Abstract
Most thyroid nodules are benign. The ideal follow-up of these patients should reflect their prognosis, which has been scarcely investigated. We aimed to evaluate the prognosis of patients with initial benign thyroid cytology. A population-based study, using the Rochester Epidemiology Project, identifying patients with benign thyroid cytology diagnosed between 2003 and 2006 and completely followed to 2014 using linked medical records. We identified 363 thyroid nodules with benign cytology in 327 patients after fine-needle aspiration biopsy. Patients were on average 53 years old (standard deviation 17), and 80 % were women. The median nodule size was 1.6 cm (interquartile range 1.2–2.4); 26 % had at least one suspicious ultrasound feature. During a median follow-up of 8 years, 54 patients (17 %) with 57 benign nodules underwent thyroidectomy, mostly due to compressive symptoms (44 %). Thyroidectomy was more likely in younger patients [hazard ratio (HR) 0.97, 95 % CI 0.96–0.99] and patients with larger nodules (HR 1.3, 95 % CI 1.16–1.48). Two patients were found to have follicular thyroid cancer in the index nodule (0.6 %) and 6 patients had papillary thyroid cancer detected in other nodules (1.8 %). No patient died from thyroid cancer. Patients with benign thyroid nodules are unlikely to suffer morbidity or mortality due to thyroid cancer. Follow-up strategies for these patients should consider this excellent prognosis and avoid causing unnecessary fear in patients and adding unneeded expense and burden to the healthcare system.
Keywords: Thyroid nodule, Fine-needle aspiration biopsy, Thyroid cancer, Benign thyroid nodule, Thyroidectomy
Introduction
The widespread use of imaging technology has partly fueled an increased detection and diagnosis of thyroid cancer over the last decade [1, 2]. This increase in thyroid cancer detection and the potential harm associated with overtreatment of indolent thyroid cancer represents only a part of the problem created by increased detection of thyroid nodules (since most nodules will be found to be benign). After a thyroid nodule is discovered, our current diagnostic strategies rely on ultrasound (US) features and ultrasound-guided fine-needle aspiration biopsy (USFNA) results to guide further management [3]. However, even in the case of benign thyroid nodules, the most common result after USFNA, there is still uncertainty regarding the ideal follow-up strategy [3, 4]. For example, two out of the three recommendations by the American Thyroid Association guidelines that address this topic are based on low-quality evidence [3].
Although a benign diagnosis on USFNA cytology is usually reassuring, there are concerns regarding the possibility of a missed diagnosis of thyroid cancer that when acted upon may result in significant worse patient outcomes [3, 5]. For this reason, current follow-up strategies recommend repeat neck US and repeat USFNA according to the initial risk of malignancy based on US features [3]. The burden of this approach to the health care system as well as to the individual patient is significant. For example, because thyroid nodules can be found in up to 70 % of patients when assessed by US, and considering that at least 60 % of USFNA are reported as benign, the number of patients entering this follow-up strategy in the United States is very large [4, 6, 7]. In addition, from an individual patient perspective there is a significant treatment burden associated with follow-up of thyroid nodules in terms of anxiety, costs, time away from work, repeat US, and USFNAs.
The long-term outcomes of patients with benign thyroid nodules have been evaluated scarcely. One large single-center study with a mean follow-up time of 8.5 years found a low rate of new diagnosis of thyroid cancer and no mortality associated with thyroid cancer during the study period [8]. An Italian, multicenter study also found a low rate of new diagnosis of thyroid cancer; however, only about half of the patients had undergone USFNA [9].
The objective of our study was to evaluate the long-term prognosis of patients with biopsy-proven benign thyroid nodules using a population-based design. We explored the rates of patient-important outcomes during follow-up to determine if less intensive and less aggressive follow-up strategies of these patients can be safely considered.
Methods
We performed a population-based study approved by the local institutional review board using the Rochester epidemiology project (REP) medical records linkage system which was initiated in 1966 to study disease epidemiology and patterns of health care among the residents of Olmsted County, Minnesota. The REP provides a research infrastructure that covers residents of all ages, regardless of socioeconomic status, ethnicity, or insurance status. The follow-up of patients is based on routine care; each time an Olmsted county resident visits a REP health care provider, this information is automatically integrated into the research infrastructure [10].
