Abstract
Aging is associated with visceral adiposity, metabolic disorders, and chronic low-grade inflammation. 17α-estradiol (17α-E2), a naturally occurring enantiomer of 17β-estradiol (17β-E2), extends life span in male mice through unresolved mechanisms. We tested whether 17α-E2 could alleviate age-related metabolic dysfunction and inflammation. 17α-E2 reduced body mass, visceral adiposity, and ectopic lipid deposition without decreasing lean mass. These declines were associated with reductions in energy intake due to the activation of hypothalamic anorexigenic pathways and direct effects of 17α-E2 on nutrient-sensing pathways in visceral adipose tissue. 17α-E2 did not alter energy expenditure or excretion. Fasting glucose, insulin, and glycosylated hemoglobin were also reduced by 17α-E2, and hyperinsulinemic-euglycemic clamps revealed improvements in peripheral glucose disposal and hepatic glucose production. Inflammatory mediators in visceral adipose tissue and the circulation were reduced by 17α-E2. 17α-E2 increased AMPKα and reduced mTOR complex 1 activity in visceral adipose tissue but not in liver or quadriceps muscle, which is in contrast to the generalized systemic effects of caloric restriction. These beneficial phenotypic changes occurred in the absence of feminization or cardiac dysfunction, two commonly observed deleterious effects of exogenous estrogen administration. Thus, 17α-E2 holds potential as a novel therapeutic for alleviating age-related metabolic dysfunction through tissue-specific effects.
Key Words: 17α-Estradiol, Adipose tissue, Hypothalamus, Inflammation, Metabolism
Human life expectancy has increased dramatically in developed countries in the past several decades, and no deceleration of this trend appears imminent (1). Healthspan extension has failed to keep pace with increasing life expectancy, thus the incidence of chronic diseases and multimorbidity has become more common in the older adult population (2,3). Physiological aging and age-related diseases can be delayed by interventions that modulate nutrient-sensing pathways (4,5). The most consistent and effective intervention among mammals is calorie restriction (CR) without malnutrition (6). CR protects against a number of age-related diseases through mechanisms that are not fully understood, although improvements in regional adiposity, ectopic lipid deposition, metabolic homeostasis, and inflammatory status likely play a role (6–9).
White adipose tissue (WAT) influences physiological aging and disease onset by modulating macronutrient homeostasis and systemic inflammation (10). Advancing age is associated with WAT insulin resistance and pro-inflammatory activity, which in turn contribute to the expansion of visceral adiposity, ectopic lipid deposition, and metabolic dysfunction (11–14). Visceral adiposity and ectopic lipid accumulation are linked with morbidity and all-cause mortality in aged mammals (15,16). Despite evidence indicating that CR improves regional adiposity, metabolic parameters, and inflammatory status in obese older adults (17–19), it remains unclear whether CR is feasible and/or appropriate in nonobese older adults (20,21). Moreover, dietary interventions that restrict calorie intake are often difficult to maintain (22). Given these limitations, attention has been directed toward selectively modulating nutrient-sensing and inflammatory pathways to enhance healthspan without modifying calorie intake. Pharmacological interventions may provide solutions to alleviate age-related dysfunction without the need for dietary restriction.
17α-estradiol (17α-E2) has emerged as a candidate compound for the extension of healthspan, as recent observations document an extension of life span in male mice fed a diet enriched with 17α-E2 (23). 17α-E2 is a naturally occurring enantiomer of 17β-estradiol (17β-E2), yet appears to be nonfeminizing due to minimal activation of classical estrogen receptors, ERα and ERβ (24,25). The signaling mechanism(s) by which 17α-E2 elicits downstream effects remains elusive despite having been investigated for several decades. Although 17α-E2 is found in several mammalian tissues, urine, and plasma from both sexes (26,27), the physiological functions of endogenous 17α-E2 are unclear. It has been reported to serve a neuroprotective role due to being consistently found, and likely generated, in the brain (28,29). Supporting this premise are studies that report 17α-E2 protects against neuronal death in models of ischemia, Alzheimer’s, and Parkinson’s diseases (30–32), although this and other potential functions remain incompletely understood. In the present set of experiments, we tested whether late-life administration of 17α-E2 positively affects age-related perturbations in regional adiposity, metabolic homeostasis, and inflammatory status.
Materials and Methods
Control and Experimental Diets
TestDiet, a division of Purina Mills (Richmond, IN), prepared all the diets. Study 1 utilized LabDiet 5LG6 (65.4% CHO, 22.4% PRO, 12.2% FAT) as the control (CON) diet and was supplemented with 17α-E2 (14.4 ppm; Steraloids, Newport, RI) for the treatment diet. Study 2 utilized LabDiet 58YP (66.4% CHO, 20.5% PRO, 13.1% FAT) as the CON diet and was supplemented 17α-E2 (14.4 ppm) for the treatment diet.
Animals
All mice (male C57BL/6) used for these studies were obtained from the National Institute on Aging and were individually housed at 24±0.5°C on a 12:12-hour light-dark cycle. Unless otherwise noted, mice had ad libitum access to food and water throughout the experimental timeframe. For Study 1, 18-month-old mice were randomized by body mass, body composition, and fasting glucose to CON or 17α-E2 treatment groups (n = 10/group). Body mass and composition were measured weekly throughout the 15-week intervention. A second cohort of mice (n = 10/group) underwent identical treatment conditions at the Yale University Mouse Metabolic Phenotyping Center (MMPC) in preparation for hyperinsulinemic-euglycemic clamp analyses following a 10-week intervention. For Study 2, 20-month-old mice were randomized by body mass, body composition, fasting glucose, and glycosylated hemoglobin (HbA1c) to CON, 17α-E2, or CR treatment groups. Body mass and food intake were measured daily, whereas body composition was measured weekly throughout the 5-week intervention. Daily food allotment for the CR treatment group was calculated based upon body mass changes in the 17α-E2 treatment group from the previous day. This design allowed for weight-matching between the 17α-E2 and CR treatment groups and averaged out to an 18% restriction over the course of the study. The CR mice received half of their daily food allotment at 6 AM and the remainder at 6 PM . Body composition was assessed by quantitative magnetic resonance using an EchoMRI-100H analyzer (Houston, TX) (33). Regional adiposity was assessed by microcomputed tomography (microCT) scanning and reconstruction using a Scanco VivaCT 40 (Wayne, PA) (34). Body temperatures were measured twice daily (~6 AM and 6 PM) during Study 2 using a Thermalert TH-8 (Physitemp, Clifton, NJ) rodent rectal thermometer. At the conclusion of each study, mice were anesthetized with isoflurane and euthanized by cervical dislocation prior to dissection. Blood was collected into EDTA-lined tubes by cardiac puncture, and plasma was collected and frozen. Tissues were excised, weighed, flash frozen, and stored at −80°C unless otherwise noted. Small sections of liver and pancreas were fixed in 4% paraformaldehyde in preparation for paraffin embedding and future analyses. Small sections of eWAT and iWAT were washed and fixed in preparation for assessing SA-βgal+. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committees at Mayo Clinic or Yale University.
Plasma Analytes
Plasma adipokines, cytokines, and chemokines were evaluated using Multiplexing LASER Bead Technology (Eve Technologies, Calgary, AB). Plasma testosterone, 17β-E2, and 17α-E2 were evaluated by LC/MS/MS (29).
Real-time PCR
Total RNA was extracted from WAT, liver, and quadriceps using Trizol (Life Technologies, Carlsbad, CA) and was reverse transcribed to cDNA with the M-MLV Reverse Transcriptase kit (Life Technologies). Real-time PCR was performed in a 7500 Fast Real Time PCR System (Applied Biosystems, Foster City, CA) using TaqMan Fast Universal PCR Master Mix (Life Technologies) and predesigned primers and probes from Applied Biosystems. Target gene expression was expressed as 2−ΔΔCT by the comparative CT method (35) and normalized to the expression of TATA-binding protein. Hypothalamic RNA was extracted and processed as previously described (36).
Senescence-associated β-Galactosidase
Cellular SA-βgal+ was assessed as previously described (37).
