Abstract
AIM
To test whether Nox1 plays a role in typhlitis induced by Salmonella enterica serovar Typhimurium (S. Tm) in a mouse model.
METHODS
Eight-week-old male wild-type (WT) and Nox1 knockout (KO) C57BL6/J (B6) mice were administered metronidazole water for 4 d to make them susceptible to S. Tm infection by the oral route. The mice were given plain water and administered with 4 different doses of S. Tm by oral gavage. The mice were followed for another 4 d. From the time of the metronidazole application, the mice were observed twice daily and weighed daily. The ileum, cecum and colon were removed for sampling at the fourth day post-inoculation. Portions of all three tissues were fixed for histology and placed in RNAlater for mRNA/cDNA preparation and quantitative real-time PCR. The contents of the cecum were recovered for estimation of S. Tm CFU.
RESULTS
We found Nox1-knockout (Nox1-KO) mice were not more sensitive to S. Tm colonization and infection than WT B6 mice. This conclusion is based on the following observations: (1) S. Tm-infection induced similar weight loss in Nox1-KO mice compared to WT mice; (2) the same S. Tm CFU was recovered from the cecal content of Nox1-KO and WT mice regardless of the inoculation dose, except the lowest inoculation dose (2 × 106 CFU) for which the Nox1-KO had one-log lower CFU than WT mice; (3) there is no difference in cecal pathology between WT and Nox1-KO groups; and (4) there are no S. Tm infection-induced changes in gene expression levels (IL-1b, TNF-α, and Duox2) between WT and Nox1-KO groups. The Alpi gene expression was more suppressed by S. Tm treatment in WT than the Nox1-KO cecum.
CONCLUSION
Nox1 does not protect mice from S. Tm colonization. Nox1-KO provides a very minor protective effect against S. Tm infection. Using NOX1-specific inhibitors for colitis therapy should not increase risks in bacterial infection.
Keywords: Knockout mouse, NADPH Oxidase-1, Salmonella typhimurium, Goblet cells, Reactive oxygen species
Core tip: Using Nox1-knockout mice (Nox1-KO), we examined the role of cecum Nox1 in Salmonella typhimurium (S. Tm) infection. Mice were rendered susceptible to infection with oral metronidazole. Four days after S. Tm inoculation, Nox1-KO mice had equal or slightly lower CFU/g cecum contents and equal or slightly less pathology by histological assessment than wild-type (WT) mice. Quantitative real-time PCR measure of mRNA levels for inflammatory cytokines Il-1β and TNF-α were significantly higher in S. Tm treated WT vs untreated mice but not in S. Tm treated Nox1-KO mice. Since Nox1 may have a role in inflammatory bowel disease, treating subjects with Nox1 inhibitors may not make patients vulnerable to pathogens.
INTRODUCTION
The generation of reactive oxygen species (ROS) during infection is an important part of host defense to fend off bacterial invasion. A well-known source of ROS is produced by NADPH oxidase (NOX)-2 in innate immune cells to kill bacteria engulfed in phagosomes[1]. Mutations in genes encoding for the NOX2 complex lead to chronic granulomatous disease due diminished bactericidal activity. Nox2-KO mice are highly susceptible to Salmonella enteric serovar Typhimurium (S. Tm) colonization and mucosal inflammation[2]. S. Tm has been widely used as a mouse colitis model to study strategies by which enteropathogenic bacteria break colonization resistance[3]. Diminished ROS generation by the NOX2 complex also is a risk factor for early-onset pediatric pancolitis and Crohn’s-like disease[4].
Two other members of NADPH oxidases, NOX1 and DUOX2, expressed in the epithelium of the intestine are linked to very-early-onset inflammatory bowel disease[5]. Both Nox1 and Duox2 gene are barely expressed in the intestine of germ-free mice and are highly elevated when colonized with commensal bacteria[6,7]. Duox2 and Duoxa2 (DUOX maturation factor-2) expression is highly elevated in Helicobacter felis-infected mouse gastric epithelium compared to uninfected mice[8]. Lack of Duox activity in Duoxa-KO mice increased Helicobacter felis colonization[8]. Duox2 protein is highly elevated in the ileum and colon mono-associated with segmented filamentous bacterium compared that in germ-free mice[7]. However, Duox2 does not appear to protect mice against S. Tm infection since there is no difference in S. Tm colonization between wild-type (WT) and Duoxa-KO mice[7].