We identified all subjects residing in Olmsted County who had undergone USFNA of thyroid nodules resulting in benign cytology between 2003 and 2006. We chose this time period to allow sufficient length of follow-up to assess the outcomes of interest. An initial search of the REP database was performed to identify patients with a procedure code of USFNA and thyroid disease and followed by a detailed chart review for each patient. Exclusion criteria included a previous history of thyroid cancer, prior biopsy of the index nodule outside the study period, and biopsy performed without US guidance. An electronic data collection form was created using REDCAP and medical records were reviewed up to October 2014 [11]. Three reviewers collected baseline clinical information at the time of benign cytology, including age, sex, nodule size, thyroid-stimulating hormone (TSH) levels, risk factors for thyroid cancer (i.e., family history of thyroid cancer and history of neck irradiation), and the presence of suspicious features on US (based on the recorded US report including: hypoechogenicity, increased vascular blood flow, calcification, irregular margins, nodules described as taller than wider). During follow-up, information regarding the number of thyroid/neck ultrasound exams, need for repeat USFNA, number of patients undergoing thyroidectomy, reason for thyroidectomy, outcomes of thyroid surgery including histological information, death from any cause and due to thyroid cancer, when applicable, was collected. Clinical relevant growth of thyroid nodules was based on the information found on medical record review (no pre-defined cut-off was used). One reviewer (one of the three initial reviewers) assessed a random sample (5 % of the cases) to verify accuracy. There was a 100 % agreement when evaluating inclusion criteria to the study and 99 % agreement when evaluating adequate outcome assessment.
Analysis
A descriptive summary analysis of baseline characteristics was performed in JMP® 10.0.0 (2012 SAS Institute Inc.) and using each nodule as the unit of analysis, with the exception of patient clinical features (age, sex, past medical history) where patients with more than one nodule were counted once. Results are expressed as mean (standard deviation) or median (interquartile range, IQR) for continuous variables and as proportions for categorical variables; differences between categorical variables were assessed using the χ2 test. The outcomes of interest were a new diagnosis of thyroid cancer, death due to thyroid cancer, and thyroidectomy. We evaluated predictors of time-to-event outcomes using Cox regression models and calculated hazard ratios and 95 % confidence intervals. The last date of follow-up was the date of death, thyroidectomy, or the last clinic visit in patients without any adverse outcomes during follow-up.
Results
Baseline features
During the 3-year study period (2003–2006), 520 USFNA biopsies were performed on residents of Olmsted County, Minnesota, and 363 (69.8 %) nodules in 327 patients were diagnosed as cytologically benign. The mean age at the time of USFNA was 53 years (standard deviation, 17) with the majority of the patients being women (n-260, 80 %). Baseline clinical characteristics are found in Table 1.
Table 1.
Baseline clinical characteristics
| Clinical feature | Percentage, mean (SD) or median (IQR) |
|---|---|
| Age at time of USFNA (years) | 53 (17) |
| % Women | 80 % |
| TSH (mIU/L) | 1.3 (0.74–2.2) |
| Nodule size (largest dimension, cm) | 1.6 (1.2–2.4) |
| Presence of at least 1 US suspicious feature | 26 % |
| Negative history of neck radiation | 97 % |
| Negative family history of thyroid cancer | 95 % |
| Negative history of thyroid disease | 81 % |
| History of hypothyroidism | 8 % |
| Presence of multiple nodules | 77 % |
USFNA ultrasound-guided fine-needle aspiration biopsy, SD standard deviation, IQR interquartile range
Patient important outcomes
Need for thyroidectomy during follow-up
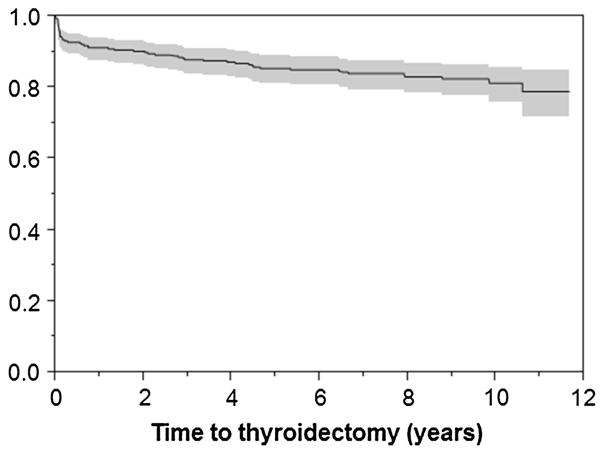
The median time of follow-up was 8.3 years (IQR 4.5–9.5). A total of 57 benign nodules in 54 patients (17 %) underwent thyroidectomy during follow-up. The development of compressive symptoms was the most common indication for surgery (n-25, 44 %). The distribution of other indications for thyroidectomy is found on Table 2 and a time-to-event analysis for thyroidectomy is shown in Fig. 1. Younger age and a larger nodule size at the time of USFNA were found to be predictors for thyroidectomy during follow-up on univariate analysis. [HR for age 0.97 (95 % CI 0.96–0.99) and 1.3 (95 % CI 1.16–1.48) for nodule size].