Ectopic Lipid Analyses
Paraffin-embedded liver sections were qualitatively evaluated for lipid accumulation by staining with osmium tetroxide (38). Liver and quadriceps triglyceride concentrations were evaluated following the extraction of total lipid as previously described (39). Final triglyceride concentrations were then measured using a spectrophotometric assay (Sigma-Aldrich).
In Vivo Metabolic Analyses
Unless otherwise noted, all experiments requiring fasting conditions were performed in the afternoon following the removal of food in the morning (~8 AM). To ensure fasting conditions, mice were transferred to clean cages with sawdust bedding. Fasting (6 hours) glucose was evaluated using a OneTouch Ultra Blood Glucose Monitoring System (Johnson & Johnson, New Brunswick, NJ). Fasting (6 hours) insulin was evaluated using a Mouse Ultrasensitive Insulin ELISA from Alpco (Salem, NH). HbA1c was assessed by A1C-Now Monitoring kits (Bayer, Whippany, NJ). Glucose tolerance tests were performed following a 6-hour fast at a dose of 1.0g/kg body mass. Blood glucose was measured as described earlier at the time of injection (time 0) and at 15, 30, 60, 90, and 120 minutes post injection. Hyperinsulinemic-euglycemic clamp analyses and follow-up procedures were performed by the Yale University MMPC as previously described (40). Indirect calorimetry and voluntary activity were measured using the Oxymax System from Columbus Instruments (Columbus, OH). Due to differences in body mass between groups, all indirect calorimetry data were normalized to lean mass (LBM).
Western Blotting
WAT, liver, and quadriceps were homogenized in Cell Lysis Buffer (Cell Signaling, Danvers, MA) with protease inhibitors (Sigma-Aldrich). Total protein was quantified using BCA Protein Assay Reagent Kit (Pierce, Rockford, IL). Proteins were separated on 4%–15% gradient SDS-PAGE gels and transferred to immuno-blot PVDF membranes (Bio-Rad, Hercules, CA). All primary antibodies were purchased from Cell Signaling and were used according to manufacturer’s suggestions. HRP-linked secondary antibodies (Santa Cruz, Santa Cruz, CA) were detected using an ECL Western Blotting Substrate Kit (Pierce).
Fecal Energy Excretion
Feces were collected and analyzed by bomb calorimetry as previously described (41).
Pancreatic Insulin Immunohistochemistry
Sections of paraffin-embedded pancreas were mounted on glass slides and processed for insulin as previously described (42). Immunoreactivity was visualized with 3,3-diaminobenzidine and counter-stained with hematoxylin. Insulin primary antibody was purchased from Dako (Carpinteria, CA). Biotinylated secondary antibody was purchased from Vector Laboratories (Burlingame, CA). HRP-conjugated biotin tertiary antibody was purchased from Vector Laboratories. Completed slides were scanned, and total pancreatic and insulin area were quantified with Image J.
Hepatic DNA Damage
Sections of paraffin-embedded liver were mounted on glass slides and processed for γH2A.X staining on telomere regions as previously described (43). γH2A.X antibody was purchased from Cell Signaling. Biotinylated secondary antibody and Fluorescein Avidin DCS were purchased from Vector Laboratories. Following γH2A.X immunofluorescence, telomere immunoFISH was performed using Cy-3–labeled telomere-specific (CCCTAA) peptide nucleic acid probe (Panagene, Daejeon, KR). Counter-staining was done with DAPI and images were taken and in-depth Z stacking was used (a minimum of 40 optical slices with ×63 objective).
U2OS Cell Transfection and Estrogen Receptor Activation
U2OS osteosarcoma cells were cultured in phenol-free αMEM with 10% FBS (Hyclone, Logan, UT) and 1% antibiotic/antimycotic (Life Technologies). Cells were plated (2.6×104 cells/cm2) in 12-well plates the day before transfection. Cells were co-transfected with 500ng of an ERα or ERβ expression construct and 500ng of an estrogen response element-luciferase reporter plasmid (44) using FuGene6 transfection reagent (Promega, Madison, WI). The following day, cells were treated with increasing concentrations of 17α-E2 and 17β-E2 in charcoal-stripped FBS-containing media. Cells were harvested 24 hours later and assayed for luciferase activity as previously described (45).
3T3-L1 Differentiation and AMPKα Activation
3T3-L1 cells were obtained from ATCC (Manassas, VA) and grown and differentiated according to vendor instructions. Following 10 days of differentiation, when more than 70% of cells had visible lipid droplets, we treated for 2 hours with vehicle (EtOH), 17α-E2 (10nM and 100nM; Steraloids), 17β-E2 (10nM and 100nM; Steraloids), or A-769662 (A76; 100 μM; Tocris, Minneapolis, MN). Cells were harvested and analyzed for AMPKα activation as described in the Western blotting section.
Cardiac Function
High-resolution ultrasound imaging was used to evaluate ventricular function and mass. Short- and long-axis views were assessed as previously described (46).
Physical Function Analyses
Forelimb grip strength and maximal treadmill endurance were evaluated as previously described (47).
Statistical Analyses
Analyses of differences between groups were performed by Student’s t test, one-way analysis of variance, or repeated-measures analysis of variance where appropriate using GraphPad Prism Software, version 6.0. Values are presented as mean ± SEM with p values less than .05 considered to be significant.
Results
17α-E2 Reduces Body Mass and Adiposity
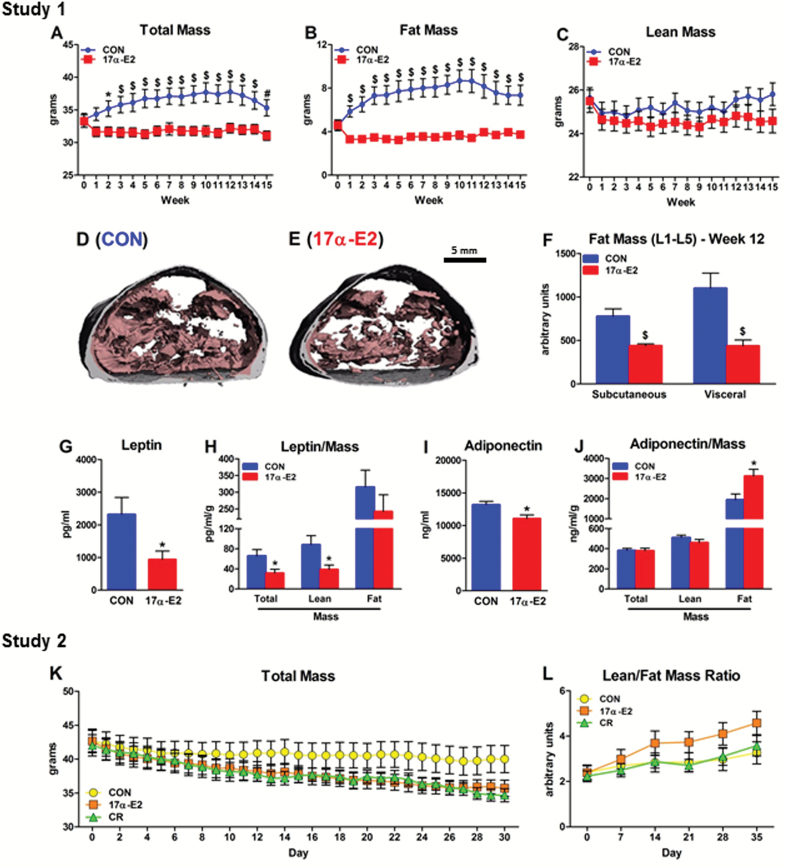
Aging is associated with increased visceral adiposity related to declines in subcutaneous WAT functional capacity (48). We tested how 17α-E2 administration affects body mass and composition in aged male mice. Two independent studies revealed that 17α-E2 prevents gains (Figure 1A and B; Study 1) or elicits reductions (Figure 1K and L; Study 2) in body mass and adiposity. Study 2 utilized a pair-feeding strategy that effectively demonstrated that 17α-E2 has direct and more robust effects on the diminishment of adiposity than mild CR treatment (18%). The reduction in whole-body adiposity was primarily due to declines in visceral adiposity (Figure 1D and F). Intraperitoneal WAT depots (epididymal [eWAT] and mesenteric [mWAT]) were diminished more than extraperitoneal depots (inguinal [iWAT], subscapular [scWAT], and perirenal [pWAT]) (Supplementary Figure 1A and B). Circulating adiponectin and leptin were decreased following 17α-E2 administration (Figure 1G and I). The reduction in leptin was anticipated because it serves as a surrogate marker of whole-body adiposity (49), which was confirmed by normalizing leptin to total adiposity (Figure 1H). The reduction in adiponectin was unexpected because it is often inversely correlated with visceral adiposity (49). However, expressing adiponectin levels relative to total adiposity revealed an increase in adiponectin per unit of fat mass (Figure 1J). 17α-E2 did not reduce lean mass (Figure 1C) and was associated with greater preservation of lean:fat mass ratios than mild CR treatment (18%; Figure 1L). With the exclusion of WAT depots, all other organ masses including liver, quadriceps, kidneys, heart, spleen, and brown adipose tissue were nearly identical between treatment groups (Supplementary Figure 1A and B).