Nox1-generated ROS can regulate intestinal epithelial cell proliferation, apoptosis, and migration[9-11]. Recently, it was shown that deficiency in Cyba/p22Phos (an obligatory partner of Nox1, Nox2 and Nox4) in intestinal epithelium resulted in protection from Citrobacter rodentium and Listeria monocytogenes[1]. The epithelium-specific Cyba-KO (CybaΔIEC-KO) mice are considered to be equivalent to Nox1ΔIEC-KO because Nox2 and Nox4 are virtually unexpressed in the intestinal epithelium. Because C. rodentium-induced Duox2 gene expression only occurs in the WT mice but not CybaΔIEC-KO mice, it was proposed that Nox1 regulates Duox2 expression in the intestinal epithelium[1].
We have previously shown that GPx1-KO and GPx2-KO mice (deficient in antioxidant glutathione peroxide-1 or -2) are more susceptible to S. Tm infection than WT mice[12]. Because Nox1-deficiency may protect mice against C. rodentium infection, we hypothesize that Nox1-produced ROS exacerbates S. Tm infection. In this manuscript, we report that Nox1-knockout (Nox1-KO) mice are equally susceptible to S. Tm colonization and infection as WT mice. We concluded that Nox1 does not play a role in S. Tm-induced colitis.
MATERIALS AND METHODS
Mice
WT and Nox1-KO (generated by Karl-Heinz Krause, Geneva University, Switzerland) mice were derived from strain C57BL/6 (B6)[11]. Animal were bred and reared in the Animal Resources Center at the City of Hope based on standards and guidelines set by the United States Department of Agriculture, approved by the National Institutes of Health (NIH), Office for Laboratory Animal Welfare, and accredited by the AAALAC. Weaned mice were fed Lab diet 5061 (LabDiet, St Louis, MO, United States), ad lib, and received water via an automated water purification system until the beginning of the study at 8 wk of age. Care and use of mice in the study conformed to NIH (United States) and AAALAC standards and were performed under protocol 11043 approved by the City of Hope BRI Institutional Animal Care and Use Committee on 1/12/12 and renewed 1/15/14. Eight-week-old male WT and Nox1-KO B6 mice were given the antibiotic metronidazole (0.75 g/L in drinking water) for 4 d to facilitate oral S. Tm infection[13]. To mask the metallic taste of metronidazole, 1 g of sucralose (Splenda®) was added to 450 mL water to prevent dehydration during the treatment. Metronidazole facilitates bacteria colonization by reducing anaerobic bacteria populations and by thinning the mucus layer in the gut[14]. After 4 d the mice were switched to regular water and orally gavaged with S. Tm in a volume of 50-100 μL phosphate buffered saline. One-inch-long 22-gauge plastic gavage needles were used to deliver the bacteria and to minimize the possibility of injury to the mice. As part of the study data collection and to ensure that the animals were not in distress prior to the endpoint all mice were observed and weighed daily from the time of placement on metronidazole to euthanasia, i.e., 4 d post-inoculation. The high risk of systemic infection in B6 mice precluded a longer study duration. Mice treated with metronidazole and then water gained about 5% body weight over the interval. Mice inoculated with S. Tm lost up to 18% body weight regardless of inoculation dose. There was no statistical difference in weight lost between the Nox1-KO and WT groups. Mice were euthanized by CO2 exposure, the recommended method under NIH and AAALAC guidelines.
Salmonella enteric S. Tm
A virulent strain of S. Tm, IR715, was obtained from Dr. Andreas J. Baumler (University of California, Davis, CA, United States), who derived this strain from isolate 14028 (American Type Culture Collection)[12]. S. Tm was grown aerobically at 37 °C in lysogeny broth (LB) containing 50 mg/mL nalidixic acid (Sigma) overnight. Cells were harvested, resuspended in PBS with 10% glycerol, then stored in aliquots at -80 °C without freeze-thaw. Between 2 × 106 to 6.2 × 108 colony-forming units (CFUs) of S. Tm were used to inoculate mice as shown (Figure 1). The titer was determined on LB agar plates containing 50 mg/mL nalidixic acid.
Figure 1.
