Abstract
von Willebrand disease (vWD), the most common inherited bleeding disorder in humans, results from abnormalities in the plasma clotting protein von Willebrand factor (vWF). Severe (type III) vWD is autosomal recessive in inheritance and is associated with extremely low or undetectable vWF levels. We report a method designed to distinguish mRNA expression from the two vWF alleles by PCR analysis of peripheral blood platelet RNA using DNA sequence polymorphisms located within exons of the vWF gene. This approach was applied to a severe-vWD pedigree in which three of eight siblings are affected and the parents and additional siblings are clinically normal. Each parent was shown to carry a vWF allele that is silent at the mRNA level. Family members inheriting both abnormal alleles are affected with severe vWD, whereas individuals with only one abnormal allele are asymptomatic. The maternal and paternal silent alleles are identical at two coding sequence polymorphisms as well as an intron 40 variable number tandem repeat, suggesting a possible common origin. Given the frequencies of the two exon polymorphisms reported here, this analysis should be applicable to approximately 70% of type I and type III vWD patients. This comparative DNA and RNA PCR-restriction fragment length polymorphism approach may also prove useful in identifying defects at the level of gene expression associated with other genetic disorders.
Full text
PDF
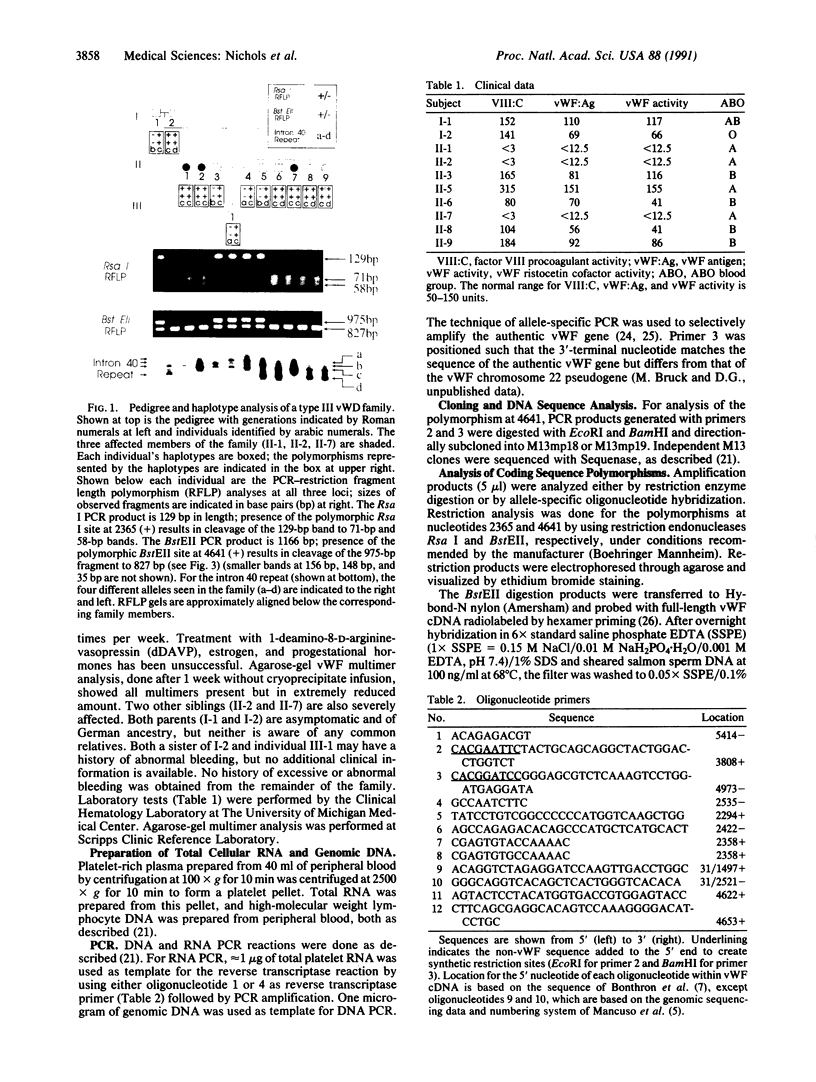
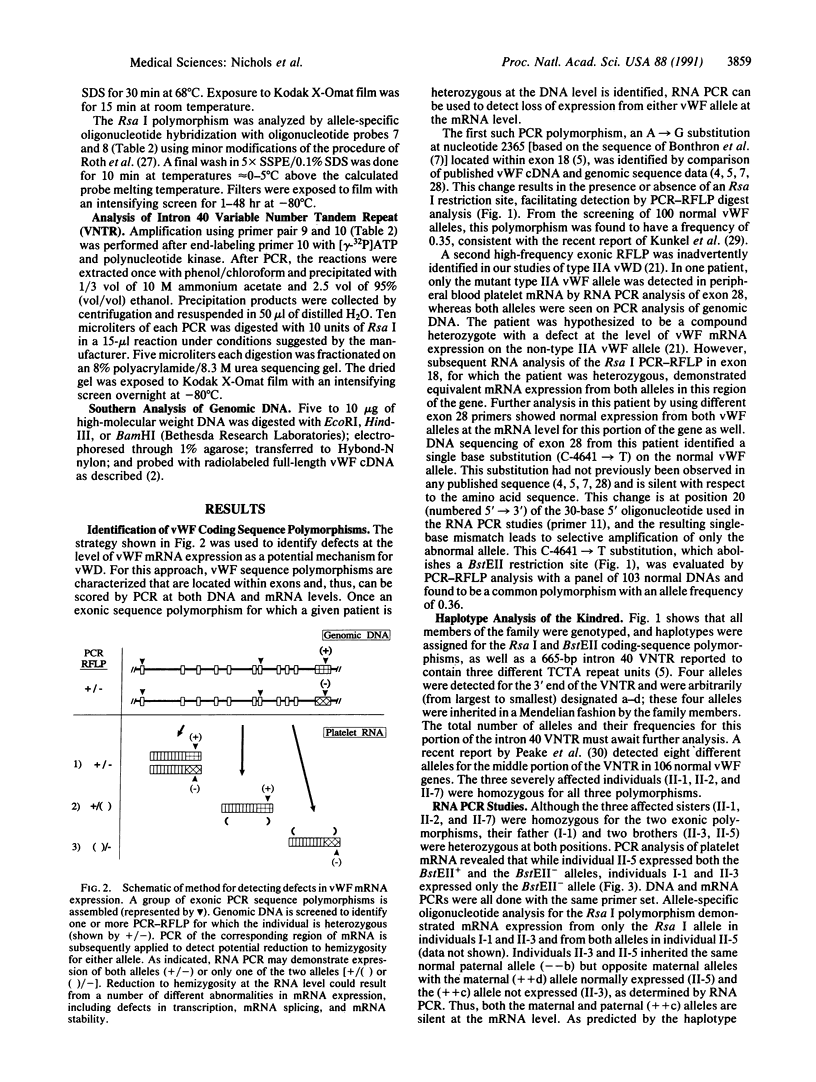


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akli S., Chelly J., Mezard C., Gandy S., Kahn A., Poenaru L. A "G" to "A" mutation at position -1 of a 5' splice site in a late infantile form of Tay-Sachs disease. J Biol Chem. 1990 May 5;265(13):7324–7330. [PubMed] [Google Scholar]
- Bahnak B. R., Lavergne J. M., Verweij C. L., Rothschild C., Pannekoek H., Larrieu M. J., Meyer D. Carrier detection in severe (type III) von Willebrand disease using two intragenic restriction fragment length polymorphisms. Thromb Haemost. 1988 Oct 31;60(2):178–181. [PubMed] [Google Scholar]
- Berliner S. A., Seligsohn U., Zivelin A., Zwang E., Sofferman G. A relatively high frequency of severe (type III) von Willebrand's disease in Israel. Br J Haematol. 1986 Mar;62(3):535–543. doi: 10.1111/j.1365-2141.1986.tb02966.x. [DOI] [PubMed] [Google Scholar]
- Bernardi F., Guerra S., Patracchini P., Volinia S., Buzzoni D., Ballerini G., Casonato A., Marchetti G. von Willebrand disease investigated by two novel RFLPs. Br J Haematol. 1988 Feb;68(2):243–248. doi: 10.1111/j.1365-2141.1988.tb06196.x. [DOI] [PubMed] [Google Scholar]
- Bignell P., Standen G. R., Bowen D. J., Peake I. R., Bloom A. L. Rapid neonatal diagnosis of von Willebrand's disease by use of the polymerase chain reaction. Lancet. 1990 Sep 8;336(8715):638–639. doi: 10.1016/0140-6736(90)93445-u. [DOI] [PubMed] [Google Scholar]