Figure 1.
17α-E2 reduces body mass and adiposity. Weekly changes in (A) total body mass, (B) fat mass, and (C) lean mass in CON (blue) and 17α-E2 (red) treatment groups from Study 1. Representative cross-sectional images of visceral (pink) and subcutaneous (gray) adiposity in the lumbar region of (D) CON and (E) 17α-E2 treatment groups at Week 12 of Study 1. (F) Quantitative analysis of regional adiposity in the lumbar region from CON and 17α-E2 treatment groups from Study 1. (G) Plasma leptin and (H) plasma leptin normalized to total, lean, and fat mass from CON and 17α-E2 treatment groups from Study 1. (I) Plasma adiponectin and (J) plasma adiponectin normalized to total, lean, and fat mass from CON and 17α-E2 treatment groups from Study 1. Daily changes in (K) total body mass and weekly changes in (L) lean:fat mass ratios in CON (yellow), 17α-E2 (orange), and calorie restriction (green) treatment groups from Study 2. All data are expressed as mean ± SEM of 7–10 mice per treatment group and were analyzed by repeated measures analysis of variance (A–C, K, L) or Student’s t test (F–J). *p < .05. # p < .01. $ p < .005.
17α-E2 Reduces Energy Intake but not Expenditure or Excretion
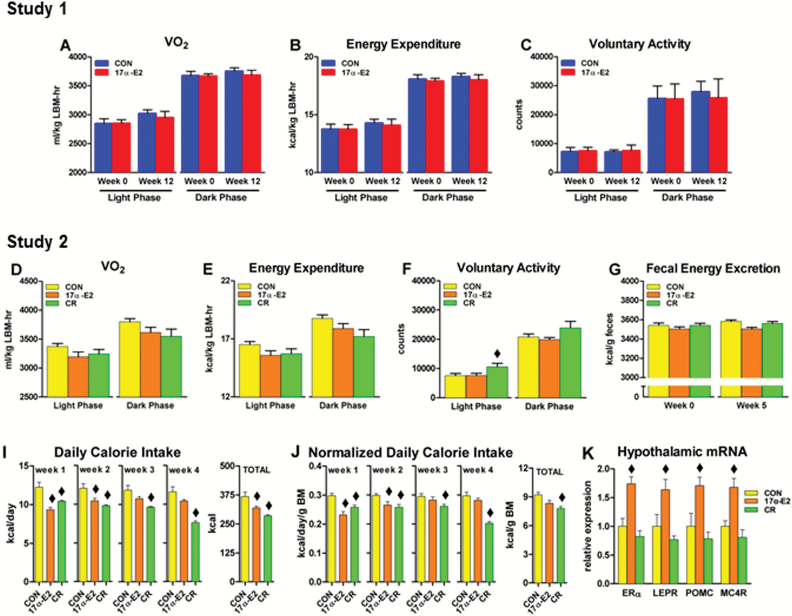
An imbalance between energy intake and expenditure can contribute to lipid redistribution and metabolic dysfunction (5). Because 17α-E2 reduced body mass and adiposity (Figure 1), we evaluated factors involved in determining body size and composition. Chronic administration of 17α-E2 did not alter oxygen consumption, energy expenditure, or voluntary activity in Studies 1 or 2 (Figure 2A–F), nor did we find changes in fecal energy excretion in Study 2 (Figure 2G). 17α-E2 did not alter body temperatures during dark or light phases (Supplementary Figure 2A and B). These findings suggest that the decline in body mass and adiposity may not be related to altered metabolic rate but may result from changes in energy intake. Consistent with this, Study 2 revealed that 17α-E2 reduced total calorie consumption (Figure 2I). Calorie intake normalized to mass was also reduced (Figure 2J), particularly within the first 2 weeks of treatment when changes in body mass and adiposity were most evident. Thereafter, calorie intake was similar to levels observed in CON mice, suggesting that 17α-E2 may alter calorie intake to maintain a lower body mass. This speculation was supported by the observation that the CR group required continually diminished allotments of food to remain weight-matched with 17α-E2–treated mice (Figure 2I and J). Assessment of hypothalamic transcripts revealed increased expression of anorexigenic mediators including the leptin receptor (LEPR), pro-opiomelanocortin (POMC), and the downstream mediator of POMC actions, the melanocortin-4 receptor (MC4R) following 17α-E2 treatment (Figure 2K). Elevated anorexigenic gene expression was associated with increased ERα transcription, which is believed to be the primary mechanism by which 17β-E2 signals in the hypothalamus to regulate feeding behavior (50). Collectively, these observations suggest that one of the mechanisms through which 17α-E2 may decrease body mass and adiposity is by acting as a satiety factor. However, additional evidence suggests that declines in caloric intake do not solely account for changes in adiposity and metabolic parameters by 17α-E2.
Figure 2.
17α-E2 reduces energy intake but not expenditure or excretion. (A) Oxygen consumption, (B) energy expenditure, and (C) voluntary activity during Weeks 0 and 12 from CON (blue) and 17α-E2 (red) treatment groups from Study 1. (D) Oxygen consumption, (E) energy expenditure, and (F) voluntary activity during Week 4 from CON (yellow), 17α-E2 (orange), and calorie restriction (CR; green) treatment groups from Study 2. (G) Fecal energy excretion during Weeks 0 and 5 from CON, 17α-E2, and CR treatment groups from Study 2. (I) Daily calorie intake per week and total calories consumed from CON, 17α-E2, and CR treatment groups from Study 2. (J) Daily calorie intake per week normalized to body mass and total calories consumed normalized to body mass from CON, 17α-E2, and CR treatment groups from Study 2. (K) Anorexigenic gene expression in the hypothalamus from CON, 17α-E2, and CR treatment groups from Study 2. All data are expressed as mean ± SEM of 7–10 mice per treatment group and were analyzed by Student’s t test (A–C) or one-way analysis of variance (D–K). ♦ p < .05 (different from nonlabeled groups).