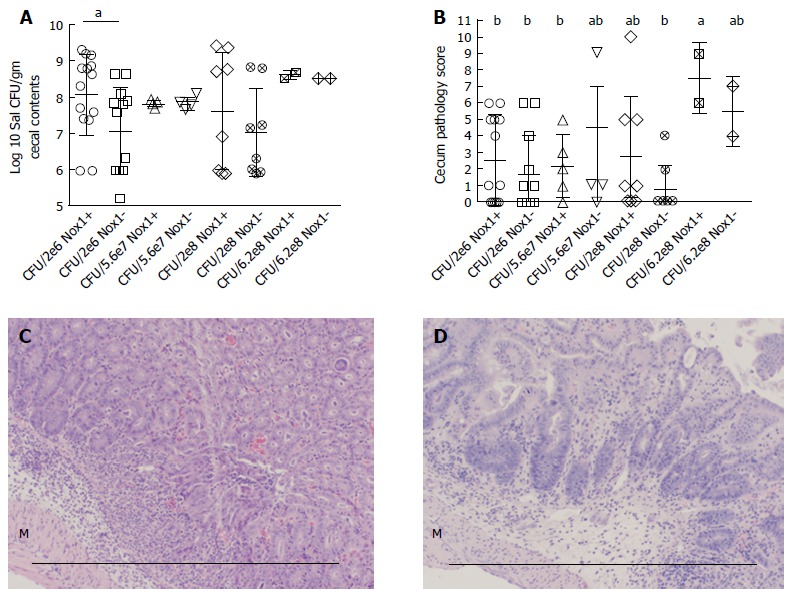
The CFU and pathology in the cecum between Nox1-knockout and wild-type mice with 4 different doses of S. Tm. A: Scatter plot of log10 transformed S. Tm CFU per gram recovered from cecal contents from WT (Nox1+) and Nox1-KO mice inoculated with 2 × 106, 5.6 × 107, 2 × 108 and 6.2 × 108 of S. Tm. The contents were plated so that 1 colony would yield a count ≥ 1 × 106 CFU/g. Zero colonies were assigned to 1 × 106 to include all mice analyzed in the panel. “a” indicates a significant difference between the Nox1 and WT groups treated with 2 × 106 S. Tm (colon-P = 0.0068; cecum-P = 0.0098); B: Scatter plot of pathology scores of mice from the groups shown in A. The groups with different letter designations in each figure are different, where a > b (α = 0.05). The groups sharing a same letter are not different; e.g., ab is not different from a or b group. Horizontal bars indicate mean ± SD; C, D: Show the worst pathology identified in Nox1-KO and WT cecum, respectively (score = 6), for mice inoculated with the lowest (2 × 106) CFU. Both groups show edema and infiltration between the muscular layers (M) and the glands. The glands are devoid of goblet cells and generally distorted. Scale bars are approximately 0.5 mm. Nox1-KO: Nox1-knockout; WT: Wild-type.
S. Tm colonization and disease parameters
Cecum luminal contents were collected sterilely, weighed, serially diluted, and plated on nalidixic acid-containing LB agar to allow detection down to approximately 1 × 106 CFU/g. The luminal contents have 2-4 log higher number of S. Tm than cecal tissue and are widely used as an indicator for bacterial colonization[12,15,16]. The inoculated S. Tm was the only bacteria capable of producing large-size colonies on nalidixic acid-containing LB agar under standard aerobic conditions[11]. To verify that the colonies were S. Tm, we performed automated-ribosomal-intergenic-spacer analysis (ARISA) PCR using ITSF (5’-GTCGTAACAAGGTAGCCGTA-3’) and ITSReub (5’-GCCAAGGCATCCACC-3’) primers on randomly selected colonies[17]. ARISA PCR produces a pattern of products with aggregate sizes characteristic of bacterial groups. We also sequenced ARISA PCR products to confirm the S. Tm identity.
The ileum, colon and cecum were excised for histology analysis. The tissues were fixed in phosphate buffered formalin. The processed slides were stained with H&E for pathology analysis and with Alcian blue counterstained with nuclear fast red for goblet cell counts. The slides were photographed, and Alcian blue stained goblet cells were counted from the sections containing full-length glands. Pathology was scored according to a 14-point system that accounts for mucin depletion, apoptosis, abscesses, and distortion of the glands[12].