- Bonthron D., Orr E. C., Mitsock L. M., Ginsburg D., Handin R. I., Orkin S. H. Nucleotide sequence of pre-pro-von Willebrand factor cDNA. Nucleic Acids Res. 1986 Sep 11;14(17):7125–7127. doi: 10.1093/nar/14.17.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caekebeke-Peerlinck K. M., Bakker E., Briet E. An infrequent DNA polymorphism associated with severe von Willebrand's disease. Br J Haematol. 1990 May;75(1):78–81. doi: 10.1111/j.1365-2141.1990.tb02619.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fischer R. R., Lerner C., Bandinelli E., Fonseca A. S., Roisenberg I. Inheritance and prevalence of von Willebrand's disease severe form in a Brazilian population. J Inherit Metab Dis. 1989;12(3):293–301. doi: 10.1007/BF01799220. [DOI] [PubMed] [Google Scholar]
- Gill J. C., Endres-Brooks J., Bauer P. J., Marks W. J., Jr, Montgomery R. R. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood. 1987 Jun;69(6):1691–1695. [PubMed] [Google Scholar]
- Ginsburg D., Handin R. I., Bonthron D. T., Donlon T. A., Bruns G. A., Latt S. A., Orkin S. H. Human von Willebrand factor (vWF): isolation of complementary DNA (cDNA) clones and chromosomal localization. Science. 1985 Jun 21;228(4706):1401–1406. doi: 10.1126/science.3874428. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Konkle B. A., Gill J. C., Montgomery R. R., Bockenstedt P. L., Johnson T. A., Yang A. Y. Molecular basis of human von Willebrand disease: analysis of platelet von Willebrand factor mRNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3723–3727. doi: 10.1073/pnas.86.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handin R. I., Wagner D. D. Molecular and cellular biology of von Willebrand factor. Prog Hemost Thromb. 1989;9:233–259. [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Hobbs H. H., Leitersdorf E., Goldstein J. L., Brown M. S., Russell D. W. Multiple crm- mutations in familial hypercholesterolemia. Evidence for 13 alleles, including four deletions. J Clin Invest. 1988 Mar;81(3):909–917. doi: 10.1172/JCI113402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T., Kadowaki H., Taylor S. I. A nonsense mutation causing decreased levels of insulin receptor mRNA: detection by a simplified technique for direct sequencing of genomic DNA amplified by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Jan;87(2):658–662. doi: 10.1073/pnas.87.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel G. R., Graham J. B., Fowlkes D. M., Lord S. T. RsaI polymorphism in von Willebrand factor (vWF) at codon 789. Nucleic Acids Res. 1990 Aug 25;18(16):4961–4961. doi: 10.1093/nar/18.16.4961-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. C., Zimmerman T. S., Collins C. J., Brown M., Morin M. J., Ling E. H., Livingston D. M. Molecular cloning of cDNA for human von Willebrand factor: authentication by a new method. Cell. 1985 May;41(1):49–56. doi: 10.1016/0092-8674(85)90060-1. [DOI] [PubMed] [Google Scholar]