17α-E2 Reduces Inflammation in WAT and the Circulation
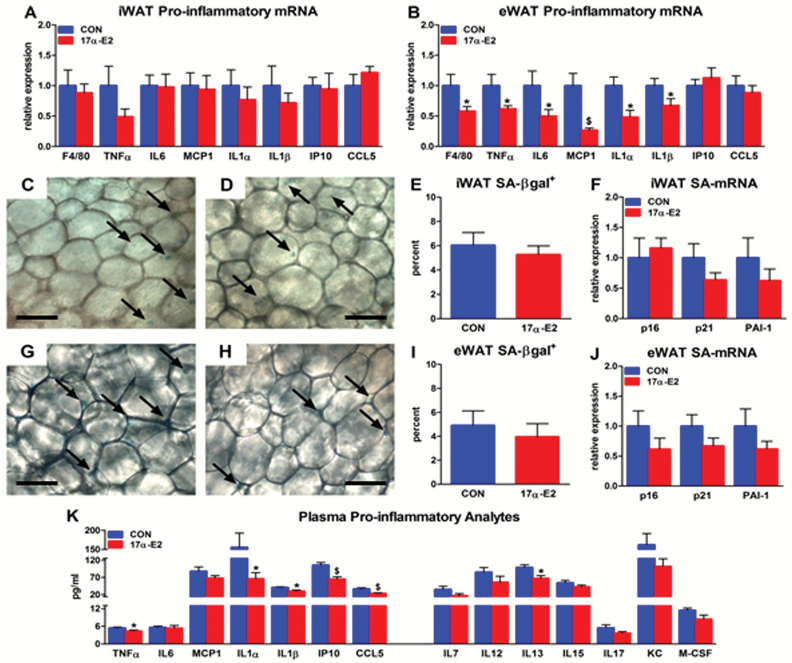
Chronic low-grade inflammation is linked with age-related metabolic dysfunction (51). WATs, particularly visceral depots, are a source of circulating pro-inflammatory mediators (52,53). Reports suggest that accumulation of senescent cells within WAT may contribute to this pro-inflammatory phenotype (37,54,55). Because 17α-E2 reduced adiposity, we determined whether these improvements were accompanied by a dampening of WAT pro-inflammatory mediators and senescent cell burden. 17α-E2 reduced expression of several pro-inflammatory transcripts in visceral (eWAT), but not subcutaneous (iWAT), WAT (Figure 3A and B). These transcripts included tumor necrosis factor-α (TNFα), monocyte chemotactic protein-1 (MCP1), interleukin (IL)-6, IL1α, IL1β, and EGF-like module-containing mucin-like hormone receptor-like 1 (F4/80), a macrophage marker (56). Although several of these cytokines and chemokines have been associated with the senescence-associated secretory phenotype (57), we did not observe significant differences in senescence-related markers, including senescence-associated β-galactosidase positivity (SA-βgal+), p16, p21, or plasminogen activator inhibitor-1 (PAI-1) transcripts in visceral or subcutaneous WAT (Figure 3C–J). However, the reduction in visceral WAT pro-inflammatory activity was associated with declines in circulating pro-inflammatory analytes. Many of the inflammatory markers suppressed in visceral WAT by 17α-E2 were also reduced in the circulation, with several additional cytokines and chemokines also trending downwards (Figure 3K). Collectively, these findings indicate an overall improvement in systemic inflammatory status.
Figure 3.
17α-E2 reduces inflammation in white adipose tissue (WAT) and the circulation. Pro-inflammatory gene expression in (A) subcutaneous (iWAT) and (B) visceral (eWAT) WAT from CON (blue) and 17α-E2 (red) treatment groups from Study 1. Representative images of SA-βgal+ in iWAT from (C) CON and (D) 17α-E2 treatment groups from Study 1. (E) Percent SA-βgal+ in iWAT from CON and 17α-E2 treatment groups from Study 1. (F) SA-mRNA expression in iWAT from CON and 17α-E2 treatment groups from Study 1. Representative images of SA-βgal+ in eWAT from (G) CON and (H) 17α-E2 treatment groups from Study 1. (I) Percent SA-βgal+ in eWAT from CON and 17α-E2 treatment groups from Study 1. (J) SA-mRNA expression in eWAT from CON and 17α-E2 treatment groups from Study 1. (K) Plasma pro-inflammatory cytokines and chemokines in CON and 17α-E2 treatment groups at Week 15 of Study 1. Arrows indicate SA-βgal+ activity in representative samples. All data are expressed as mean ± SEM of 8–10 mice per treatment group and were analyzed by Student’s t test. *p < .05. # p < .01. $ p < .005. Scale bars: 50 µm.
17α-E2 Reverses Age-related Lipid Redistribution and Restores Metabolic Flexibility
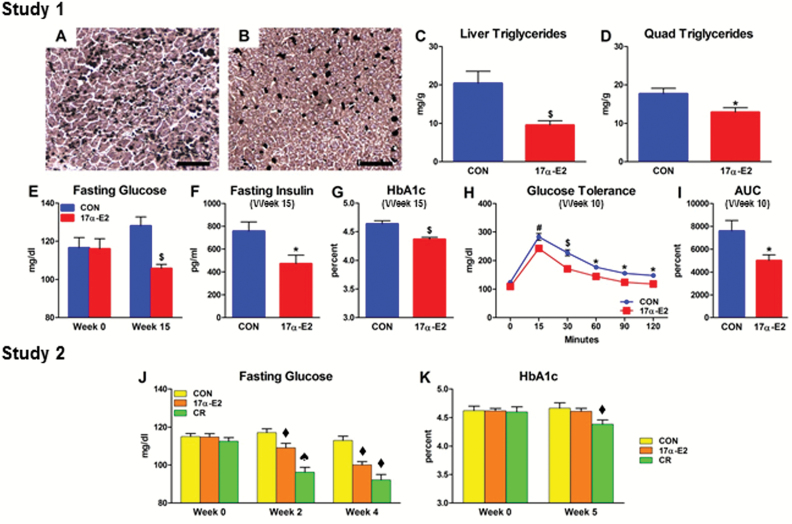
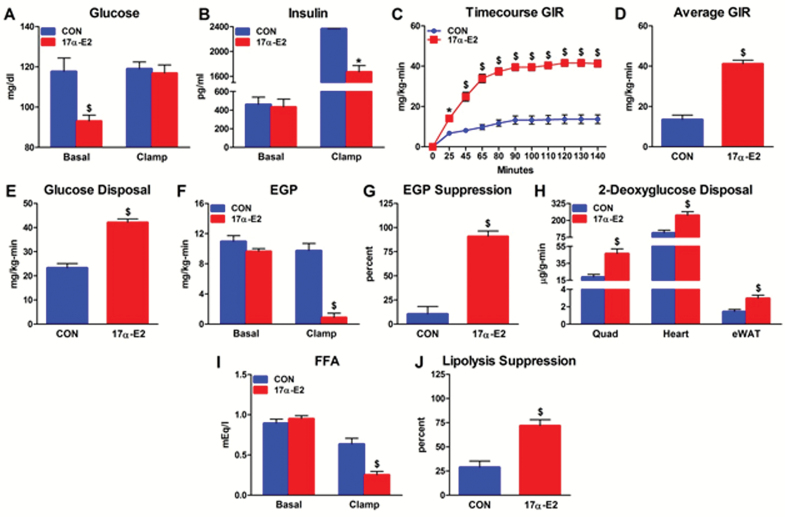
Metabolic dysfunction in older adults is associated with ectopic lipid deposition, glucose intolerance, and insulin resistance (11–13). Age-related changes in regional adiposity and systemic inflammation are predictors of metabolic decline (10,48). Given that 17α-E2 reduced adiposity and inflammatory status (Figures 1 and 3), we tested whether 17α-E2 improved metabolic health. Consistent with reduced visceral adiposity (Figure 1), 17α-E2 suppressed lipid accumulation in liver and quadriceps (Figure 4A–D). This was accompanied by improvements in glucose homeostasis, as indicated by declines in fasting glucose, fasting insulin, and HbA1c, coupled with enhanced glucose tolerance (Figure 4E–I). Studies utilizing hyperinsulinemic-euglycemic clamps revealed that 17α-E2 restored whole-body insulin sensitivity (Figure 5A–D). Improved whole-body insulin sensitivity was accounted for by increased rates of insulin-stimulated glucose disposal and reduced rates of hepatic glucose production (Figure 5E and F). Furthermore, tissue-specific rates of insulin-stimulated glucose transport were increased in quadriceps, heart, and visceral (eWAT) WAT (Figure 5H). Clamps further revealed that 17α-E2 enhanced metabolic plasticity in liver and WAT in that the suppression of hepatic glucose production and lipolysis under insulin-stimulated conditions were improved (Figure 5G and J). 17α-E2 treatment also reduced pancreatic β-cell area (Supplementary Figure 3A–D), suggesting that improvements in glucose homeostasis reduced pancreatic β-cell demand. This is commonly observed in mammals with improved peripheral insulin sensitivity due to reversal of hyperinsulinemic compensation (58). Study 2 also indicated enhancements in glucose homeostasis by 17α-E2 to an extent similar to that observed following mild CR treatment (Figure 4J and K). Given that hyperglycemia and hepatic lipid accumulation are associated with cellular senescence in the liver (59,60), we evaluated telomere-associated DNA damage, an indicator of senescence onset (43). Improvements in hepatic lipid deposition and metabolic homeostasis by 17α-E2 were associated with reduced hepatocyte DNA damage foci (Supplementary Figure 4A–D). We speculate that 17a-E2 treatment may improve overall liver function by alleviating age-related lipid accumulation and impaired glucose homeostasis.