Messenger RNA was prepared from the cecum, where S. Tm colonization is most reliably detected. Quantitative real-time PCR (qRT-PCR) was performed on mouse cecal mRNA. A segment of the cecum isolated from mice treated with 2 × 106 CFU of S. Tm was treated with RNALater (Ambion), and then RNA was isolated using the Triazol Kit (Thermo Scientific). Two μg of RNA was used to make cDNA with reagents from Promega and random hexamer primers (0.4 μg) from Invitrogen. PCR primers and probes (Thermo Scientific) used are shown in Table 1. The qRT-PCR was performed on a BioRad CFX96 instrument for 40 cycles. The ΔΔCt method was used to analyze differences in mRNA levels among groups, normalized with β-actin.
Table 1.
Taqman primer and probe IDs for quantitative real-time polymerase chain reaction
| Gene name (gene symbol) | Catalog number1 | Amplicon size |
| β-Actin (Actb) | Mm00607939_s1 | 115 |
| Dual oxidase-2 (Duox2) | Mm01326247_m1 | 65 |
| Il1b | Mm00434228_m1 | 90 |
| Intestinal alkaline phosphatase (Alpi) | Mm01285814_g1 | 60 |
| Tnfα | Mm00443258_m1 | 81 |
| Villin-1 (Vil1) | Mm004944146_m1 | 55 |
Catalog number from Thermo Fisher Scientific Inc.
Statistical analysis
GraphPad Prism version 6 was used for statistical analysis. All groups were compared in pair-wise t-tests except the cecum pathology score, which was analyzed by pair-wise Mann-Whitney tests. S. Tm CFU from cecal contents was analyzed from log 10 transformed data, which renders the data into parametric sets. The statistical analysis was reviewed and approved by Lianlian Du, MSci, biostatistician, Beijing Rehabilitation Hospital of Capital Medical University, Beijing, China.
RESULTS
Nox1-KO cecum and colon has a 7.5% and 20% higher number of goblet cells than WT counterparts
Nox1 is highly expressed in the colon, and Nox1-generated ROS can activate Notch1 signaling presumably by activating the metalloproteases (Mmp2 and Mmp9), which are involved in generation of Notch ligands[18]. Notch1 signaling promotes differentiation of epithelial cells into absorptive cells. Compared to WT mouse colon, Coant et al[18] reported that Nox1-KO colon had a 2-fold higher number of goblet cells and a 50% reduction of colonocytes due to decreases in Notch1 signaling. Because goblet cells are specialized to produce a mucus layer over the epithelial surface that acts as a barrier to intestinal microbes, changing the number of goblet cells could affect cell resistance to bacterial colonization. Therefore, we compared the number of goblet cells in the cecum and colon of Nox1-KO and WT mice (Figure 2). We found that Nox1-KO colon has a 20% increase in goblet cells compared to WT colon; however, we observed only a 7.5% increase in the Nox1-KO cecum, which is the major site of inflammation. This difference may have been overlooked in CybaΔIEC-KO mice, which do not have Nox1 activity[1].
Figure 2.
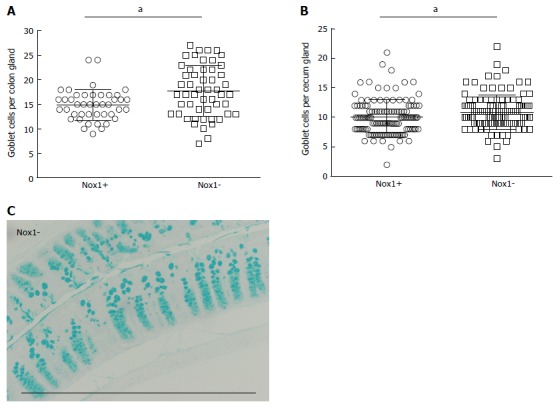
Goblets per colon (A) and cecum gland (B) counted from cross sections stained with Alcian blue and nuclear fast red (C). Each point in panels A and B represents a separate gland. Between 3-15 and 7-14 glands (obtained from 8 WT and 9 Nox1-KO mice) were counted from colon and cecum, respectively. Scale bar approximately 0.5 mm. "a" indicates a statistically significant difference between the 2 groups.
