Abstract
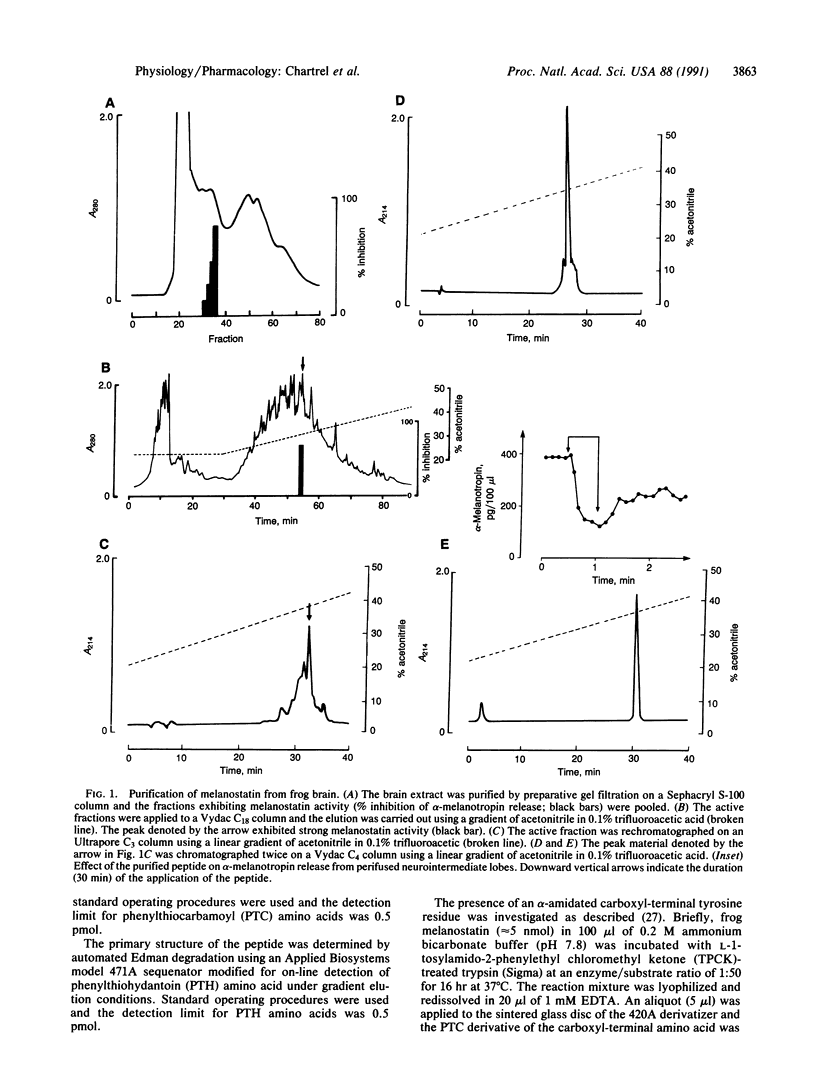
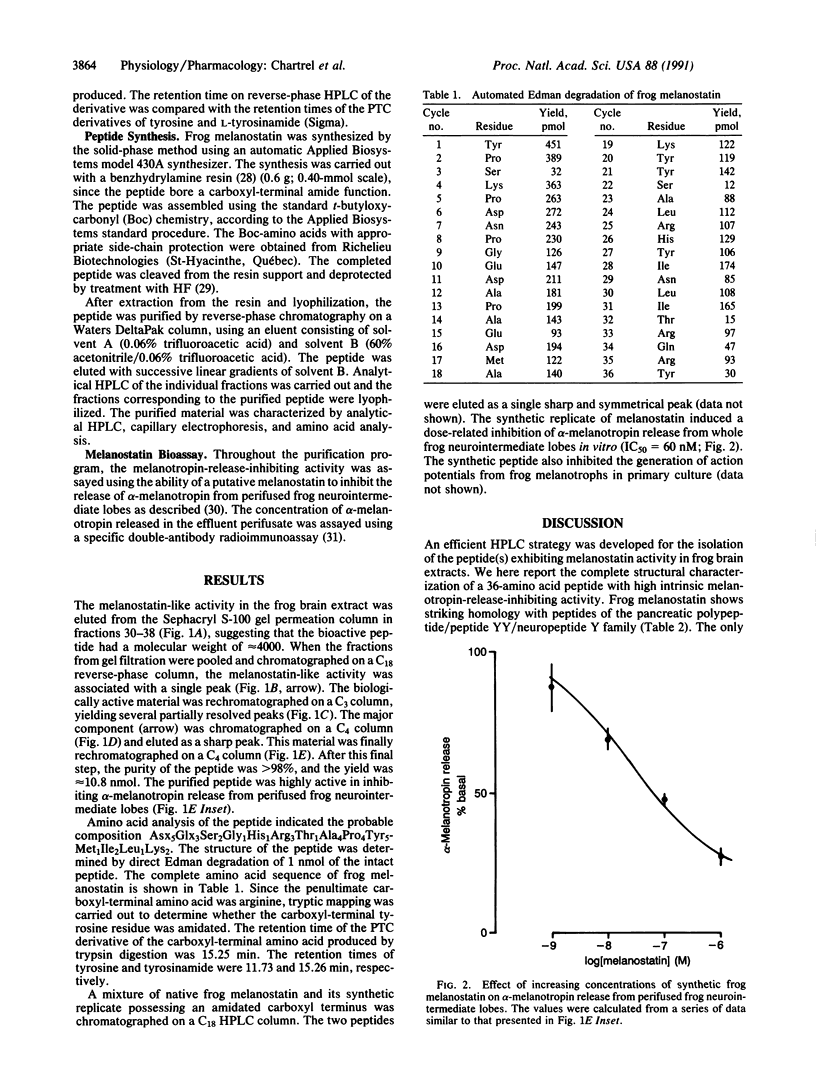
A polypeptide was purified from frog brain extracts on the basis of its ability to inhibit alpha-melanotropin release from perifused frog neurointermediate lobes. Based on Edman degradation, amino acid analysis, and peptide mapping, the primary structure of this frog melanotropin-release-inhibiting factor (melanostatin) was determined to be H-Tyr-Pro-Ser-Lys-Pro-Asp-Asn-Pro-Gly-Glu-Asp-Ala-Pro-Ala-Glu-Asp-Met- Ala-Lys-Tyr-Tyr-Ser-Ala-Leu-Arg-His-Tyr-Ile-Asn-Leu-Ile-Thr-Arg-Gln-Arg- Tyr-NH2 . Frog melanostatin belongs to the pancreatic polypeptide/neuropeptide Y/peptide YY family, and the structure of this peptide differs from that of human neuropeptide Y by only one amino acid substitution in position 19. A synthetic replicate of frog melanostatin is coeluted with the native peptide on HPLC and is highly potent in inhibiting alpha-melanotropin secretion in vitro (IC50 = 60 nM).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bower A., Hadley M. E., Hruby V. J. Biogenic amines and control of melanophore stimulating hormone release. Science. 1974 Apr 5;184(4132):70–72. doi: 10.1126/science.184.4132.70. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Burgus R., Butcher M., Ling N., Monahan M., Rivier J., Fellows R., Amoss M., Blackwell R., Vale W., Guillemin R. Structure moléculaire du facteur hypothalamique (LRF) d'origine ovine contrôlant la sécrétion de l'hormone gondadotrope hypophysaire de lutéinisation. C R Acad Sci Hebd Seances Acad Sci D. 1971 Nov 3;273(18):1611–1613. [PubMed] [Google Scholar]
- Burgus R., Dunn T. F., Desiderio D., Guillemin R. Structure moléculaire du facteur hypothalamique hypophysiotrope TRF d'origine ovine: mise en évidence par spectrométrie de masse de la séquence PCA-His-Pro-NH2. C R Acad Sci Hebd Seances Acad Sci D. 1969 Nov 12;269(19):1870–1873. [PubMed] [Google Scholar]
- Celis M. E., Taleisnik S., Walter R. Regulation of formation and proposed structure of the factor inhibiting the release of melanocyte-stimulating hormone. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1428–1433. doi: 10.1073/pnas.68.7.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis M. E., Taleisnik S., Walter R. Release of pituitary melanocyte-stimulating hormone by the oxytocin fragment, H-Cys-Tyr-Ile-Gln-Asn-Oh. Biochem Biophys Res Commun. 1971 Nov 5;45(3):564–569. doi: 10.1016/0006-291x(71)90454-2. [DOI] [PubMed] [Google Scholar]