Figure 4.
17α-E2 reverses age-related lipid redistribution and improves glucose homeostasis. Representative images of total hepatic lipid content from (A) CON and (B) 17α-E2 treatment groups from Study 1. Triglyceride accumulation in (C) liver and (D) quadriceps from CON (blue) and 17α-E2 (red) treatment groups from Study 1. (E) Fasting glucose at Weeks 0 and 15 in CON and 17α-E2 treatment groups from Study 1. (F) Fasting insulin and (G) Glycosylated hemoglobin (HbA1c) at Week 15 in CON and 17α-E2 treatment groups from Study 1. (H) Glucose tolerance at Week 10 in CON and 17α-E2 treatment groups from Study 1. (I) Glucose tolerance area under the curve at Week 10 in CON and 17α-E2 treatment groups from Study 1. (J) Fasting glucose at Weeks 0, 2, and 4 in CON (yellow), 17α-E2 (orange), and calorie restriction (CR; green) treatment groups from Study 2. (K) HbA1c at Weeks 0 and 5 in CON, 17α-E2, and CR treatment groups from Study 2. All data are expressed as mean ± SEM of 8–10 mice per treatment group and were analyzed by Student’s t test (C–G), repeated measures analysis of variance (ANOVA; H), or one-way ANOVA (J–K). *p < .05. # p < .01. $ p < .005. ♦ p < .05 (different from nonlabeled groups); ♠ p < .05 (different from nonlabeled and ♦-labeled groups). Scale bars: 200 µm.
Figure 5.
17α-E2 improves systemic insulin sensitivity. (A) Glucose and (B) insulin under basal and clamped conditions from CON (blue) and 17α-E2 (red) treatment groups from Study 1. (C) Real-time and (D) average glucose infusion rates (GIRs) from CON and 17α-E2 treatment groups from Study 1. (E) Whole-body glucose disposal, (F) endogenous glucose production (EGP) under basal and clamped conditions, and (G) hyperinsulinemia-induced percent EGP suppression from CON and 17α-E2 treatment groups from Study 1. (H) Tissue-specific disposal of 2-deoxyglucose during hyperinsulinemia from CON and 17α-E2 treatment groups from Study 1. (I) Circulating free fatty acids (FFAs) under basal and clamped conditions and (J) percent lipolysis suppression during hyperinsulinemia from CON and 17α-E2 treatment groups from Study 1. All data are expressed as mean ± SEM of 7 mice per treatment group and were analyzed by Student’s t test (A, B, D–J) or repeated-measures analysis of variance (c). *p < .05. $ p < .005.
17α-E2 Selectively Modulates Nutrient-sensing Pathways
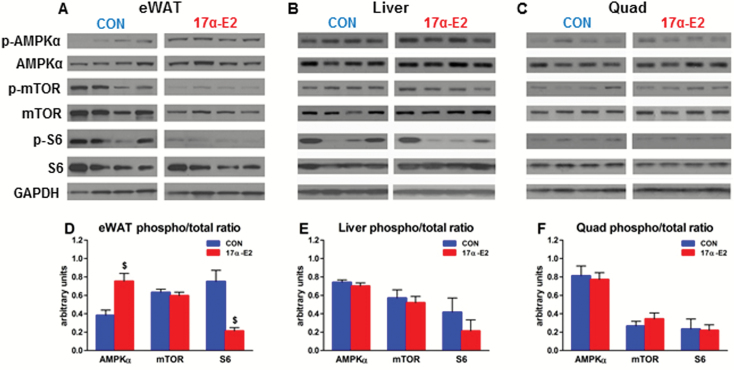
Dysregulation of nutrient-sensing pathways contributes to the aging process and onset of age-related diseases (4,5). Many dietary, pharmacological, and genetic interventions that extend life span and delay or alleviate age-related dysfunction directly or indirectly modulate AMPKα and/or mTOR signaling (6). 17α-E2 increased AMPKα and reduced mTOR complex 1 activity (reduced p-S6) in visceral (eWAT) WAT (Figure 6A and D). We did not observe similar outcomes in liver (Figure 6B and E) or quadriceps (Figure 6C and F), which are known to respond to CR and exercise through AMPKα and mTOR modulation (5,6). Thus, unlike CR, 17α-E2 selectively modulates AMPKα and mTOR activity in a tissue-specific manner. Treatment of cultured 3T3-L1 adipocytes revealed that acute administration of 17α-E2 activates AMPKα (Supplementary Figure 5A and B), suggesting that 17α-E2 may have direct effects in WAT. Moreover, these effects appear greater than those observed following 17β-E2 treatment (Supplementary Figure 5A and B). Collectively, our findings suggest that 17α-E2 may preferentially modulate nutrient-sensing pathways in WAT, which is in contrast to the global effects of CR.
Figure 6.
17α-E2 selectively activates AMPKα and suppresses mTOR complex 1. Representative Western blots of p-AMPKα, AMPKα, p-mTOR, mTOR, p-S6, S6, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from (A) visceral adipose (eWAT), (B) liver, and (C) quadriceps from CON and 17α-E2 treatment groups from Study 1. Densitometry results for p-AMPKα:AMPKα, p-mTOR:mTOR, and p-S6:S6 ratios from (D) visceral (eWAT), (E) liver, and (F) quadriceps from CON (blue) and 17α-E2 (red) treatment groups from Study 1. All data are expressed as mean ± SEM of 7–10 mice per treatment group and were analyzed by Student’s t test (D–F). $ p < .005.
17α-E2 Does not Feminize Male Mice
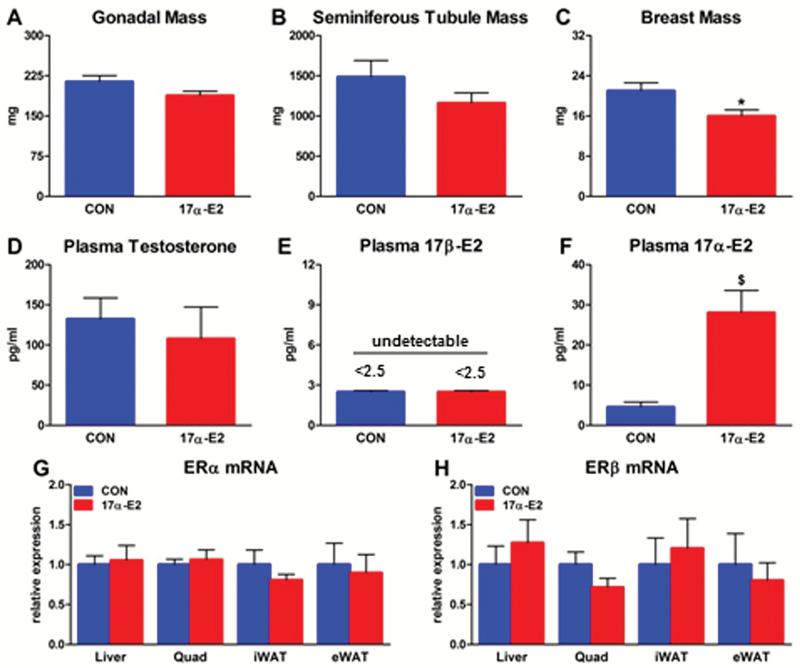
Several studies have shown that 17α-E2 has less binding affinity and activation potential of ERα and ERβ compared with 17β-E2 (24,25), and thus is unlikely to induce feminine characteristics (26,30,32). However, this has not been evaluated in long-term in vivo studies in which exogenous 17α-E2 was administered. We examined several characteristics that would indicate whether chronic (15 weeks) 17α-E2 administration feminizes male mice. 17α-E2 did not alter gonadal or seminiferous tubule mass, whereas breast mass was reduced (Figure 7A–C). No differences were found in circulating testosterone or 17β-E2 between treatment groups (Figure 7D and E). Differences would be expected if 17α-E2 were feminizing because oral estrogen therapies reduce bioavailable testosterone and increase 17β-E2 in the circulation (61). As expected, circulating 17α-E2 was increased by exogenous administration (Figure 7F). Given that 17β-E2 can modulate the expression of its own receptors (62), we evaluated ERα and ERβ transcription in several tissues and found them to be unchanged by 17α-E2 (Figure 7G and H). This is in contrast to our findings in the hypothalamus (Figure 2K), suggesting that 17α-E2 may modulate classical ER expression in a tissue-specific manner. To confirm that 17α-E2 elicits less ERα and ERβ activity than 17β-E2, we transfected U2OS cells with each receptor and exposed them to escalating doses of both hormones to evaluate reporter gene activity. In agreement with a previous report (25), ER activation by 17α-E2 required ~20-fold higher concentrations to replicate reporter activity induced by 17β-E2 (Supplementary Figure 6A and B). Another potential deleterious effect associated with exogenous estrogen administration is cardiovascular disease progression due to chronic activation of classical ERs (63). We evaluated cardiac function (Supplementary Figure 7A–C) and physical function parameters (Figure 8A and B) in male mice chronically exposed to 17α-E2 and found no difference between treatment groups. Collectively, our observations suggest that 17α-E2 is nonfeminizing and does not impair cardiac or physical function parameters in male mice at the given dose and duration of treatment used here. Additional studies in female mice are needed to evaluate sex-specific responses to 17α-E2.