- Mancuso D. J., Tuley E. A., Westfield L. A., Worrall N. K., Shelton-Inloes B. B., Sorace J. M., Alevy Y. G., Sadler J. E. Structure of the gene for human von Willebrand factor. J Biol Chem. 1989 Nov 25;264(33):19514–19527. [PubMed] [Google Scholar]
- Mannucci P. M., Lattuada A., Castaman G., Lombardi R., Colibretti M. L., Ciavarella N., Rodeghiero F. Heterogeneous phenotypes of platelet and plasma von Willebrand factor in obligatory heterozygotes for severe von Willebrand disease. Blood. 1989 Nov 15;74(7):2433–2436. [PubMed] [Google Scholar]
- Nakano T., Suzuki K. Genetic cause of a juvenile form of Sandhoff disease. Abnormal splicing of beta-hexosaminidase beta chain gene transcript due to a point mutation within intron 12. J Biol Chem. 1989 Mar 25;264(9):5155–5158. [PubMed] [Google Scholar]
- Ngo K. Y., Glotz V. T., Koziol J. A., Lynch D. C., Gitschier J., Ranieri P., Ciavarella N., Ruggeri Z. M., Zimmerman T. S. Homozygous and heterozygous deletions of the von Willebrand factor gene in patients and carriers of severe von Willebrand disease. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2753–2757. doi: 10.1073/pnas.85.8.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. C., Liepnieks J. J., McKusick V. A., Benson M. D. Direct sequencing of the gene for Maryland/German familial amyloidotic polyneuropathy type II and genotyping by allele-specific enzymatic amplification. Genomics. 1989 Oct;5(3):535–540. doi: 10.1016/0888-7543(89)90020-7. [DOI] [PubMed] [Google Scholar]
- Okayama H., Curiel D. T., Brantly M. L., Holmes M. D., Crystal R. G. Rapid, nonradioactive detection of mutations in the human genome by allele-specific amplification. J Lab Clin Med. 1989 Aug;114(2):105–113. [PubMed] [Google Scholar]
- Peake I. R., Bowen D., Bignell P., Liddell M. B., Sadler J. E., Standen G., Bloom A. L. Family studies and prenatal diagnosis in severe von Willebrand disease by polymerase chain reaction amplification of a variable number tandem repeat region of the von Willebrand factor gene. Blood. 1990 Aug 1;76(3):555–561. [PubMed] [Google Scholar]
- Peake I. R., Liddell M. B., Moodie P., Standen G., Mancuso D. J., Tuley E. A., Westfield L. A., Sorace J. M., Sadler J. E., Verweij C. L. Severe type III von Willebrand's disease caused by deletion of exon 42 of the von Willebrand factor gene: family studies that identify carriers of the condition and a compound heterozygous individual. Blood. 1990 Feb 1;75(3):654–661. [PubMed] [Google Scholar]