Table 2.
Indications for thyroidectomy and repeat USFNA
| Indications for thyroidectomy N (%) | Total nodules (N = 57) | Nodules with repeat US (N = 27) | Nodules without repeat US (N = 30) | |
|---|---|---|---|---|
| Compressive symptoms | 25 (44) | 16 (59) | 9 (30) | |
| Management of a different nodule | 11 (19) | 1 (4) | 10 (33) | |
| Physician’s concern (other than size) | 6 (10) | 3 (11) | 3 (10) | |
| Concerns due to size | 6 (10) | 2 (7) | 4 (13) | |
| Patient’s desire | 5 (9) | 4 (15) | 1 (3) | |
| Other | 4 (7) | 1 (4) | 3 (10) | |
|
| ||||
| Indications for repeat USFNA N (%) | Total N = 42 | |||
|
| ||||
| Nodule growth | 26 (62) | |||
| Physician concern | 11 (26) | |||
| Other (patient desire, unclear) | 5 (12) | |||
USFNA ultrasound-guided fine-needle aspiration biopsy
Fig. 1.
Time to thyroidectomy (Kaplan–Meier). Left axis represents the proportion of nodules without thyroidectomy. Shaded area represents 95 % confidence interval
New diagnosis of thyroid cancer
From the 57 nodules undergoing surgery (in 54 patients), 2 cases of follicular thyroid cancer were found. None had distant metastatic disease at the time of diagnosis. One case was a 62-year-old male with a 7.5 × 6.5 × 2.7 cm nodule that underwent surgery 3 months after initial USFNA due to size concerns. The pathology showed a minimally invasive follicular carcinoma (both capsular and vascular invasion); after 6 years of follow-up there is no evidence of recurrent or metastatic disease. In the other case, a 23-year-old female underwent thyroidectomy due to nodule growth during follow-up (initial nodule size 0.5 × 1 × 1.1 cm and 2 years after follow-up 1 × 1.6 × 1.7 cm). Her pathology showed a minimally invasive grade 1 follicular carcinoma (vascular invasion). She has had no evidence of recurrence after 7 years of follow-up.
Overall, the rate of malignancy in the index nodule was 2/57 when using histology as the gold standard (3.5 %) and 2/363 (0.5 %) when using histology and long-term follow-up as the gold standard. In addition, surgery/histology revealed incidental papillary thyroid cancer in 6 out of the 54 patients that underwent thyroidectomy (11 %). Three of these cases were micropapillary thyroid cancer (size range 0.1–0.5 cm). The size in the other cases ranged from 1.2 to 1.8 cm. Positive cervical lymph nodes were found in 2 cases (lateral and central neck in one case and only central neck in the other) and surgical margins were negative in all cases. One patient received radioactive iodine ablation treatment. There was no evidence of recurrence or distant metastasis in any of these incidental tumors.
Mortality attributed to thyroid cancer
No cases of mortality attributed to thyroid cancer or thyroid disease were reported. Twenty-seven patients died of other causes during follow-up.