Figure 7.
17α-E2 does not feminize male mice. (A) Gonadal, (B) seminiferous tubule, and (C) breast mass during necropsy from CON (blue) and 17α-E2 (red) treatment groups from Study 1. Plasma (D) testosterone, (E) 17β-E2, and (F) 17α-E2 in CON and 17α-E2 treatment groups at Week 15 of Study 1. (G) ERα and (H) ERβ mRNA expression in liver, quadriceps, subcutaneous and visceral WAT from CON and 17α-E2 treatment groups from Study 1. All data are expressed as mean ± SEM of 8–10 mice per treatment group and were analyzed by Student’s t test. *p < .05. $ p < .005.
Discussion
17α-E2, a nonfeminizing enantiomer of 17β-E2, was recently found to extend life span in male mice through unknown mechanisms (23). 17β-E2 is reported to protect females from metabolic declines through central and peripheral actions (64,65). We hypothesized that 17α-E2 may elicit similar effects in male mice and improve healthspan. We provide evidence that 17α-E2 alleviates metabolic and inflammatory dysfunction in old male mice by reducing calorie intake and beneficially altering nutrient-sensing and inflammatory pathways in visceral WAT. Our data indicate that 17α-E2 reduces visceral adiposity and chronic inflammation, both of which are associated with morbidity and mortality in older adults (16,66). We speculate that 17α-E2 may be an attractive therapy to improve healthspan in older adults with metabolic and/or inflammatory dysfunction.
Age-related declines in WAT function promote lipid redistribution to favor ectopic deposition and visceral adiposity (11,67). These changes are linked with insulin resistance and the development of type 2 diabetes (68,69). We found that 17α-E2 reduced overall body mass and adiposity, with declines in visceral fat, hepatic lipid, and muscle triglyceride. Our findings are in some ways like those seen in mammals undergoing chronic CR (6,7). We anticipated that the reduction in body mass and adiposity by 17α-E2 might occur in response to altered metabolic balance to favor an energy deficit, as seen with CR. Assessment of oxygen consumption, activity, and body temperatures did not reveal evidence of increased energy expenditure in 17α-E2–treated mice. We also considered decreased digestive efficiency as a potential contributing mechanism to reduced body mass and adiposity by 17α-E2. However, fecal energy content did not change in response to any treatment. Thus, in contrast to 17β-E2 (70), 17α-E2 does not appear to increase energy expenditure or alter nutrient bioavailability. Rather, our studies demonstrate reduced calorie intake in response to 17α-E2 treatment, but this alone does not explain all of our findings, particularly why 17α-E2 selectively affects adipose tissue to a different extent than muscle or liver. The reduction in calorie intake was sustained during the first 2 weeks of administration when body mass and adiposity were most robustly reduced. 17α-E2 altered calorie intake to maintain this lower body mass. These observations suggest that 17α-E2 treatment improved body composition partially through reducing calorie intake, potentially to establish a new body mass set-point.
Feeding behavior is regulated in part by hypothalamic mechanisms, whereby anorexigenic (POMC/CART-expressing) neuronal populations suppress calorie intake and metabolic output. These neuronal populations act largely in response to peripheral regulators, including insulin and leptin (71). Leptin activation of POMC expressing neurons in response to a meal promotes the release of α-melanocyte-stimulating hormone (αMSH) (72). In turn, αMSH promotes satiety through selective binding to melanocortin-3 (MC3) and MC4 receptors, acting within the paraventricular and lateral nuclei of the hypothalamus (73). Assessment of hypothalamic expression of LEPR, POMC, and MC4R indicated an upregulation of these transcripts, and thus, the anorexigenic pathway, suggesting that 17α-E2 might suppress calorie intake by improving leptin-mediated satiety effects. Aging is associated with progressive central leptin resistance (74). Resistance to leptin-mediated satiety signaling within the hypothalamus may compound weight gain (75). Given the rise in LEPR mRNA expression in response to 17α-E2 treatment, we speculate that 17α-E2 may selectively improve hypothalamic leptin signaling to reduce calorie intake. Although the observed rise in LEPR mRNA expression supports this speculation, it is yet to be directly assessed.
In brain, 17β-E2 modulates calorie intake by acting primarily through ERα (50). ERα is abundantly expressed in POMC neurons (64), and deletion of ERα results in reduced hypothalamic POMC expression (76). Given the observed increase in hypothalamic ERα expression in 17α-E2–treated mice, selective activation of POMC-mediated satiety signaling may have been facilitated through enhanced hypothalamic ERα signaling, and thus direct 17α-E2 effects. Deletion of ERα in POMC neurons results in hyperphagia without directly changing energy expenditure (50), suggesting that 17α-E2 may selectively modulate POMC-mediated calorie intake without altering energy expenditure. Combined observations from Studies 1 and 2 support this. We propose that 17α-E2 may at least partially reduce adiposity by promoting satiety.
In conjunction with declines in body mass and visceral adiposity, 17α-E2 elicited significant improvements in several systemic variables associated with age-related morbidity. Pro-inflammatory mediators in visceral WAT and the circulation were reduced by 17α-E2. Interestingly, subcutaneous WAT inflammatory markers remained unchanged. It remains unresolved whether 17α-E2 action is limited to visceral WAT, but this seems plausible given that previous studies show 17β-E2 has preferential effects on visceral WAT inflammatory processes (77,78). The activation of AMPKα and suppression of mTOR activity by 17α-E2 in visceral WAT support this contention. Increased AMPKα and reduced mTOR activity blunt pro-inflammatory pathways by regulating nuclear factor kappa B (NFκB) activity (4,5). NFκB is the master regulator of several pro-inflammatory mediators commonly elevated with aging (79). Suppression of pro-inflammatory processes in visceral WAT is likely to improve systemic inflammatory status because visceral WAT is a major source of circulating cytokines and chemokines (52,53). Thus, the observed reduction in circulating pro-inflammatory analytes in response to 17α-E2 treatment may be related to improved visceral WAT inflammatory status. Despite declines in several pro-inflammatory mediators, we did not find evidence of reduced cellular senescence in WAT. Accumulation of senescent cells is believed to contribute to age-related chronic inflammation due to the pro-inflammatory senescence-associated secretory phenotype (57,80). This is particularly true in WAT (37,54,55), which is susceptible to inflammation-mediated functional declines (10,48). 17α-E2 may suppress the senescence-associated secretory phenotype independent of changes of senescent cell accumulation, which has been shown with metformin (81) and rapamycin (82). Alternatively, 17α-E2 could suppress visceral WAT pro-inflammatory activity by reducing immune cell infiltration, which is increased with aging (83). The reduction in visceral WAT F4/80 expression supports this idea, although additional studies will be required to test this.
