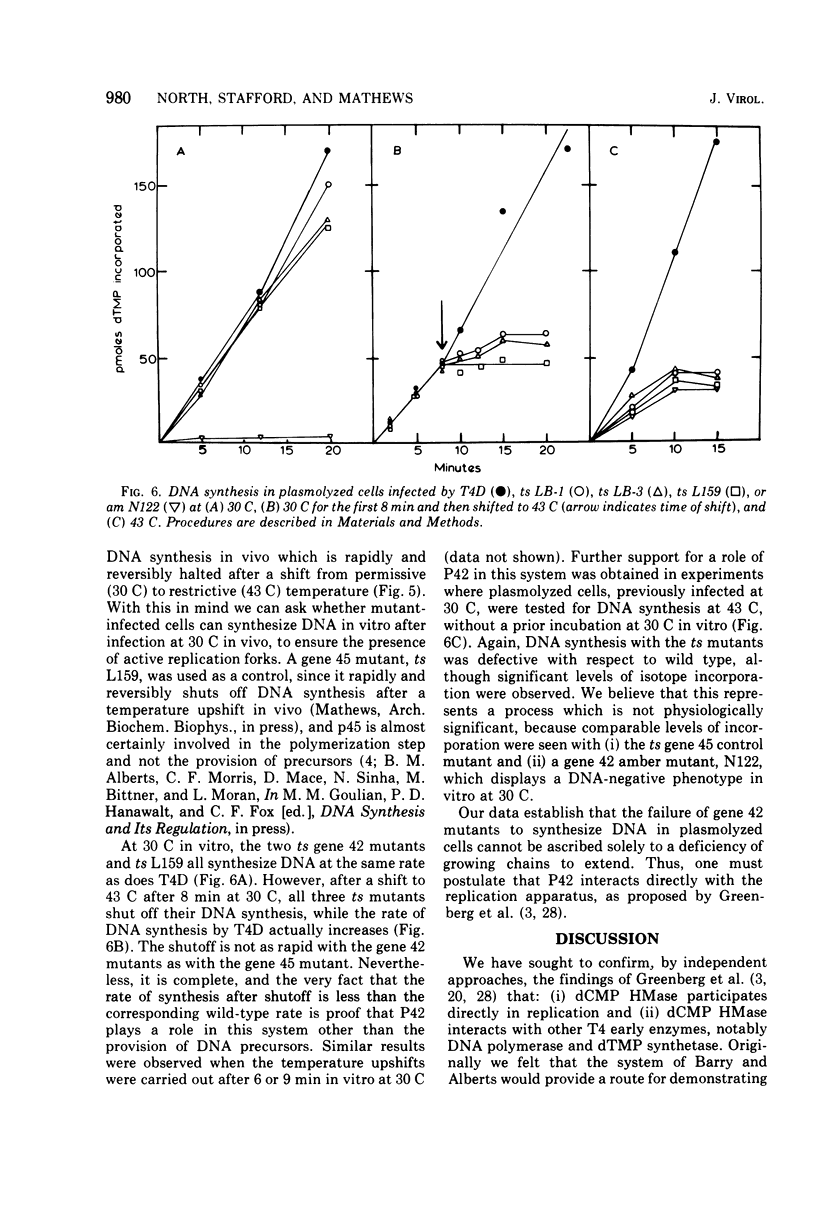
Bacteriophage T4 gene 1 and 42 amber mutants (defective in deoxynucleoside monophosphate kinase and deoxycytidylate hydroxymethylase, respectively) are able to synthesize DNA in cell-free lysates prepared as described by Barry and Alberts (1972), in contrast to their inabliity to do so in plasmolyzed and toluenized cell systems. Addition of extracts containing an active gene 1 or 42 product has no effect on synthesis in lysates defective in the respective gene. Thus, if these enzymes do play additional direct roles in replication, these roles are not manifest in the lysed-cell system. The gene 42 mutant am N122/m, a double mutant bearing an additional defect in DNA polymerase, is unable to synthesize DNA in these lysates. This inability is overcome by addition of extracts containing an active T4 DNA polymerase. m is a leaky amber mutation which reduces DNA polymerase activity to a very low level. However, this level is high enough to allow positive genetic complementation tests with gene 43 mutants. Two other gene 42 amber mutants contain additional defects: am 269 induces only half the normal level of DNA polymerase, and am C87 fails to induce a detectable level of thymidylate synthetase. These defects do not result from pleiotropic effects of the gene 42 mutations. In plasmolyzed cells, temperature-sensitive gene 42 mutants fail to synthesize DNA under conditions where replication forks and 5-hydroxymethyl-dCTP are present. This supports the idea that the gene 42 protein is directly involved in DNA synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry J., Alberts B. In vitro complementation as an assay for new proteins required for bacteriophage T4 DNA replication: purification of the complex specified by T4 genes 44 and 62. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2717–2721. doi: 10.1073/pnas.69.9.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C. S., Greenberg G. R. Evidence for a possible direct role of dCMP hydroxymethylase in T4 phage DNA synthesis. Cold Spring Harb Symp Quant Biol. 1968;33:351–359. doi: 10.1101/sqb.1968.033.01.041. [DOI] [PubMed] [Google Scholar]
- Collinsworth W. L., Mathews C. K. Biochemistry of DNA-defective amber mutants of bacteriophage T4. IV. DNA synthesis in plasmolyzed cells. J Virol. 1974 Apr;13(4):908–915. doi: 10.1128/jvi.13.4.908-915.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waard A., Paul A. V., Lehman I. R. The structural gene for deoxyribonucleic acid polymerase in bacteriophages T4 and T5. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1241–1248. doi: 10.1073/pnas.54.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicou L., Cozzarelli N. R. Bacteriophage T4-directed DNA synthesis in toluene-treated cells. J Virol. 1973 Dec;12(6):1293–1302. doi: 10.1128/jvi.12.6.1293-1302.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H., Bessman M. J. The enzymology of virus-infected bacteria. X. A biochemical-genetic study of the deoxynucleotide kinase induced by wild type and amber mutants of phage T4. J Biol Chem. 1967 Jun 25;242(12):2877–2885. [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- Holmes G. E. Intragenic complementation by gene 42 amber mutations of bacteriophage T4. J Virol. 1975 Oct;16(4):1085–1089. doi: 10.1128/jvi.16.4.1085-1089.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam J. D., O'Donnell P. V. Suppression of amber mutations of bacteriophage T4 gene 43 (DNA polymerase) by translational ambiguity. J Virol. 1973 Jun;11(6):933–945. doi: 10.1128/jvi.11.6.933-945.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. K. Biochemistry of deoxyribonucleic acid-defective amber mutant of bacteriophage T4. I. Ribonucleic acid metabolism. J Biol Chem. 1968 Nov 10;243(21):5610–5615. [PubMed] [Google Scholar]
- Mathews C. K. Biochemistry of deoxyribonucleic acid-defective amber mutants of bacteriophage T4. 3. Nucleotide pools. J Biol Chem. 1972 Nov 25;247(22):7430–7438. [PubMed] [Google Scholar]
- Mathews C. K., Hewlett M. J. T-even bacteriophage-tolerant mutants of Escherichia coli B. II. Nucleic acid metabolism. J Virol. 1971 Sep;8(3):275–285. doi: 10.1128/jvi.8.3.275-285.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. K., Kessin R. H. Control of bacteriophage-induced enzyme synthesis in cells infected with a temperature-sensitive mutant. J Virol. 1967 Feb;1(1):92–96. doi: 10.1128/jvi.1.1.92-96.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. E., Mathews C. K. Addition of nucleotides to parental DNA early in infection by bacteriophage T4. J Mol Biol. 1969 Sep 14;44(2):233–248. doi: 10.1016/0022-2836(69)90172-7. [DOI] [PubMed] [Google Scholar]
- PIZER L. I., COHEN S. S. Virus-induced acquisition of metabolic function. V. Purification and properties of the deoxycytidylate hydroxymethylase and studies on its origin. J Biol Chem. 1962 Apr;237:1251–1259. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- WIBERG J. S., BUCHANAN J. M. STUDIES ON LABILE DEOXYCYTIDYLATE HYDROXYMETHYLASES FROM ESCHERICHIA COLI B INFECTED WITH TEMPERATURE-SENSITIVE MUTANTS OF BACTERIOPHAGE T4. Proc Natl Acad Sci U S A. 1964 Mar;51:421–428. doi: 10.1073/pnas.51.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Barnes J. E. Deoxyribonucleic acid synthesis in Escherichia coli infected with some deoxyribonucleic acid polymerase-less mutants of bacteriophage T4. Virology. 1966 Jan;28(1):100–107. doi: 10.1016/0042-6822(66)90310-2. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Incorporation of uracil-14C into nucleic acids in Escherichia coli infected with bacteriophage T4 and T4 amber mutants. Virology. 1967 Nov;33(3):376–384. doi: 10.1016/0042-6822(67)90113-4. [DOI] [PubMed] [Google Scholar]
- Werner R. Mechanism of DNA replication. Nature. 1971 Apr 30;230(5296):570–572. doi: 10.1038/230570a0. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