Outcomes based on follow-up strategies
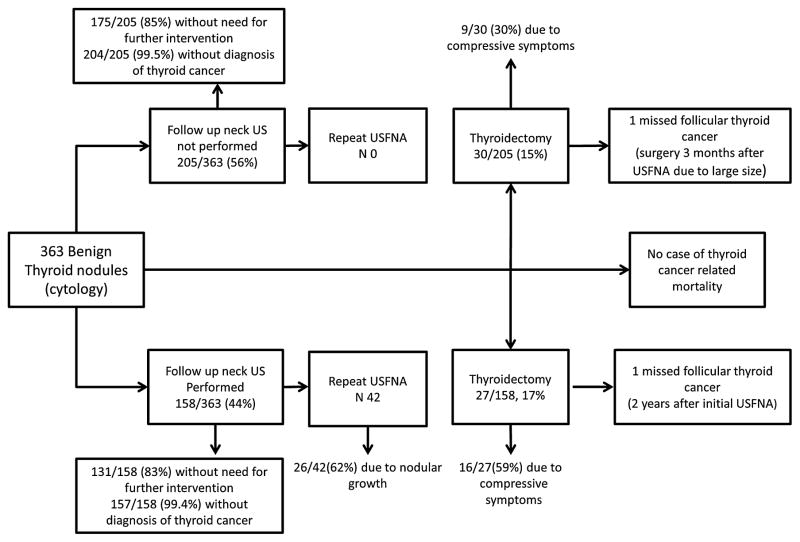
A follow-up strategy based on repeat US occurred in 44 % of nodules (n-158) and follow-up without repeat US occurred in 56 % (n-205).(Table 3) Among the group who received a follow-up US, the median time to US was 2.2 years (IQR 1.0–4.6). Furthermore, 42 of these patients had a repeat USFNA (12 %) and 27 underwent thy-roidectomy (17 %); the most common indication for thyroidectomy was the presence of compressive symptoms (59 %).
Table 3.
Baseline characteristics according to repeat US status (yes/no)
| Clinical feature | Nodules with follow-up US (N = 158) |
Nodules without follow-up US (N = 205) |
p value |
|---|---|---|---|
| Age | 49 (15.2) | 56.2 (17) | <0.001 |
| Female | 80 % | 79 % | 0.719 |
| Size (cm) | 1.7 (1.2–2.8) | 1.5 (1.2–2.2) | 0.175 |
| TSH (IU/ml) | 1.4 (0.8–2.25) | 1.2 (0.69–2.1) | 0.150 |
| Thyroidectomy during follow-up | 18 % | 15 % | 0.475 |
| Presence of at least one suspicious US feature | 24 % | 28 % | 0.364 |
Data reported as mean (standard deviation), median (interquartile range), or percentages
In the cases without a repeat neck US during follow-up, no repeat USFNA was conducted, and 31 nodules underwent surgery (15 %); the most common indication for thyroidectomy was the management of a different thyroid nodule (33 %) with second most common indication being the development of compressive symptoms (30 %).
TSH was re-measured in 78 % of the patients during follow-up (median 5 measurements, interquartile range 2–8). The distribution of patient-important outcomes according to follow-up strategy is shown in Fig. 2.
Fig. 2.
Distribution of outcomes according to follow-up strategy
Discussion
We performed a population-based study evaluating the prognosis of benign thyroid nodules. We identified a cohort of 363 benign nodules in 327 residents of Olmsted County, Minnesota that were on average middle-aged women without significant risk factors for thyroid cancer and with 1 out of 3 nodules presenting at least one suspicious US feature for malignancy at baseline. During a median follow-up time of 8 years, no deaths due to thyroid cancer were identified and 17 % of the patients underwent thy-roidectomy. A new diagnosis of thyroid cancer was found in only 2 patients, none presenting with metastatic disease at baseline or developing recurrence during follow-up. These findings are compatible with previous reports (Table 4) suggesting an overall excellent prognosis for patients with a benign thyroid nodule using different sampling methodologies (e.g., single-center, multicenter, and population-based studies) and follow-up times [8, 9, 12–15].
Table 4.