A decline in metabolic homeostasis is one of the more common morbidities associated with advancing age (11,84). The underlying mechanisms are multifactorial, yet prevailing evidence suggests ectopic lipid deposition due to declines in WAT functional capacity and inflammatory status likely play a pivotal role (10,48). The accumulation of lipid in liver and muscle has been shown to impair insulin signaling and thereby promote hyperglycemia (14). Given that 17α-E2 reduced visceral adiposity and pro-inflammatory mediators, we hypothesized lipid redistribution and macronutrient homeostasis would also be improved. Indeed, 17α-E2 reversed age-related ectopic lipid accumulation and enhanced glycemic control and insulin sensitivity. These observations may be attributed to a combination of factors. First, WAT from 17α-E2–treated mice was more sensitive to insulin and thus exhibited greater insulin-mediated control of lipolysis. This, coupled with reduced calorie intake, potentially contributed to declines in ectopic lipid accumulation in 17α-E2–treated mice. Perry and colleagues previously reported that WAT insulin resistance, and thus impaired suppression of lipolysis, promotes hepatic lipid accumulation and insulin resistance (85). 17α-E2 treatment enhanced liver and muscle insulin responsiveness, as evidenced by improved control of hepatic glucose production and peripheral glucose disposal, respectively. Although improved liver and muscle function occurs in response to reduced lipid accumulation (14,85), it remains unclear whether 17α-E2 directly mediates these effects. Another potential contributor to the observed enhancements in liver function is the reduction in hepatocyte DNA damage. Hepatic lipid accumulation and age-related liver diseases are linked with DNA damage and cellular senescence (60,86). Although 17α-E2 reduced telomere-associated DNA damage, which is unrepairable and a good predictor of senescence onset (43), it remains unclear whether these results represent a potential mechanism that contributes to improvements in metabolic health, or serve as a surrogate marker of hepatic health.
A major caveat associated with exogenous administration of estrogenic compounds is the potential for adverse biological consequences. Excess 17β-E2 activity can cause feminization (87) and increase cardiovascular disease risk (88,89). Therefore, the challenge exists of how best to exploit the beneficial effects of 17β-E2 while circumventing the adverse biological consequences of exogenous administration. Studies in rodents and humans indicate that short-term administration of 17α-E2, the naturally occurring enantiomer of 17β-E2, does not induce deleterious effects (26,27). However, the emergence of deleterious effects following long-term 17α-E2 treatment is yet to be tested. We found that chronic 17α-E2 treatment did not impair cardiac function, exercise endurance, or muscular strength. Moreover, increased circulating 17α-E2 following oral administration was not associated with changes in sex organ mass or circulating testosterone or 17β-E2 levels in our male mice. This is consistent with the possibility that the absence of feminization by 17α-E2 results from a lack of classical ER (ERα and ERβ) activation, which is supported by our transfection experiments and prior reports (24,25).
In summary, we provide evidence that 17α-E2 is a potential therapeutic to improve healthspan through selective modulation of pathways that control calorie intake in the hypothalamus and nutrient sensing in visceral adipose tissue. Improvements in systemic health in 17α-E2–treated mice reflected a reduction in pro-inflammatory status, attributed predominantly to improvements in visceral WAT inflammation and metabolic regulation. The beneficial effects of 17α-E2 appear to occur in the absence of deleterious effects on cardiac function and feminization. 17α-E2 may prove to have clinical application for alleviating age-related chronic diseases.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by the National Institutes of Health (P01 AG041122; J.L.K.), Robert and Arlene Kogod Career Development Award in Aging Research (M.B.S.), Pilot Research Funding from the Minnesota Obesity Center (P30 DK050456; M.B.S.), and FAPESP 2015/05801-7 (R.S.S.). Core facilities used for this work include the OSU CPMPSR Histology/IHC Core Laboratory (P30 CA106058), UAB Small Animal Phenotyping Core (P30 DK56336, and P30 DK079626), and Yale University MMPC (U24 DK059635, R01 DK40936, P30 DK45735, and K01 DK099402).
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
The authors thank Dr. Richard Miller for guidance with study designs and Alan Flechtner, Jesse Lamsam, Daniel Fraser, Kristin Mantz, Kurt Johnson, Dr. Roy Dyer, and Dr. Maria Johnson for technical support.
References
- 1. Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. doi:10.1016/S0140-6736(09)61460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc. 2015;16:640–647. doi:10.1016/j.jamda.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fabbri E, An Y, Zoli M, et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci. 2015;70:63–70. doi:10.1093/gerona/glu127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi:10.1038/nature11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–241. doi:10.1016/j.arr.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 6. Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. doi:10.1016/j.cell.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Masoro EJ. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790:1040–1048. doi:10.1016/j.bbagen.2009.02.011 [DOI] [PubMed] [Google Scholar]
- 8. Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petersen KF, Dufour S, Morino K, Yoo PS, Cline GW, Shulman GI. Reversal of muscle insulin resistance by weight reduction in young, lean, insulin-resistant offspring of parents with type 2 diabetes. Proc Natl Acad Sci USA. 2012;109:8236–8240. doi:10.1073/pnas.1205675109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stout M, Tchkonia T, Kirkland J. The aging adipose organ: lipid redistribution, inflammation, and cellular senescence. In: Fantuzzi G, Braunschweig C, eds. Adipose Tissue and Adipokines in Health and Disease (Nutrition and Health). New York, NY: Humana Press; 2014:69–80. [Google Scholar]
- 11. Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8:339–348. doi:10.1016/j.arr.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 12. Flannery C, Dufour S, Rabøl R, Shulman GI, Petersen KF. Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes. 2012;61:2711–2717. doi:10.2337/db12-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi:10.1126/science.1082889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. New Engl J Med. 2014;371:2237–2238. doi:10.1056/NEJMc1412427 [DOI] [PubMed] [Google Scholar]
- 15. Muzumdar R, Allison DB, Huffman DM, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi:10.1111/j.1474-9726.2008.00391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity. 2006;14:336–341. doi:10.1038/oby.2006.43 [DOI] [PubMed] [Google Scholar]
- 17. Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–866. doi:10.1001/archinte.166.8.860 [DOI] [PubMed] [Google Scholar]
- 18. Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab. 2009;297:E999–E1003. doi:10.1152/ajpendo.00377.2009 [DOI] [PubMed] [Google Scholar]
- 19. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. New Engl J Med. 2011;364:1218–1229. doi:10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rolland Y, Kim MJ, Gammack JK, Wilson MM, Thomas DR, Morley JE. Office management of weight loss in older persons. Am J Med. 2006;119:1019–1026. doi:10.1016/j.amjmed.2006.02.039 [DOI] [PubMed] [Google Scholar]
- 21. Morley JE, Chahla E, Alkaade S. Antiaging, longevity and calorie restriction. Curr Opin Clin Nutrition Metab Care. 2010;13:40–45. doi:10.1097/MCO.0b013e3283331384 [DOI] [PubMed] [Google Scholar]
- 22. Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev. 2006;127:1–7. doi:10.1016/j.mad.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 23. Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi:10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anstead GM, Carlson KE, Katzenellenbogen JA. The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids. 1997;62:268–303. [DOI] [PubMed] [Google Scholar]
- 25. Littlefield BA, Gurpide E, Markiewicz L, McKinley B, Hochberg RB. A simple and sensitive microtiter plate estrogen bioassay based on stimulation of alkaline phosphatase in Ishikawa cells: estrogenic action of delta 5 adrenal steroids. Endocrinology. 1990;127:2757–2762. doi:10.1210/endo-127-6-2757 [DOI] [PubMed] [Google Scholar]
- 26. Dykens JA, Moos WH, Howell N. Development of 17alpha-estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann NY Acad Sci. 2005;1052:116–135. doi:10.1196/annals.1347.008 [DOI] [PubMed] [Google Scholar]
- 27. Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann NY Acad Sci. 2005;1052:136–144. doi:10.1196/annals.1347.009 [DOI] [PubMed] [Google Scholar]
