Abstract
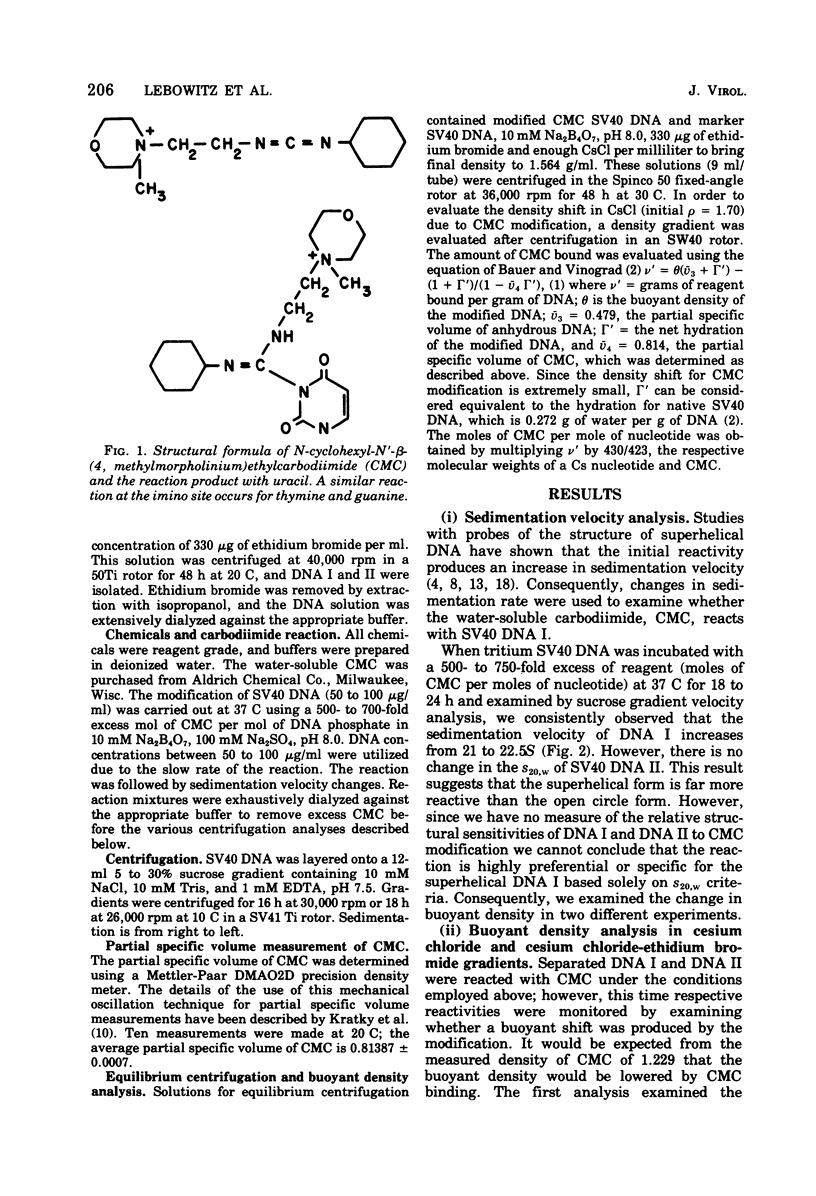
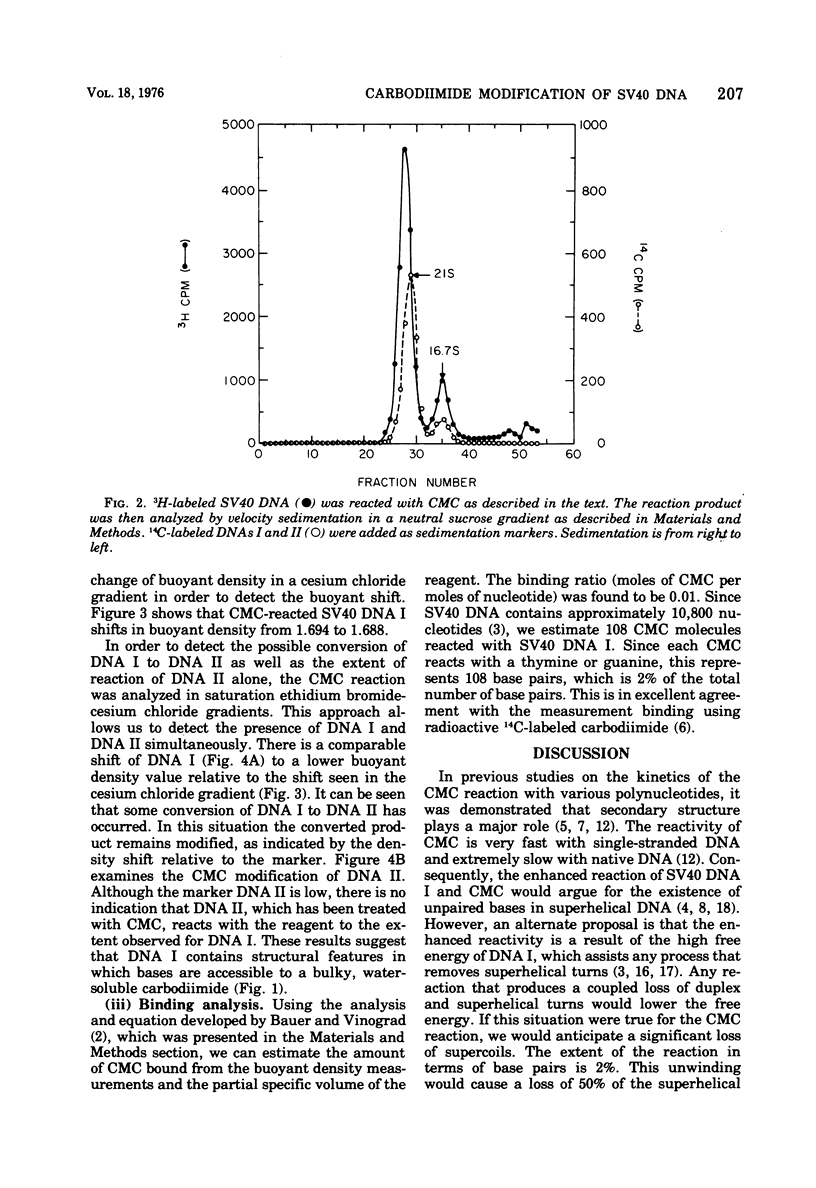
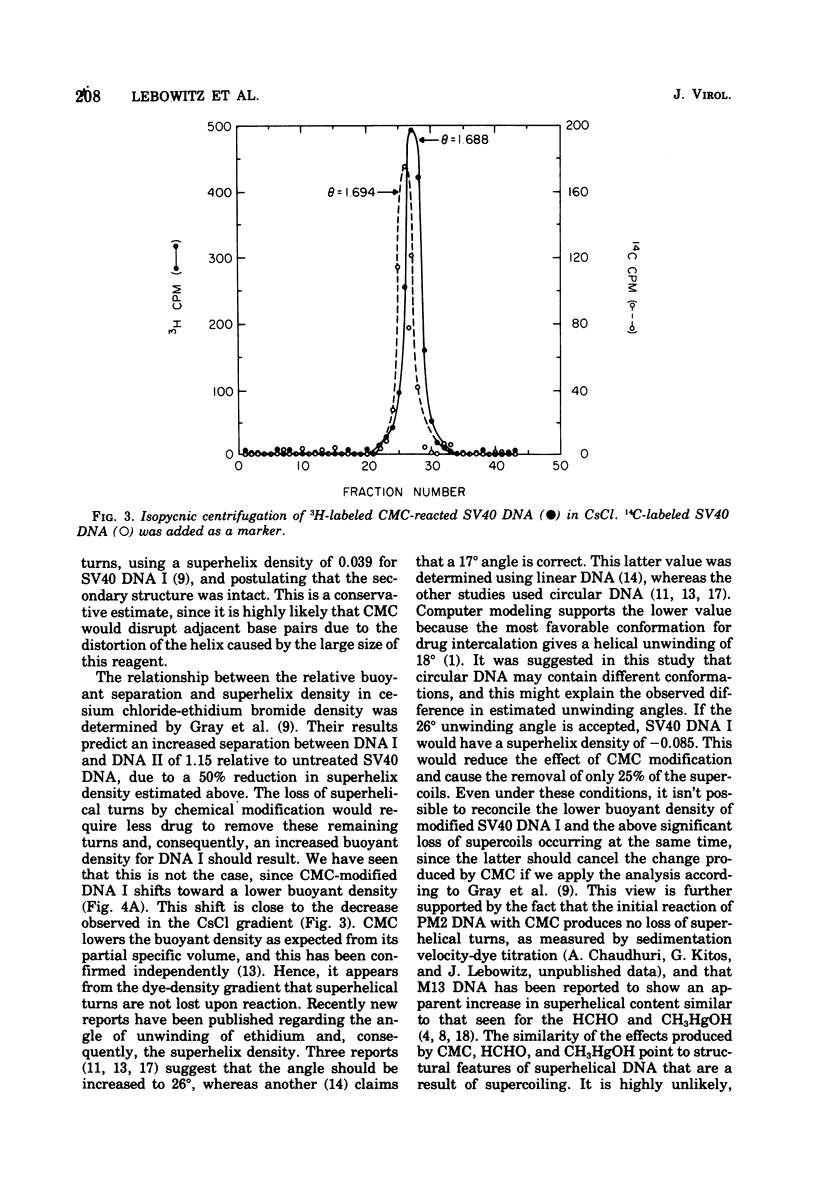
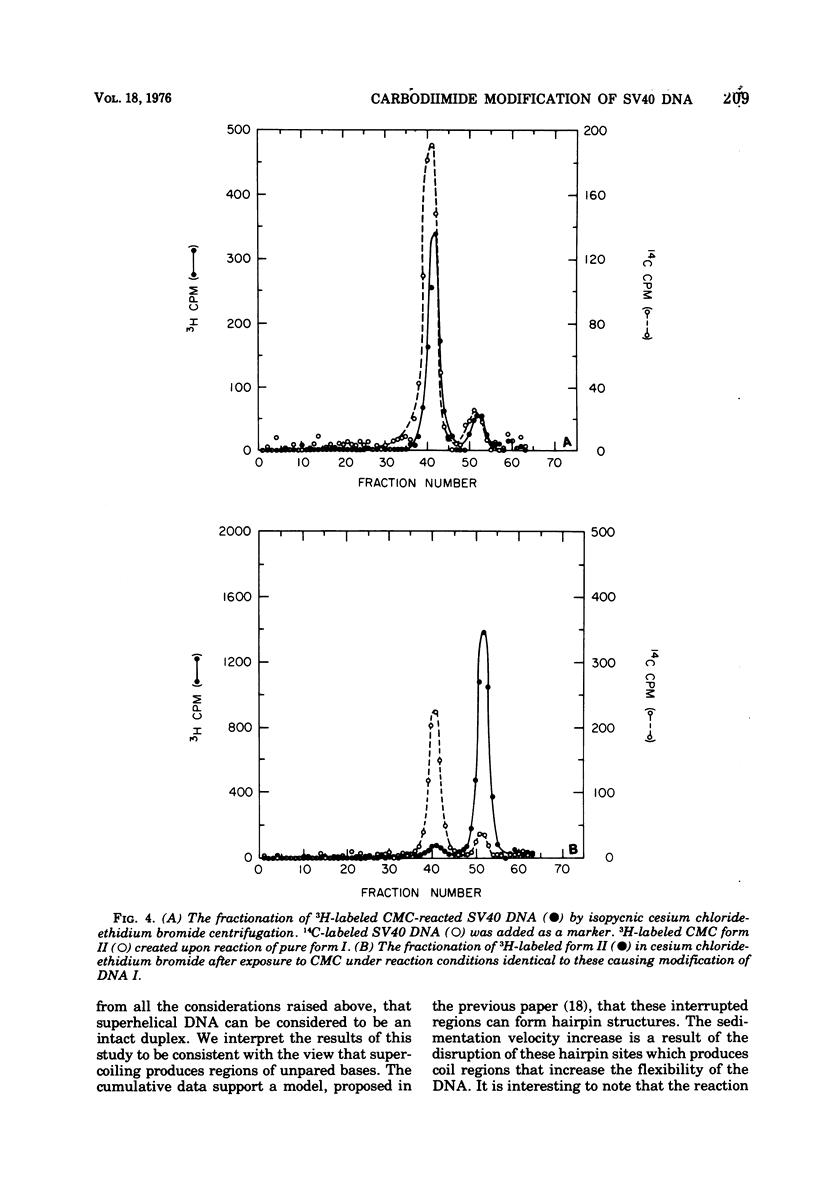
Superhelical simian virus 40 (SV40) DNA I can be modified with N-cyclohexyl-N'-beta-(4 methylmorpholinium)ethylcarbodiimide (CMC). The reaction produces an increase in the sedimentation velocity of DNA I from 21 to 22.5S and a decrease in its buoyant density in CsCl from 1.694 to 1.688. A comparable shift in buoyant density is observed in a saturated ethidium bromide-cesium chloride gradient where form II, which has been exposed to CMC, shows no shift. The CsCl-buoyant density data allows us to estimate that 108 mol of CMC are bound per mol of SV40 DNA I. In the subsequent paper an alternative procedure has been used to locate CMC sites, and the extent of the regions available to bind CMC have been measured.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alden C. J., Arnott S. Visualization of planar drug intercalations in B-DNA. Nucleic Acids Res. 1975 Oct;2(10):1701–1717. doi: 10.1093/nar/2.10.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman T. A., Lebowitz J. Further analysis of the altered secondary structure of superhelical DNA. Sensitivity to methylmercuric hydroxide a chemical probe for unpaired bases. J Mol Biol. 1973 Sep 25;79(3):451–470. doi: 10.1016/0022-2836(73)90398-7. [DOI] [PubMed] [Google Scholar]
- Chen M., Lebowitz J., Salzman N. P. Hin D restriction mapping of upaired regions in simian virus 40 superhelical DNA I: considerations regarding structure-function relationships. J Virol. 1976 Apr;18(1):211–217. doi: 10.1128/jvi.18.1.211-217.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean W. W., Lebowitz J. Partial alteration of secondary structure in native superhelical DNA. Nat New Biol. 1971 May 5;231(18):5–8. [PubMed] [Google Scholar]
- Goad W. B., Cann J. R. V. Chemically interacting systems. I. Theory of sedimentation of interacting systems. Ann N Y Acad Sci. 1969 Nov 7;164(1):192–225. doi: 10.1111/j.1749-6632.1969.tb14039.x. [DOI] [PubMed] [Google Scholar]
- Gray H. B., Jr, Upholt W. B., Vinograd J. A buoyant method for the determination of the superhelix density of closed circular DNA. J Mol Biol. 1971 Nov 28;62(1):1–19. doi: 10.1016/0022-2836(71)90127-6. [DOI] [PubMed] [Google Scholar]
- Kratky O., Leopold H., Stabinger H. The determination of the partial specific volume of proteins by the mechanical oscillator technique. Methods Enzymol. 1973;27:98–110. doi: 10.1016/s0076-6879(73)27007-6. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. On the degree of unwinding of the DNA helix by ethidium. II. Studies by electron microscopy. Biochim Biophys Acta. 1975 Jul 23;395(4):401–412. [PubMed] [Google Scholar]
- Metz D. H., Brown G. L. The investigation of nucleic acid secondary structure by means of chemical modification with a carbodiimide reagent. II. The reaction between N-cyclohexyl-N'-beta-(4-methylmorpholinium)ethylcarbodiimide and transfer ribonucleic acid. Biochemistry. 1969 Jun;8(6):2329–2342. doi: 10.1021/bi00834a013. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Morgan A. R. The sense of naturally occurring superhelices and the unwinding angle of intercalated ethidium. J Mol Biol. 1975 Jan 5;91(1):1–13. doi: 10.1016/0022-2836(75)90368-x. [DOI] [PubMed] [Google Scholar]
- Thoren M. M., Sebring E. D., Salzman N. P. Specific initiation site for simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):462–468. doi: 10.1128/jvi.10.3.462-468.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichadou J. L., Genest D., Wahl P., Aubel-Sabron G. The use of fluorescence anisotropy decay of poly d(A-T) ethidium bromide complex to estimate the unwinding angle of the double helix. Biophys Chem. 1975 Apr;3(2):142–146. doi: 10.1016/0301-4622(75)80003-2. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interactions between twisted DNAs and enzymes: the effects of superhelical turns. J Mol Biol. 1974 Aug 25;87(4):797–816. doi: 10.1016/0022-2836(74)90085-0. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Woodworth-Gutai M., Lebowitz J. Introduction of interrupted secondary structure in supercoiled DNA as a function of superhelix density: consideration of hairpin structures in superhelical DNA. J Virol. 1976 Apr;18(1):195–204. doi: 10.1128/jvi.18.1.195-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