- Corder R., Emson P. C., Lowry P. J. Purification and characterization of human neuropeptide Y from adrenal-medullary phaeochromocytoma tissue. Biochem J. 1984 May 1;219(3):699–706. doi: 10.1042/bj2190699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danger J. M., Leboulenger F., Guy J., Tonon M. C., Benyamina M., Martel J. C., Saint-Pierre S., Pelletier G., Vaudry H. Neuropeptide Y in the intermediate lobe of the frog pituitary acts as an alpha-MSH-release inhibiting factor. Life Sci. 1986 Sep 29;39(13):1183–1192. doi: 10.1016/0024-3205(86)90350-4. [DOI] [PubMed] [Google Scholar]
- Dierickx K., Vandesande F. Immuno-enzyme cytochemical demonstration of mesotocinergic nerve fibres in the pars intermedia of the amphibian hypophysis. Cell Tissue Res. 1976 Oct 22;174(1):25–33. doi: 10.1007/BF00222148. [DOI] [PubMed] [Google Scholar]
- Forest M., Martel J. C., St-Pierre S., Quirion R., Fournier A. Structural study of the N-terminal segment of neuropeptide tyrosine. J Med Chem. 1990 Jun;33(6):1615–1619. doi: 10.1021/jm00168a014. [DOI] [PubMed] [Google Scholar]
- Grant N. H., Clark D. E., Rosanoff E. I. Evidence that Pro-Leu-Gly-NH 2 , tocinoic acid, and des-Cys-tocinoic acid do not affect secretion of melanocyte stimulating hormone. Biochem Biophys Res Commun. 1973 Mar 5;51(1):100–106. doi: 10.1016/0006-291x(73)90513-5. [DOI] [PubMed] [Google Scholar]
- Guillemin R., Brazeau P., Böhlen P., Esch F., Ling N., Wehrenberg W. B. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science. 1982 Nov 5;218(4572):585–587. doi: 10.1126/science.6812220. [DOI] [PubMed] [Google Scholar]
- Hruby V. J., Smith C. W., Bower A., Hadley M. E. Melanophore stimulating hormone: release inhibition by ring structures of neurohypophysial hormones. Science. 1972 Jun 23;176(4041):1331–1332. doi: 10.1126/science.176.4041.1331. [DOI] [PubMed] [Google Scholar]
- Jacobs G. F., Michielsen R. P., Kühn E. R. Thyroxine and triiodothyronine in plasma and thyroids of the neotenic and metamorphosed axolotl Ambystoma mexicanum: influence of TRH injections. Gen Comp Endocrinol. 1988 Apr;70(1):145–151. doi: 10.1016/0016-6480(88)90103-7. [DOI] [PubMed] [Google Scholar]
- Kastin A. J., Schally A. V., Viosca S. Inhibition of MSH release in frogs by direct application of L-proplyl-L-leucylglycinamide to the pituitary. Proc Soc Exp Biol Med. 1971 Sep;137(4):1437–1439. doi: 10.3181/00379727-137-35805. [DOI] [PubMed] [Google Scholar]
- Lamacz M., Hindelang C., Tonon M. C., Vaudry H., Stoeckel M. E. Three distinct thyrotropin-releasing hormone-immunoreactive axonal systems project in the median eminence-pituitary complex of the frog Rana ridibunda. Immunocytochemical evidence for co-localization of thyrotropin-releasing hormone and mesotocin in fibers innervating pars intermedia cells. Neuroscience. 1989;32(2):451–462. doi: 10.1016/0306-4522(89)90093-6. [DOI] [PubMed] [Google Scholar]
- Lamacz M., Tonon M. C., Leboulenger F., Héry F., Idres S., Verhofstad A. J., Pelletier G., Vaudry H. Effect of serotonin on alpha-melanocyte-stimulating hormone secretion from perifused frog neurointermediate lobe: evidence for the presence of serotonin-containing cells in the frog pars intermedia. J Endocrinol. 1989 Jul;122(1):135–146. doi: 10.1677/joe.0.1220135. [DOI] [PubMed] [Google Scholar]
- Lamacz M., Tonon M. C., Louiset E., Cazin L., Strosberg D., Vaudry H. Acetylcholine stimulates alpha-melanocyte-stimulating hormone release from frog pituitary melanotrophs through activation of muscarinic and nicotinic receptors. Endocrinology. 1989 Aug;125(2):707–714. doi: 10.1210/endo-125-2-707. [DOI] [PubMed] [Google Scholar]
- Minth C. D., Bloom S. R., Polak J. M., Dixon J. E. Cloning, characterization, and DNA sequence of a human cDNA encoding neuropeptide tyrosine. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4577–4581. doi: 10.1073/pnas.81.14.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemura M., Cote T. E., Tsuruta K., Eskay R. L., Kebabian J. W. The dopamine receptor in the intermediate lobe of the rat pituitary gland: pharmacological characterization. Endocrinology. 1980 Dec;107(6):1676–1683. doi: 10.1210/endo-107-6-1676. [DOI] [PubMed] [Google Scholar]
- Pietta P. G., Cavallo P. F., Takahashi K., Marshall G. R. Preparation and use of benzhydrylamine polymers in peptide synthesis. II. Syntheses of thyrotropin releasing hormone, thyrocalcitonin 26-32, and eledoisin. J Org Chem. 1974 Jan 11;39(1):44–48. doi: 10.1021/jo00915a008. [DOI] [PubMed] [Google Scholar]
- Rivier J., Spiess J., Thorner M., Vale W. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature. 1982 Nov 18;300(5889):276–278. doi: 10.1038/300276a0. [DOI] [PubMed] [Google Scholar]
- Schimchowitsch S., Stoeckel M. E., Klein M. J., Garaud J. C., Schmitt G., Porte A. Oxytocin-immunoreactive nerve fibers in the pars intermedia of the pituitary in the rabbit and hare. Cell Tissue Res. 1983;228(2):255–263. doi: 10.1007/BF00204877. [DOI] [PubMed] [Google Scholar]