- 28. Ikeda T, Makino Y, Yamada MK. 17α-estradiol is generated locally in the male rat brain and can regulate GAD65 expression and anxiety. Neuropharmacology. 2015;90:9–14. doi:10.1016/j.neuropharm.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 29. Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. 17alpha-estradiol: a brain-active estrogen? Endocrinology. 2005;146:3843–3850. doi:10.1210/en.2004-1616 [DOI] [PubMed] [Google Scholar]
- 30. Perez E, Liu R, Yang SH, Cai ZY, Covey DF, Simpkins JW. Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain Res. 2005;1038:216–222. doi:10.1016/j.brainres.2005.01.026 [DOI] [PubMed] [Google Scholar]
- 31. Ozacmak VH, Sayan H. The effects of 17beta estradiol, 17alpha estradiol and progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemia. Physiol Res. 2009;58:909–912. [DOI] [PubMed] [Google Scholar]
- 32. Green PS, Simpkins JW. Estrogens and estrogen-like non-feminizing compounds. Their role in the prevention and treatment of Alzheimer’s disease. Ann NY Acad Sci. 2000;924:93–98. [DOI] [PubMed] [Google Scholar]
- 33. Akasaki Y, Ouchi N, Izumiya Y, Bernardo BL, Lebrasseur NK, Walsh K. Glycolytic fast-twitch muscle fiber restoration counters adverse age-related changes in body composition and metabolism. Aging Cell. 2014;13:80–91. doi:10.1111/acel.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lublinsky S, Luu YK, Rubin CT, Judex S. Automated separation of visceral and subcutaneous adiposity in in vivo microcomputed tomographies of mice. J Digital Imaging. 2009;22:222–231. doi:10.1007/s10278-008-9152-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 36. Morselli E, Fuente-Martin E, Finan B, et al. Hypothalamic PGC-1alpha protects against high-fat diet exposure by regulating ERalpha. Cell Reports. 2014;9:633–645. doi:10.1016/j.celrep.2014.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi:10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Angermuller S, Fahimi HD. Imidazole-buffered osmium-tetroxide—an excellent stain for visualization of lipids in transmission electron-microscopy. Histochem J. 1982;14:823–835. doi:10.1007/Bf01033631 [DOI] [PubMed] [Google Scholar]
- 39. Stout MB, Liu LF, Belury MA. Hepatic steatosis by dietary-conjugated linoleic acid is accompanied by accumulation of diacylglycerol and increased membrane-associated protein kinase C epsilon in mice. Mol Nutrition Food Res. 2011;55:1010–1017. doi:10.1002/mnfr.201000413 [DOI] [PubMed] [Google Scholar]
- 40. Kliewer KL, Ke JY, Lee HY, et al. Short-term food restriction followed by controlled refeeding promotes gorging behavior, enhances fat deposition, and diminishes insulin sensitivity in mice. J Nutritional Biochem. 2015;26:721–728. doi:10.1016/j.jnutbio.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnson MS, Jumbo-Lucioni P, Watts AJ, Allison DB, Nagy TR. Effect of dairy supplementation on body composition and insulin resistance in mice. Nutrition. 2007;23:836–843. doi:10.1016/j.nut.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 42. Marshall JM, Flechtner AD, La P, erle KM, Gunn JS. Visualization of extracellular matrix components within sectioned Salmonella biofilms on the surface of human gallstones. PLoS One. 2014;9:e89243. doi:10.1371/journal.pone.0089243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jurk D, Wilson C, Passos JF, et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nature Commun. 2014;2:4172. doi:10.1038/ncomms5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Monroe DG, Johnsen SA, Subramaniam M, et al. Mutual antagonism of estrogen receptors alpha and beta and their preferred interactions with steroid receptor coactivators in human osteoblastic cell lines. J Endocrinol. 2003;176:349–357. [DOI] [PubMed] [Google Scholar]
- 45. Modder UI, Oursler MJ, Khosla S, Monroe DG. Wnt10b activates the Wnt, notch, and NFkappaB pathways in U2OS osteosarcoma cells. J Cell Biochem. 2011;112:1392–1402. doi:10.1002/jcb.23048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roos CM, Hagler M, Zhang B, Oehler EA, Arghami A, Miller JD. Transcriptional and phenotypic changes in aorta and aortic valve with aging and MnSOD deficiency in mice. Am J Physiol Heart Circul Physiol. 2013;305:H1428–H1439. doi:10.1152/ajpheart.00735.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. List EO, Berryman DE, Funk K, et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155:1793–1805. doi:10.1210/en.2013-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi:10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature Rev Immunol. 2011;11:85–97. doi:10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi:10.1016/j.cmet.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Michaud M, Balardy L, Moulis G, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14:877–882. doi:10.1016/j.jamda.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 52. Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 2009;64:723–730. doi:10.1093/gerona/glp046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cartier A, Cote M, Lemieux I, et al. Age-related differences in inflammatory markers in men: contribution of visceral adiposity. Metab Clin Exp. 2009;58:1452–1458. doi:10.1016/j.metabol.2009.04.025 [DOI] [PubMed] [Google Scholar]
- 54. Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi:10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stout MB, Tchkonia T, Pirtskhalava T, et al. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging. 2014;6:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lin HH, Stacey M, Stein-Streilein J, Gordon S. F4/80: the macrophage-specific adhesion-GPCR and its role in immunoregulation. Adv Exp Med Biol. 2010;706:149–156. [DOI] [PubMed] [Google Scholar]
- 57. Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi:10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meier JJ, Bonadonna RC. Role of reduced beta-cell mass versus impaired beta-cell function in the pathogenesis of type 2 diabetes. Diabetes Care. 2013;36(suppl 2):S113–S119. doi:10.2337/dcS13-2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64:2289–2298. doi:10.2337/db14-1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aravinthan A, Scarpini C, Tachtatzis P, et al. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J Hepatol. 2013;58:549–556. doi:10.1016/j.jhep.2012.10.031 [DOI] [PubMed] [Google Scholar]
- 61. Gower BA, Nyman L. Associations among oral estrogen use, free testosterone concentration, and lean body mass among postmenopausal women. J Clin Endocrinol Metab. 2000;85:4476–4480. doi:10.1210/jcem.85.12.7009 [DOI] [PubMed] [Google Scholar]
- 62. Castles CG, Oesterreich S, Hansen R, Fuqua SAW. Auto-regulation of the estrogen receptor promoter. J Steroid Biochem Mol Biol. 1997;62:155–163. doi:10.1016/S0960-0760(97)00023-X [DOI] [PubMed] [Google Scholar]
- 63. Mosca L, Collins P, Herrington DM, et al. Hormone replacement therapy and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:499–503. [DOI] [PubMed] [Google Scholar]
- 64. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocrine Rev. 2013;34:309–338. doi:10.1210/er.2012-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lopez M, Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol Metab. 2015;26:411–421. doi:10.1016/j.tem.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 66. Reuben DB, Cheh AI, Harris TB, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50:638–644. [DOI] [PubMed] [Google Scholar]
- 67. Slawik M, Vidal-Puig AJ. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev. 2006;5:144–164. doi:10.1016/j.arr.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 68. Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Int Med. 2005;165:777–783. doi:10.1001/archinte.165.7.777 [DOI] [PubMed] [Google Scholar]
- 69. Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. [DOI] [PubMed] [Google Scholar]
- 70. Camporez JP, Jornayvaz FR, Lee HY, et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology. 2013;154:1021–1028. doi:10.1210/en.2012-1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity. 2008;16(suppl 3):S11–S22. doi:10.1038/oby.2008.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi:10.1038/35078085 [DOI] [PubMed] [Google Scholar]
- 73. Millington GW. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutrition Metab. 2007;4:18. doi:10.1186/1743-7075-4-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ma XH, Muzumdar R, Yang XM, Gabriely I, Berger R, Barzilai N. Aging is associated with resistance to effects of leptin on fat distribution and insulin action. J Gerontol A Biol Sci Med Sci. 2002;57:B225–B231. [DOI] [PubMed] [Google Scholar]
- 75. Sainz N, Barrenetxe J, Moreno-Aliaga MJ, Martinez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metab Clin Exp. 2015;64:35–46. doi:10.1016/j.metabol.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 76. Hirosawa M, Minata M, Harada KH, Hitomi T, Krust A, Koizumi A. Ablation of estrogen receptor alpha (ERalpha) prevents upregulation of POMC by leptin and insulin. Biochem Biophys Res Commun. 2008;371:320–323. doi:10.1016/j.bbrc.2008.04.073 [DOI] [PubMed] [Google Scholar]
- 77. Davis KE, