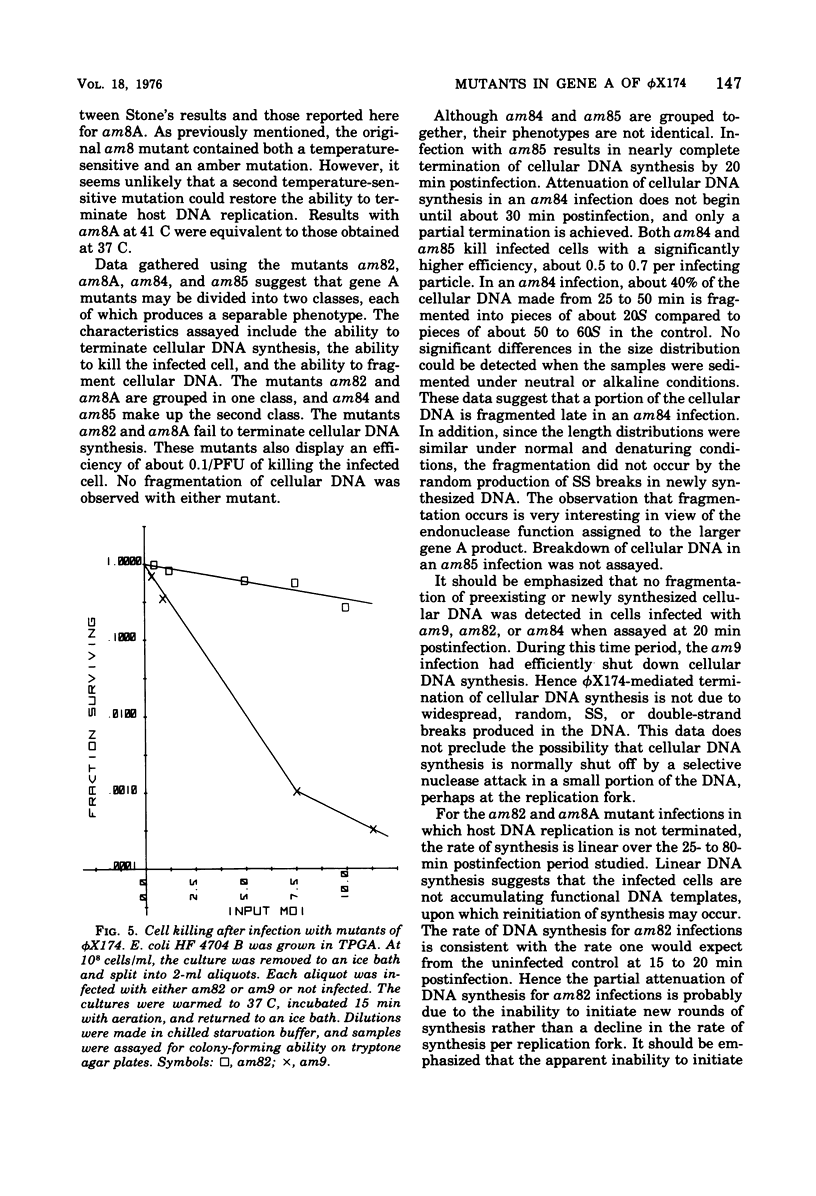
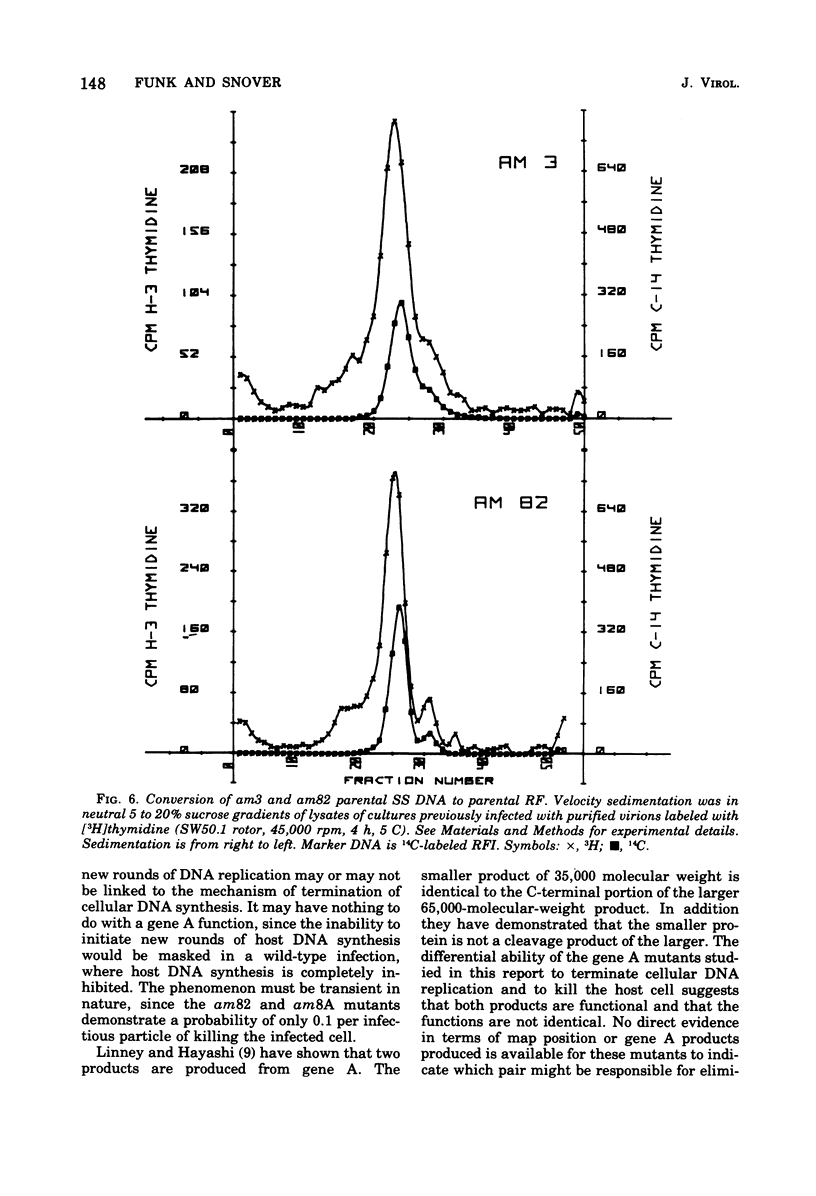
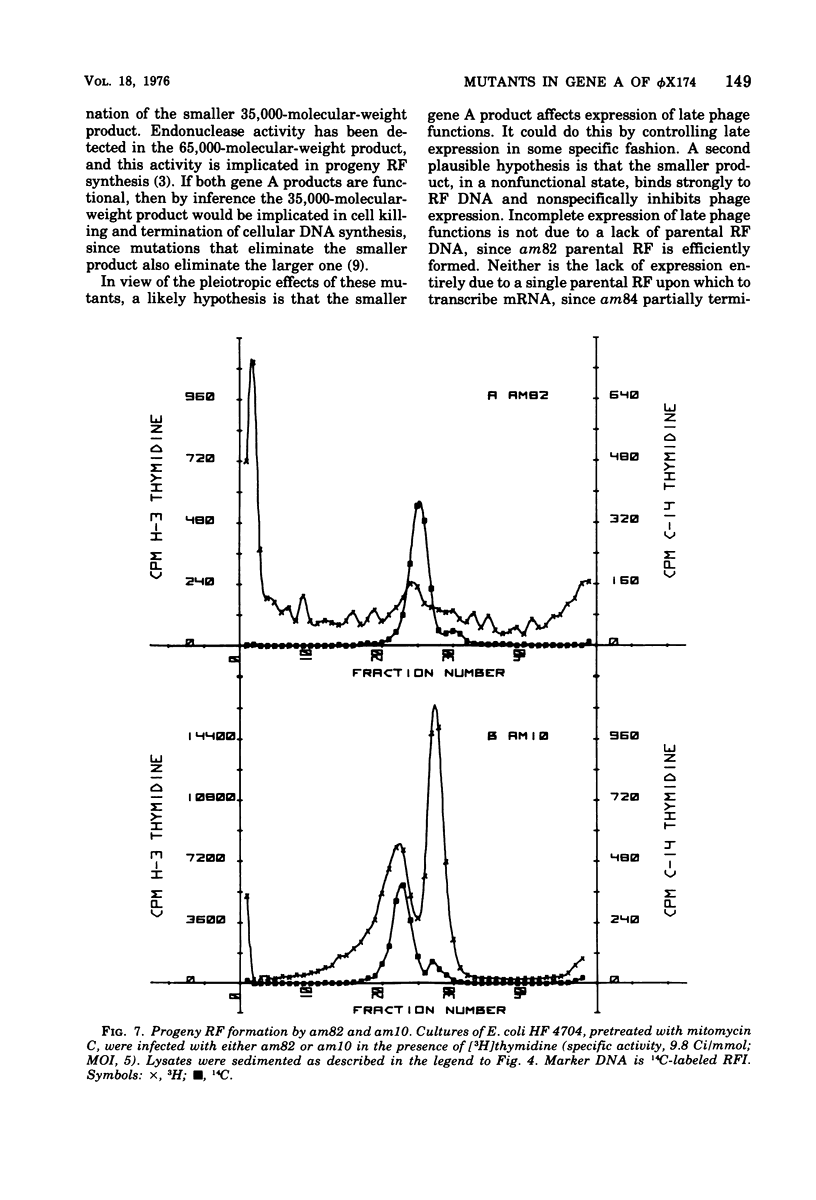
Abstract
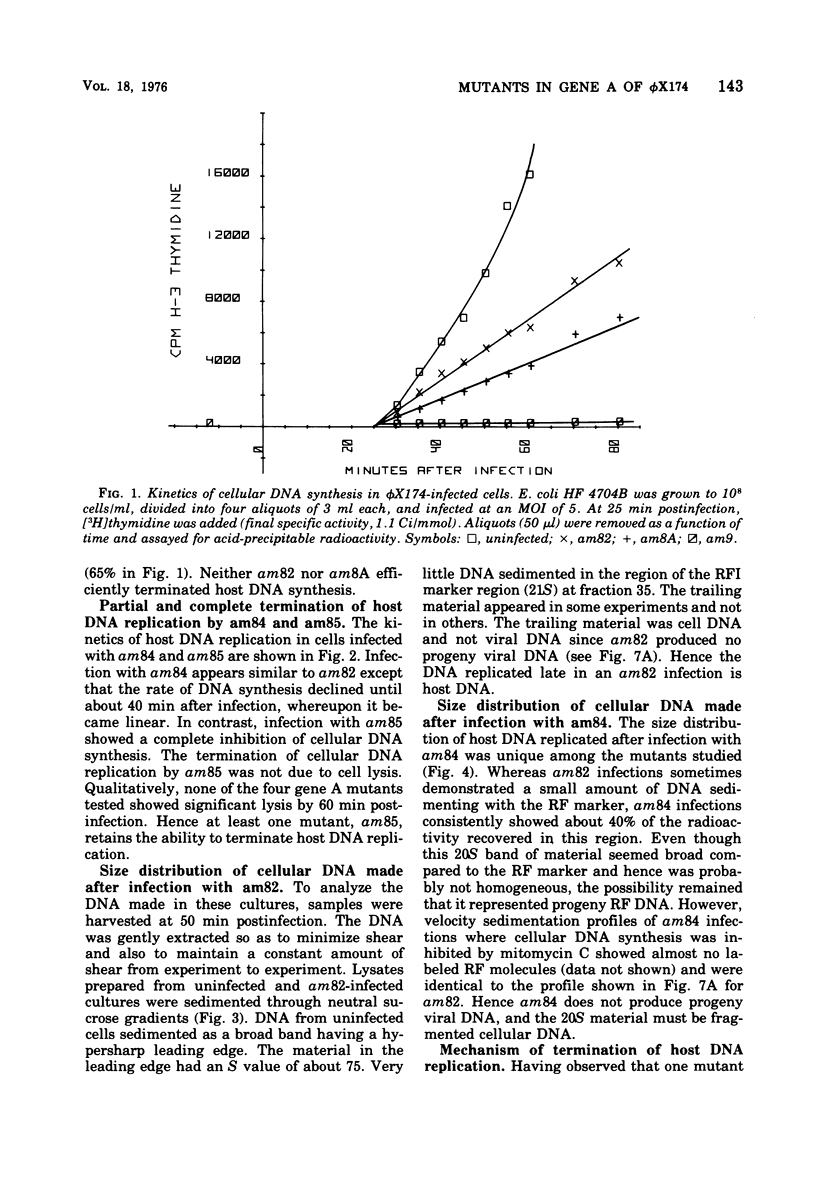
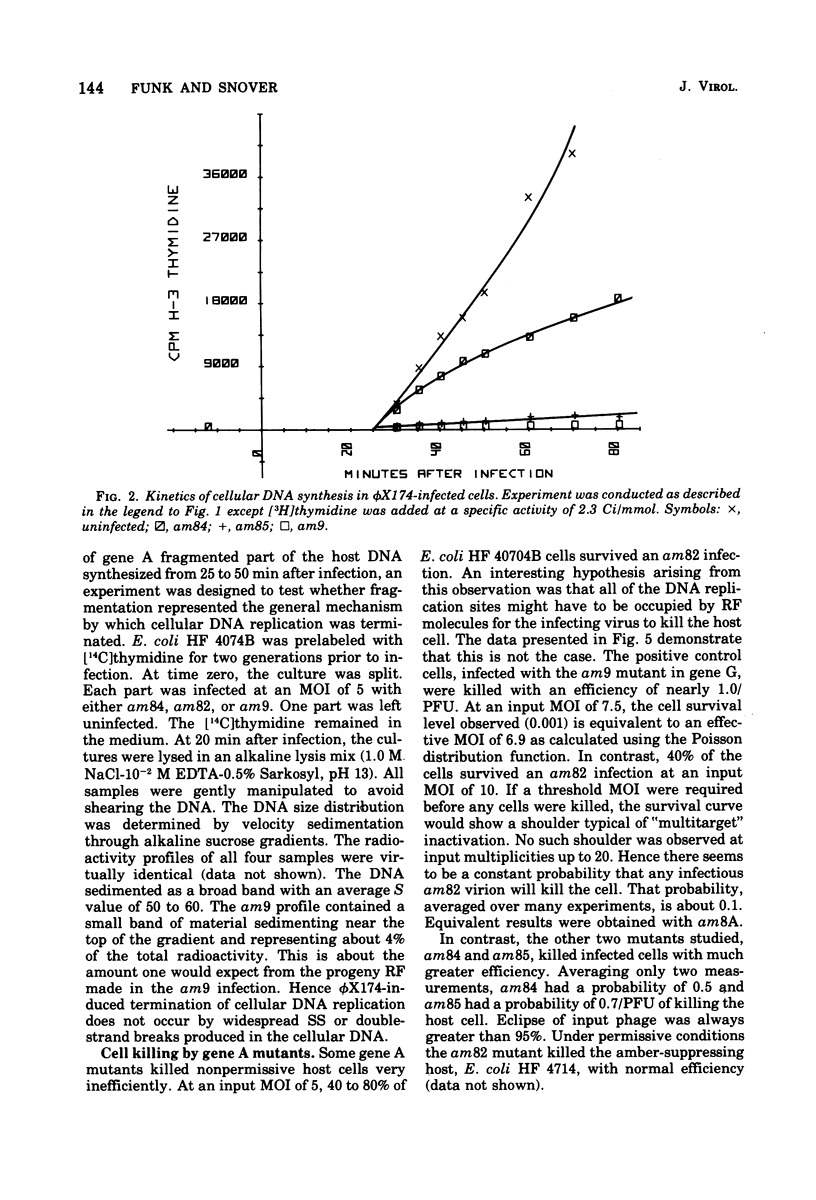
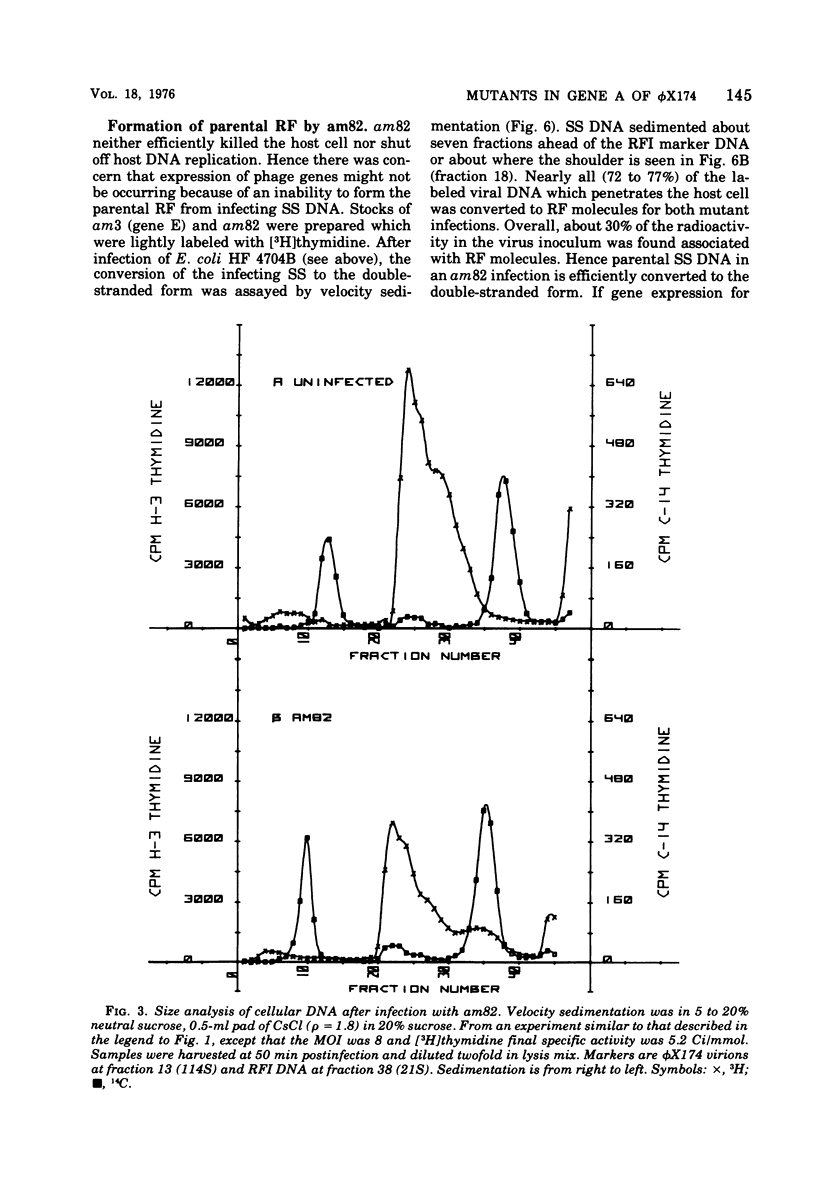
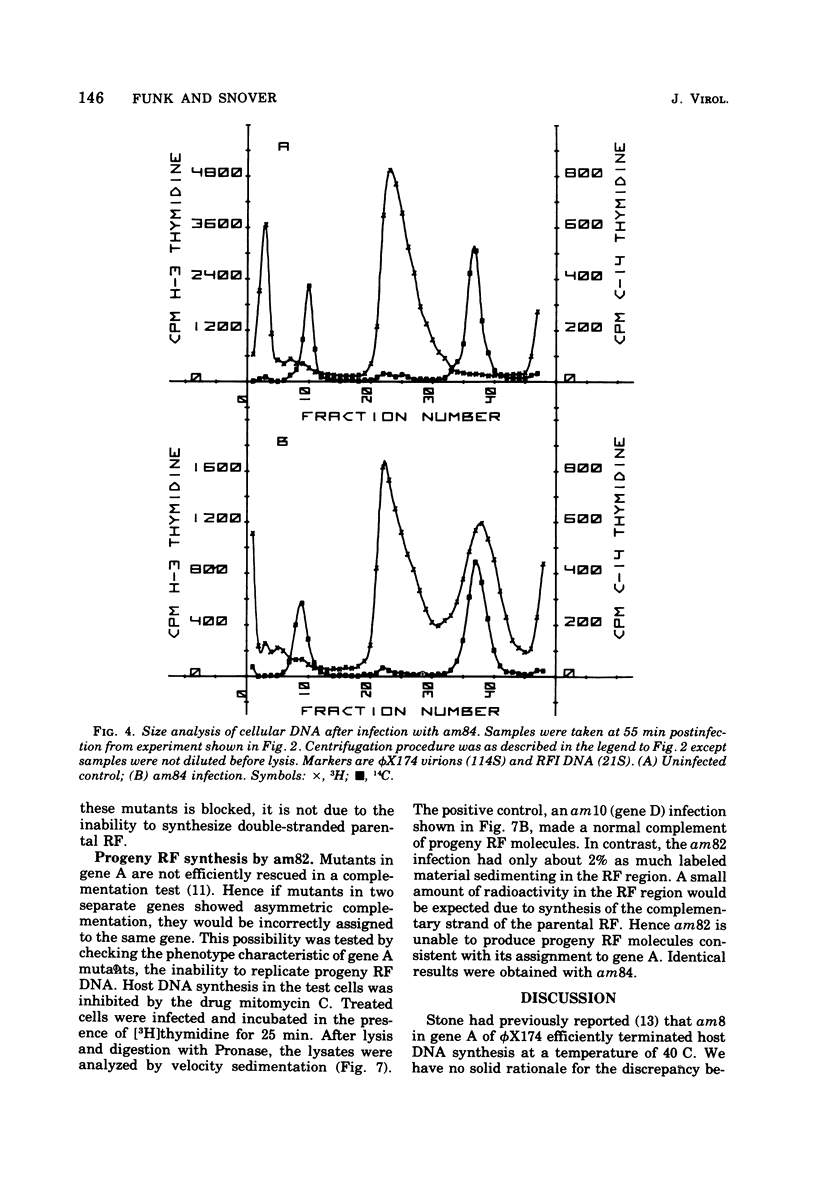
It has previously been established that the functional gene A product of phi chi X 174 is required for double-stranded DNA replication and that mutants in gene A affect the lysis of the host cell. We report here other alterations of normal phenotype for a subset of gene A mutants suggesting additional functions of gene A. Mutants in the subset failed to terminate cellular DNA synthesis and were unable to efficiently inactivate the colony-forming ability of the host. Two mutants in a second group retained the ability to kill the infected cell, although only one of these mutants efficiently terminated cellular DNA synthesis. Normal termination of cellular DNA synthesis did not occur by the production of random multiple breaks in the DNA, although it may have occurred by the selective production of breaks in newly synthesized DNA. It has previously been shown that two protein products are produced from the gene A region, the smaller of which is a C-terminal fragment of the larger. The separate phenotypes reported here for the two groups of mutants in gene A are consistent with separate functions for the two gene products previously reported.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Funk F. D., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XXXV. Cistron 8. J Virol. 1970 Jul;6(1):12–19. doi: 10.1128/jvi.6.1.12-19.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk F., Sinsheimer R. L. Process of infection with bacteriophage phiX174. 33. Templates for the synthesis of single-stranded deoxyribonucleic acid. J Virol. 1970 Mar;5(3):282–288. doi: 10.1128/jvi.5.3.282-288.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T. J., Knippers R. Isolation and function of the gene A initiator of bacteriophage phi-chi 174, a highly specific DNA endonuclease. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1549–1553. doi: 10.1073/pnas.71.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. X. Mutations in a phi-X Lysis gene. J Mol Biol. 1966 Jul;18(3):429–447. doi: 10.1016/s0022-2836(66)80035-9. [DOI] [PubMed] [Google Scholar]
- Karam J. D., O'Donnell P. V. Suppression of amber mutations of bacteriophage T4 gene 43 (DNA polymerase) by translational ambiguity. J Virol. 1973 Jun;11(6):933–945. doi: 10.1128/jvi.11.6.933-945.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XIV. Studies on macromolecular synthesis during infection with a lysis-defective mutant. J Mol Biol. 1967 Aug 28;28(1):87–94. doi: 10.1016/s0022-2836(67)80079-2. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XV. Bacteriophage DNA synthesis in abortive infections with a set of conditional lethal mutants. J Mol Biol. 1967 Nov 28;30(1):69–80. doi: 10.1016/0022-2836(67)90244-6. [DOI] [PubMed] [Google Scholar]
- Linney E., Hayashi M. Two proteins of gene A of psiX174. Nat New Biol. 1973 Sep 5;245(140):6–8. doi: 10.1038/newbio245006a0. [DOI] [PubMed] [Google Scholar]
- Martin D. F., Godson G. N. Identification of a phiX174 coded protein involved in the shut-off of host DNA replication. Biochem Biophys Res Commun. 1975 Jul 8;65(1):323–330. doi: 10.1016/s0006-291x(75)80096-9. [DOI] [PubMed] [Google Scholar]
- Person S., Osborn M. The conversion of amber suppressors to ochre suppressors. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1030–1037. doi: 10.1073/pnas.60.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsheimer R. L., Knippers R., Komano T. Stages in the replication of bacteriophage phi X174 DNA in vivo. Cold Spring Harb Symp Quant Biol. 1968;33:443–447. doi: 10.1101/sqb.1968.033.01.051. [DOI] [PubMed] [Google Scholar]
- Stone A. B. Protein synthesis and the arrest of bacterial DNA synthesis by bacteriophage phi-X174. Virology. 1970 Sep;42(1):171–181. doi: 10.1016/0042-6822(70)90250-3. [DOI] [PubMed] [Google Scholar]
- Yarus M. J., Sinsheimer R. L. The process of infection with bacteriophage phiX174. 8. Evidence for an essential bacterial "site". J Virol. 1967 Feb;1(1):135–144. doi: 10.1128/jvi.1.1.135-144.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