Summary of studies evaluating long-term outcomes of patients with benign thyroid nodules
| Author year | Study design, country | Sample size | Mean age (years) | Mean nodule size (cm) | Patients with surgery during follow (%) | Reasons for surgery (%) | New diagnosis of Thyroid cancer (rate per patient) | Death due to thyroid cancer | Follow-up time description |
|---|---|---|---|---|---|---|---|---|---|
| Lee 2013 | Single center, USA | 738 patients | 50a | 2.3a | 136 (18.4 %) (92 immediate and 44 during follow-up) | 57 % symptomatic nodule | 2 (0.3 %) | NA | 140 patients with ≥3 years of follow |
| Negro 2014 | Single center, Italy | 249 patients | 55 | 1.6 | 6 (2.4 %) | NA | 0 | NA | Outcome assessed at5 years |
| Nou 2014 | Single center, USA | 1369 patients (2010 nodules) | 50 | 2.4 | 325 (24 %) | 60 % symptomatic nodule | 18 (1.3 %) | None | 8.5 years, mean follow-up time |
| Yeon Kim2014 | Single center, Korea | 819 patients (854 nodules) | 47.9 | 2.0 | 76 (9 %) | 22 % nodule growth | 10 (1.2 %) | NA | 4 years, mean follow-up time |
| Ajmal 2015 | Single center, USA | 263 patients | 53 | 2.7 | 129 (49 %) (48 immediate and 81 during follow-up) | 67 % symptomatic nodule | 13 (5 %) | NA | 3.0 years, mean follow-up time |
| Durante 2015 | Multicenter Italy | 579 patients | 52b | 1.4b | 32 (6 %) | NA | 4 (0.7 %) | None | Outcome assessed at5 years |
| Present study | Population based, USA | 327 patients with 363 nodules | 53 | 1.6a | 54 (17 %) | 44 %, symptomatic nodule | 2 (0.6 %) | None | 8.3 years, median follow-up time |
NA not available
Median
Complete cohort of 992 patients with 579 having biopsy-proven benign nodules
In clinical practice, when a diagnosis of a benign thyroid nodule is made, there is reassurance to the patient that a diagnosis of thyroid cancer is unlikely. This is based on the low but not negligible false-negative rates that have been reported in patients with benign thyroid nodules, which vary significantly between institutions [3, 4]. Due to concerns of a missed diagnosis of thyroid cancer, revisiting the diagnosis (e.g., repeat USFNA) in cases where the clinical features do not match the benign cytological results is recommended (e.g., suspicious pattern for malignancy on US) and appears to be the first step in management [3, 5]. In the rest of the cases (low risk of thyroid malignancy at baseline based on US features and a benign biopsy), our current strategies for identifying patients with adverse outcomes include a repeat US (assessing for growth, new nodules, or change in US features) and/or repeating an USFNA according to the risk of malignancy [3]. However, high-quality evidence to support nodule growth as a predictor of malignancy after a benign biopsy is lacking [16]. In addition, reports from multiple centers have shown that the results of repeat US are usually unchanged. As a result, using US demonstrated growth as an indication for repeat USFNA may be associated with a high number of false-positive results, as the final rate of malignancy in those who undergo surgery due to changes on the repeat USFNA is low [5, 17–19].
A large number of patients with benign thyroid nodules on biopsy undergo follow-up to detect a missed diagnosis of thyroid cancer, which appears to be quite rare [8, 9]. The current strategies employed for this follow-up are not proven to result in significant benefit for the patient. In addition, thyroid cancer cases that are missed in the initial evaluation do not appear to be associated with worse treatment outcomes. In our cohort, there was no evidence of recurrent or metastatic disease during the follow-up of the 2 patients that had a new diagnosis of thyroid cancer. Similarly, in the study by Nou et al., none of the 18 cases of new diagnosis of thyroid cancer was associated with recurrent or metastatic disease [8]. These unproven strategies can also result in direct harm. For example, repeat US and USFNA may increase the number of false-positive results (growth on US or USFNA suspicious diagnosis) after which patients will be referred for unnecessary surgery or more intense follow-up (associated with side effects, cost, anxiety).
Overall, the ideal framework to determine the subsequent follow-up of patients with thyroid nodules appears to be based on a combination of clinical and US features in addition to USFNA results [3]. Interestingly, the most common indication for thyroidectomy during follow-up in many series is the development of compressive symptoms, which is an event unrelated to diagnostic uncertainty of the USFNA [8, 13, 14]. This suggests that routine clinical follow-up without repeat US or USFNA might be enough to identify patients in which further treatment is required.
The main limitations of our study are based on its retrospective nature, which resulted in different length of follow-up in our patients. Nevertheless, the main focus of our study, which was patient important outcomes, could be assessed even when repeat US or USFNA were not performed in all patients. We also relied on clinical documentation when evaluating the presence of nodular growth or compressive symptoms during follow-up. In addition, because the number of adverse outcomes was low during the study period, we could not perform analysis of time-to-event occurrence in the cases of death due to thyroid cancer or identify predictors for all of the proposed outcomes. Moreover, the pathology for most of these patients was read at a center with a very low false-negative rate, which might not be representative of cytopathology evaluation in other areas [20].
Important strengths of our study include our population-based design which decreases the chances of referral bias and allowed us to have comprehensive follow-up of the included cases [10, 21]. In addition, our follow-up time, although still not ideal (e.g., 15–20 years, as thyroid cancer mortality is low) is the second longest when compared to other series. Most of the other available series have focused on the false-negative rate after an initially benign USFNA, have had shorter follow-up and have not provided information on mortality.
Given the significant number of patients with benign thyroid nodules, an adequate management strategy both at the time of initial diagnosis and follow-up is urgently needed. To improve our diagnostic accuracy at the time of initial diagnosis, large prospective studies are required evaluating different frameworks to determine probabilities of malignancy on the basis of the available tools (clinical features, US features, USFNA result). To determine the best follow-up strategy of these patients, given the rarity of adverse outcomes, large multicenter studies would be required to compare different management strategies (different frequency and timing of initial US, indications for repeat biopsy during follow-up, and safety of follow-up based only on routine clinical evaluation). Until such studies are available, the current evidence makes it difficult to provide clear and confident guidance to clinicians and patients with benign thyroid nodules, but support the following suggestions. Physicians should counsel patients with thyroid nodules with benign cytology about their excellent prognosis and engage them in shared decision making about follow-up modality, intensity, and duration. Due to the uncertainty surrounding the benefits and risks of standard follow-up, it is reasonable to suggest follow-up protocols tailored to each patient’s risk of thyroid malignancy and patient context (e.g., personal and social situation, competing risks, health status and life expectancy). For example, a healthy young patient with a high-risk nodule on ultrasound and benign cytology should be offered further evaluation given the high post-test probability of malignancy even with a benign USFNA result. Patients with nodules with benign features on US and benign cytology, the most common situation, who opt to receive follow-up should be offered clinical and ultrasound examination 3 years after the initial biopsy or earlier if symptomatic.
Conclusion
In a population-based study of patients with benign thyroid nodules, there were no deaths related to thyroid cancer and only two missed diagnoses of thyroid cancer, both of which presented without metastatic disease or recurrence during follow-up. Thyroidectomy most commonly indicated by the development of compressive symptoms was required in 17 % of the cases. These findings suggest that a benign cytological diagnosis of a thyroid nodule carries a very good prognosis in terms of patient-important outcomes and should encourage us to reconsider the goals of follow-up strategies in patients without significant risk factors for thyroid cancer to avoid overwhelming the medical system and individual patients.
Supplementary Material
Acknowledgments
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Compliance with ethical standards
Conflict of interest
The authors have nothing to disclose.