Nox1 does not significantly affect S. Tm colonization and inflammation in mice
We inoculated mice with 4 different doses of S. Tm (2 × 106, 5.6 × 107, 2 × 108, and 6.2 × 108) to compare the CFU and pathology in the cecum between Nox1-KO and WT mice (Figure 1). When inoculated with the lowest number of S. Tm (2 × 106), Nox1-KO mice had a significantly lower number of S. Tm CFUs in the cecum than WT mice (P = 0.034). No differences in the cecal S. Tm CFUs were found between Nox1-KO and WT mice when inoculated with higher doses of S. Tm (Figure 1A). Also, the CFUs of S. Tm in mouse cecum remained the same regardless of the inoculation doses, which is consistent with previous work[19].
We analyzed cecum pathology scores to determine the effect of Nox1. Scores of 0-6 generally reflect with presence of crypt apoptosis, hyperproliferation, and mucin depletion without overt signs of inflammation. Scores of above 6 are considered inflamed, showing immune infiltration, crypt distortion, goblet cell depletion, and elevated apoptosis in the epithelium. The worst cecal histology from a Nox1-KO and a WT mouse inoculated with a low dose of S. Tm (2 × 106) is shown in Figure 1C and 1D. Only WT mice inoculated with the highest dose (6.2 × 108) of S. Tm had a median score above 6, which is significantly higher than four groups of WT and Nox1-KO mice inoculated with lower doses of bacteria (P ≤ 0.025) (Figure 1B). However, there was no difference between the WT and Nox1-KO groups inoculated with the same high dose. These results indicate that Nox1 does not have significant impact on S. Tm infection.
Nox1 does not affect mucosal gene expression altered by S. Tm infection
Although the pathology scores are low in the mice inoculated with 2 × 106 CFU of S. Tm, these mice tend to have higher IL-1β and TNF-α mRNA levels compared to control mice that received metronidazole only (Figure 3A and B). However, we observed no difference in IL-1β and TNF-α mRNA levels between Nox1-KO and WT mice treated with metronidazole and S. Tm or untreated.
Figure 3.
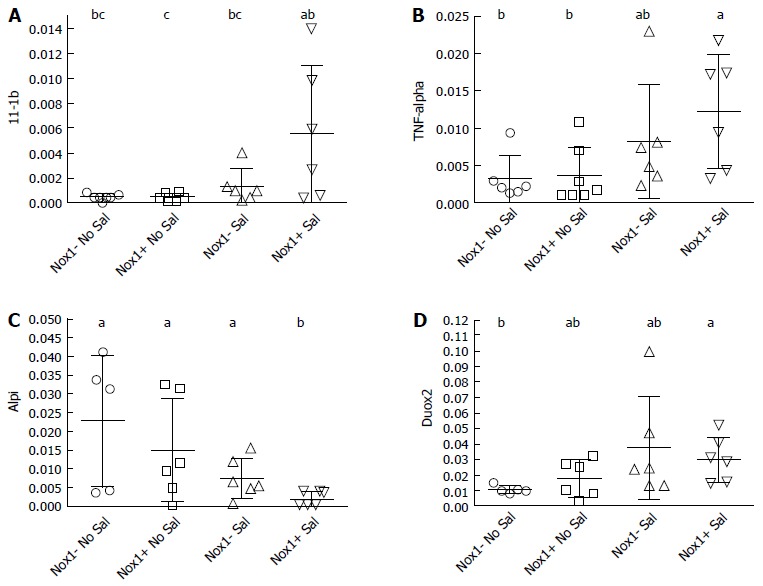
Quantitative real-time polymerase chain reaction analysis of cecum mRNA from the 2 × 106 CFU-treated wild-type and Nox1-knockout mice to measure inflammation markers IL-β (A), TNF-α (B), a brush border marker for the integrity of the epithelium, Alpi (C); and Duox2 levels (D). Horizontal bars are mean ± SD. The groups with different letter designations in each figure are different, where a > b > c (α = 0.05). The groups sharing a same letter are not different; e.g., bc is not different from b or ab group. Nox1-KO: Nox1-knockout; WT: Wild-type.