- Schimchowitsch S., Stoeckel M. E., Schmitt G., Porte A. Contrôle stimulant par l'ocytocine (ou un peptide analogue) du lobe intermédiaire de l'hypophyse chez le lapin. Rôle inhibiteur de la sérotonine. C R Acad Sci III. 1985;300(7):283–286. [PubMed] [Google Scholar]
- Schmidt W. E., Conlon J. M., Mutt V., Carlquist M., Gallwitz B., Creutzfeldt W. Identification of the C-terminally alpha-amidated amino acid in peptides by high-performance liquid chromatography. Eur J Biochem. 1987 Feb 2;162(3):467–472. doi: 10.1111/j.1432-1033.1987.tb10663.x. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. GABA directly affects electrophysiological properties of pituitary pars intermedia cells. Nature. 1982 Oct 21;299(5885):733–734. doi: 10.1038/299733a0. [DOI] [PubMed] [Google Scholar]
- Tatemoto K. Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2514–2518. doi: 10.1073/pnas.79.8.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thody A. J., Lever de Vries C. H., Tilders F. J. The failure of L-prolyl-L-leucylglycinamide to inhibit the release of alpha-melanocyte-stimulating hormone in the rat. Acta Endocrinol (Copenh) 1980 Mar;93(3):300–305. doi: 10.1530/acta.0.0930300. [DOI] [PubMed] [Google Scholar]
- Tonon M. C., Cuet P., Lamacz M., Jégou S., Côté J., Gouteaux L., Ling N., Pelletier G., Vaudry H. Comparative effects of corticotropin-releasing factor, arginine vasopressin, and related neuropeptides on the secretion of ACTH and alpha-MSH by frog anterior pituitary cells and neurointermediate lobes in vitro. Gen Comp Endocrinol. 1986 Mar;61(3):438–445. doi: 10.1016/0016-6480(86)90231-5. [DOI] [PubMed] [Google Scholar]
- Tonon M. C., Leroux P., Stoeckel M. E., Jegou S., Pelletier G., Vaudry H. Catecholaminergic control of alpha-melanocyte-stimulating hormone (alpha MSH) release by frog neurointermediate lobe in vitro: evidence for direct stimulation of alpha MSH release by thyrotropin-releasing hormone. Endocrinology. 1983 Jan;112(1):133–141. doi: 10.1210/endo-112-1-133. [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vaudry H., Tonon M. C., Delarue C., Vaillant R., Kraicer J. Biological and radioimmunological evidence for melanocyte stimulating hormones (MSH) of extrapituitary origin in the rat brain. Neuroendocrinology. 1978;27(1-2):9–24. doi: 10.1159/000122796. [DOI] [PubMed] [Google Scholar]
- Verburg-van Kemenade B. M., Jenks B. G., Danger J. M., Vaudry H., Pelletier G., Saint-Pierre S. An NPY-like peptide may function as MSH-release inhibiting factor in Xenopus laevis. Peptides. 1987 Jan-Feb;8(1):61–67. doi: 10.1016/0196-9781(87)90166-5. [DOI] [PubMed] [Google Scholar]