References
- 1.Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ. 2013;347:f4706. doi: 10.1136/bmj.f4706. [DOI] [PubMed] [Google Scholar]
- 2.Sosa JA, Hanna JW, Robinson KA, Lanman RB. Increases in thyroid nodule fine-needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery. 2013;154(6):1420–1426. doi: 10.1016/j.surg.2013.07.006. discussion 1426–1427. [DOI] [PubMed] [Google Scholar]
- 3.Haugen BRM, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE, Pacini F, Randolph G, Sawka A, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward D, Tuttle RMM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015 doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19(11):1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 5.Kwak JY, Koo H, Youk JH, Kim MJ, Moon HJ, Son EJ, Kim EK. Value of US correlation of a thyroid nodule with initially benign cytologic results. Radiology. 2010;254(1):292–300. doi: 10.1148/radiol.2541090460. [DOI] [PubMed] [Google Scholar]
- 6.Cronan JJ. Thyroid nodules: is it time to turn off the US machines? Radiology. 2008;247(3):602–604. doi: 10.1148/radiol.2473072233. [DOI] [PubMed] [Google Scholar]
- 7.Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154(16):1838–1840. doi: 10.1001/archinte.154.16.1838. [DOI] [PubMed] [Google Scholar]
- 8.Nou E, Kwong N, Alexander LK, Cibas ES, Marqusee E, Alexander EK. Determination of the optimal time interval for repeat evaluation after a benign thyroid nodule aspiration. J Clin Endocrinol Metab. 2014;99(2):510–516. doi: 10.1210/jc.2013-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durante C, Costante G, Lucisano G, Bruno R, Meringolo D, Paciaroni A, Puxeddu E, Torlontano M, Tumino S, Attard M, Lamartina L, Nicolucci A, Filetti S. The natural history of benign thyroid nodules. JAMA. 2015;313(9):926–935. doi: 10.1001/jama.2015.0956. [DOI] [PubMed] [Google Scholar]
- 10.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, Rocca WA. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajmal S, Rapoport S, Batlle HR, Mazzaglia PJ. The natural history of the benign thyroid nodule: what is the appropriate follow-up strategy? J Am Coll Surg. 2015;220(6):987–992. doi: 10.1016/j.jamcollsurg.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Kim SY, Han KH, Moon HJ, Kwak JY, Chung WY, Kim EK. Thyroid nodules with benign findings at cytologic examination: results of long-term follow-up with US. Radiology. 2014;271(1):272–281. doi: 10.1148/radiol.13131334. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Skelton TS, Zheng F, Schwartz KA, Perrier ND, Lee JE, Bassett RL, Ahmed S, Krishnamurthy S, Busaidy NL, Grubbs EG. The biopsy-proven benign thyroid nodule: is long-term follow-up necessary? J Am Coll Surg. 2013;217(1):81–88. doi: 10.1016/j.jamcollsurg.2013.03.014. discussion 88–89. [DOI] [PubMed] [Google Scholar]
- 15.Negro R. What happens in a 5-year follow-up of benign thyroid nodules. J Thyroid Res. 2014;2014:459791. doi: 10.1155/2014/459791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh Ospina N, Maraka S, Espinosa De Ycaza A, O’Keeffe D, Brito JP, Gionfriddo MR, Castro MR, Morris JC, Erwin P, Montori VM. Diagnostic accuracy of thyroid nodule growth to predict malignancy in thyroid nodules with benign cytology: Systematic review and meta-analysis. Clin Endocrinol (Oxf) 2015 doi: 10.1111/cen.12975. [DOI] [PubMed] [Google Scholar]
- 17.Singh Ospina N, Sebo TJ, Morris JC, Castro MR. The value of repeat thyroid fine-needle aspiration biopsy in patients with a previously benign result: how often does it alter management? Thyroid. 2015;25(10):1121–1126. doi: 10.1089/thy.2015.0146. [DOI] [PubMed] [Google Scholar]
- 18.Gabalec F, Cap J, Ryska A, Vasatko T, Ceeova V. Benign fine-needle aspiration cytology of thyroid nodule: to repeat or not to repeat? Eur J Endocrinol. 2009;161(6):933–937. doi: 10.1530/EJE-09-0514. [DOI] [PubMed] [Google Scholar]
- 19.Oertel YC, Miyahara-Felipe L, Mendoza MG, Yu K. Value of repeated fine needle aspirations of the thyroid: an analysis of over ten thousand FNAs. Thyroid. 2007;17(11):1061–1066. doi: 10.1089/thy.2007.0159. [DOI] [PubMed] [Google Scholar]
- 20.Porterfield JR, Jr, Grant CS, Dean DS, Thompson GB, Farley DR, Richards ML, Reading CC, Charboneau JW, Vollrath BK, Sebo TJ. Reliability of benign fine needle aspiration cytology of large thyroid nodules. Surgery. 2008;144(6):963–968. doi: 10.1016/j.surg.2008.09.006. discussion 968–969. [DOI] [PubMed] [Google Scholar]
- 21.Kokmen E, Ozsarfati Y, Beard CM, O’Brien PC, Rocca WA. Impact of referral bias on clinical and epidemiological studies of Alzheimer’s disease. J Clin Epidemiol. 1996;49(1):79–83. doi: 10.1016/0895-4356(95)00031-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.