S. Tm infected intestine has decreased brush-border enzyme activities and gene expression levels including intestinal alkaline phosphatase (Alpi), sucrose-isomaltase, and maltose[19]. We found that S. Tm infected WT mouse cecum had decreased levels of Alpi gene expression (P ≥ 0.044), while the Alpi mRNA levels in the S. Tm-infected Nox1-KO mice were not significantly lower than the un-treated control mice (Figure 3C). Villin mRNA levels were not affected by S. Tm (data not shown). Villin is an actin-binding protein that regulates actin dynamics and organization of the brush border of enterocytes[20]. Loss of Villin mRNA is indicative of gross destruction of the gland surface architecture, reflected by pathology scores of 8 and above as observed in S. Tm treated Gpx1-KO and Gpx2-KO mice[12]. The overall trend indicates that the Nox1-KO mice tend to respond more mildly to S. Tm infection than WT mice, although not in a significant or uniform way.
We have previously shown that Duox2 gene expression was elevated 30-fold in the cecum of S. Tm-inoculated GPx1-KO mice (mean pathology scores of 10) compared to infected WT mice[12]. Here, the S. Tm-infected WT and Nox1-KO mouse cecum had 2- and 4-fold higher Duox2 gene expression than the non-infected respective controls (Figure 3D). No difference in the Duox2 mRNA levels between the WT and Nox1-KO cecum were observed.
DISCUSSION
Nox1 expressed in intestinal epithelium plays an important role in cell signaling, regulating many cellular events, including differentiation, proliferation, apoptosis, and migration[10,11,18]. Recently, Nox1 was shown to exacerbate pathogenic bacterial infection, including C. rodentium and L. monocytogenes studied in CybaΔIEC-KO mice[1]. In the present study, we compared S. Tm colonization and S. Tm-induced colitis in Nox1-KO and WT mice inoculated at different doses and found that Nox1 plays a very minor role in S. Tm infectivity. Because we found that Nox1-KO mice have a significant increase of goblet cells in the cecum and colon and because goblet cells secrete mucus to form a barrier to fend off bacterial infection[14,16], the protective effect of Nox1-deficiency is likely due to the increase in goblet cells rather than a direct effect of ROS production. The strong protective effect of Cyba deficiency against C. rodentium and L. monocytogenes may have contributions from multiple Nox deficiencies, because Nox2 is induced in the intestinal epithelium by serotonin, a neuroendocrine secreted by enterochromaffin cells[21].
We have confirmed that S. Tm infection suppresses intestinal alkaline phosphatase (Alpi) expression in WT mice and likely in Nox1-KO mice. Mice deficient in Alpi suffer from dysbiosis[22]. The antibiotic-induced susceptibility to S. Tm or Clostridium difficile can be prevented by oral supplement of calf Alpi[15]. It remains unclear how S. Tm inhibits Alpi gene expression and activity.
We have reported that intestinal inflammation, as observed in mice deficient in GPx1 and GPx2 [GPx1/2-double knockout (DKO)], have elevated Nox1 gene expression in the ileum[11]. Nox1 deletion completely abolished GPx1/2-DKO intestinal inflammation. Because NOX1 and NOX2 are the major sources of ROS in the artery wall for conditions such as hypertension, hypercholesterolemia, and diabetes, NOX inhibitors are being developed to treat ROS-associated diseases[23]. A clinical relevance of this study is that when targeting Nox1, it is unlikely that the anti-Nox1 therapy will increase risks of bacterial infection.
Nox1 is important for symbiotic-lactobacilli-induced cell proliferation in the ileum[10]; it also promotes restitution of colons damaged by dextran sulfate sodium[9,24]. Whether anti-Nox1 therapy has adverse effects other than bacterial infection needs to be further investigated.
In conclusion, we demonstrated that Nox1-KO mice are not more susceptible to S. Tm colonization than WT mice. The clinical relevance of this and other studies is that anti-Nox1 therapy should not present a major risk for bacterial infection, such as by S. Tm, Citrobacter rodentium or Listeria monocytogenes. However, because Nox1 also has a positive role in cell proliferation and tissue restitution, more studies are needed to clarify other potential risks of anti-Nox1 therapy.
ACKNOWLEDGMENTS
We thank Dr. Qiang Gao, Department of Gastroenterology and Hepatology, Beijing Rehabilitation Hospital of Capital Medical University, for his insightful comments during the drafting of the manuscript.
COMMENTS
Background
Generation of reactive oxygen species (ROS) is implicated in the pathology of inflammatory bowel disease, fibrosis, hypertension, stroke, and atherogenesis and may play role in tumorigenesis. NADPH oxidase 1 (Nox1) appears to be a major generator of ROS in many of these cases. Therefore, identifying specific Nox1 inhibitors may lead to new therapeutic agents. ROS can also be generated by Nox2 as part of the innate immune response to pathogenic microflora, and it has been speculated that Nox1 participates in the control of gut microflora. The authors and others are testing the idea that Nox1 may play a vital role in defense against gut pathogens by colonizing Nox1-KO mouse gut with pathogens, such as Salmonella enterica serovar Typhimurium (S. Tm) in this study.
Research frontiers
The role of Nox1 in disease has just recently been explored, and the search for potent and specific inhibitors is emerging as a major research focus. In support of using Nox1 inhibition as a therapy, it is important learn if there are major risks from pathogens or other complications such as existing damage to the gut. Wound healing and epithelial restitution may be impaired by inhibition of Nox1.
Innovations and breakthroughs
The study shows that Nox1 expression levels do not significantly affect colonization by S. Tm in the gut. Together with studies on Listeria and Citrobacter, this work suggests that inhibition of Nox1 activity poses little risk to the subject for bacterial infection.
Applications
Studies of this type will help define the risks inherent in the use of Nox1 inhibitors as therapeutic agents.
Terminology
Nox1 is a member of a family of oxidases that generate either superoxide or hydrogen peroxide (ROS) using NADPH as the electron donor. Nox1 generates superoxide. S. Tm is an enteropathogenic bacteria commonly used to explore microbial pathology in rodent models.
Peer-review
The authors investigated the role of Nox1 in S. Tm colonization and infection in a mouse model. The paper is interesting and adds to our general understanding of S. Tm infection and demonstrates that Nox1 does not play an important role in S. Tm colonization.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: Care and use of mice in this study conformed to NIH (USA) and Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) standards and were performed under protocol 11043 approved by the City of Hope BRI Institutional Animal Care and Use Committee on 1/12/12 and renewed 1/15/14. Animals were bred and reared in the Animal Resources Center at the City of Hope based on standards and guidelines set by the United States Department of Agriculture; approved by the National Institutes of Health, Office for Laboratory Animal Welfare; and accredited by the AAALAC International.
Conflict-of-interest statement: The authors declare no conflicts of interests.
Peer-review started: July 25, 2016
First decision: September 20, 2016
Article in press: November 16, 2016
P- Reviewer: Chen CJ, Chirullo B S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
References
- 1.Pircalabioru G, Aviello G, Kubica M, Zhdanov A, Paclet MH, Brennan L, Hertzberger R, Papkovsky D, Bourke B, Knaus UG. Defensive Mutualism Rescues NADPH Oxidase Inactivation in Gut Infection. Cell Host Microbe. 2016;19:651–663. doi: 10.1016/j.chom.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Felmy B, Songhet P, Slack EM, Müller AJ, Kremer M, Van Maele L, Cayet D, Heikenwalder M, Sirard JC, Hardt WD. NADPH oxidase deficient mice develop colitis and bacteremia upon infection with normally avirulent, TTSS-1- and TTSS-2-deficient Salmonella Typhimurium. PLoS One. 2013;8:e77204. doi: 10.1371/journal.pone.0077204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhillon SS, Fattouh R, Elkadri A, Xu W, Murchie R, Walters T, Guo C, Mack D, Huynh HQ, Baksh S, et al. Variants in nicotinamide adenine dinucleotide phosphate oxidase complex components determine susceptibility to very early onset inflammatory bowel disease. Gastroenterology. 2014;147:680–689.e2. doi: 10.1053/j.gastro.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Hayes P DS, O’Neill K, Thoeni C, Hui KY, Elkadri A, Guo CH, Kovacic L, Aviello G, Alvarez LA, Griffiths AM, et al. Defects in NADPH Oxidase Genes NOX1 and DUOX2 in Very Early Onset Inflammatory Bowel Disease. Cell Mol Gastroenterol Hepatol. 2015;1:14. doi: 10.1016/j.jcmgh.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommer F, Bäckhed F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunol. 2015;8:372–379. doi: 10.1038/mi.2014.74. [DOI] [PubMed] [Google Scholar]