- Rodeghiero F., Castaman G., Dini E. Epidemiological investigation of the prevalence of von Willebrand's disease. Blood. 1987 Feb;69(2):454–459. [PubMed] [Google Scholar]
- Roth M. S., Antin J. H., Bingham E. L., Ginsburg D. Use of polymerase chain reaction-detected sequence polymorphisms to document engraftment following allogeneic bone marrow transplantation. Transplantation. 1990 Apr;49(4):714–720. doi: 10.1097/00007890-199004000-00012. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. von Willebrand factor and von Willebrand disease. Blood. 1987 Oct;70(4):895–904. [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton-Inloes B. B., Chehab F. F., Mannucci P. M., Federici A. B., Sadler J. E. Gene deletions correlate with the development of alloantibodies in von Willebrand disease. J Clin Invest. 1987 May;79(5):1459–1465. doi: 10.1172/JCI112974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton-Inloes B. B., Titani K., Sadler J. E. cDNA sequences for human von Willebrand factor reveal five types of repeated domains and five possible protein sequence polymorphisms. Biochemistry. 1986 Jun 3;25(11):3164–3171. doi: 10.1021/bi00359a014. [DOI] [PubMed] [Google Scholar]
- Sultan Y., Simeon J., Caen J. P. Detection of heterozygotes in both parents of homozygous patients with Von Willebrand's disease. J Clin Pathol. 1975 Apr;28(4):309–316. doi: 10.1136/jcp.28.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltkamp J. J., van Tilburg N. H. Detection of heterozygotes for recessive von Willebrand's disease by the assay of antihemophilic-factor-like antigen. N Engl J Med. 1973 Oct 25;289(17):882–885. doi: 10.1056/NEJM197310252891703. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Diergaarde P. J., Hart M., Pannekoek H. Full-length von Willebrand factor (vWF) cDNA encodes a highly repetitive protein considerably larger than the mature vWF subunit. EMBO J. 1986 Aug;5(8):1839–1847. doi: 10.1002/j.1460-2075.1986.tb04435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., Quadt R., Briët E., Dubbeldam K., van Ommen G. B., Pannekoek H. Genetic linkage of two intragenic restriction fragment length polymorphisms with von Willebrand's disease type IIA. Evidence for a defect in the von Willebrand factor gene. J Clin Invest. 1988 Apr;81(4):1116–1121. doi: 10.1172/JCI113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J., Ball A. P., Mannucci P. M. Incidence of severe von Willebrand's disease. N Engl J Med. 1982 Jul 8;307(2):127–127. doi: 10.1056/NEJM198207083070222. [DOI] [PubMed] [Google Scholar]
- Zhang B., Edenberg H. J., Crabb D. W., Harris R. A. Evidence for both a regulatory mutation and a structural mutation in a family with maple syrup urine disease. J Clin Invest. 1989 Apr;83(4):1425–1429. doi: 10.1172/JCI114033. [DOI] [PMC free article] [PubMed] [Google Scholar]