- 7.Grasberger H, Gao J, Nagao-Kitamoto H, Kitamoto S, Zhang M, Kamada N, Eaton KA, El-Zaatari M, Shreiner AB, Merchant JL, et al. Increased Expression of DUOX2 Is an Epithelial Response to Mucosal Dysbiosis Required for Immune Homeostasis in Mouse Intestine. Gastroenterology. 2015;149:1849–1859. doi: 10.1053/j.gastro.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasberger H, El-Zaatari M, Dang DT, Merchant JL. Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent Helicobacter felis infection and inflammation in mice. Gastroenterology. 2013;145:1045–1054. doi: 10.1053/j.gastro.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RM, Luo L, Ardita CS, Richardson AN, Kwon YM, Mercante JW, Alam A, Gates CL, Wu H, Swanson PA, et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 2013;32:3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esworthy RS, Kim BW, Chow J, Shen B, Doroshow JH, Chu FF. Nox1 causes ileocolitis in mice deficient in glutathione peroxidase-1 and -2. Free Radic Biol Med. 2014;68:315–325. doi: 10.1016/j.freeradbiomed.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esworthy RS, Kim BW, Wang Y, Gao Q, Doroshow JH, Leto TL, Chu FF. The Gdac1 locus modifies spontaneous and Salmonella-induced colitis in mice deficient in either Gpx2 or Gpx1 gene. Free Radic Biol Med. 2013;65:1273–1283. doi: 10.1016/j.freeradbiomed.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barthel M, Hapfelmeier S, Quintanilla-Martínez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Rüssmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wlodarska M, Willing B, Keeney KM, Menendez A, Bergstrom KS, Gill N, Russell SL, Vallance BA, Finlay BB. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect Immun. 2011;79:1536–1545. doi: 10.1128/IAI.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam SN, Yammine H, Moaven O, Ahmed R, Moss AK, Biswas B, Muhammad N, Biswas R, Raychowdhury A, Kaliannan K, et al. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann Surg. 2014;259:715–722. doi: 10.1097/SLA.0b013e31828fae14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarepour M, Bhullar K, Montero M, Ma C, Huang T, Velcich A, Xia L, Vallance BA. The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect Immun. 2013;81:3672–3683. doi: 10.1128/IAI.00854-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esworthy RS, Smith DD, Chu FF. A Strong Impact of Genetic Background on Gut Microflora in Mice. Int J Inflam. 2010;2010:986046. doi: 10.4061/2010/986046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coant N, Ben Mkaddem S, Pedruzzi E, Guichard C, Tréton X, Ducroc R, Freund JN, Cazals-Hatem D, Bouhnik Y, Woerther PL, et al. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol. 2010;30:2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Symonds EL, O’Mahony C, Lapthorne S, O’Mahony D, Sharry JM, O’Mahony L, Shanahan F. Bifidobacterium infantis 35624 protects against salmonella-induced reductions in digestive enzyme activity in mice by attenuation of the host inflammatory response. Clin Transl Gastroenterol. 2012;3:e15. doi: 10.1038/ctg.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lhocine N, Arena ET, Bomme P, Ubelmann F, Prévost MC, Robine S, Sansonetti PJ. Apical invasion of intestinal epithelial cells by Salmonella typhimurium requires villin to remodel the brush border actin cytoskeleton. Cell Host Microbe. 2015;17:164–177. doi: 10.1016/j.chom.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regmi SC, Park SY, Ku SK, Kim JA. Serotonin regulates innate immune responses of colon epithelial cells through Nox2-derived reactive oxygen species. Free Radic Biol Med. 2014;69:377–389. doi: 10.1016/j.freeradbiomed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Malo MS, Moaven O, Muhammad N, Biswas B, Alam SN, Economopoulos KP, Gul SS, Hamarneh SR, Malo NS, Teshager A, et al. Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates. Am J Physiol Gastrointest Liver Physiol. 2014;306:G826–G838. doi: 10.1152/ajpgi.00357.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato M, Marumo M, Nakayama J, Matsumoto M, Yabe-Nishimura C, Kamata T. The ROS-generating oxidase Nox1 is required for epithelial restitution following colitis. Exp Anim. 2016;65:197–205. doi: 10.1538/expanim.15-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]


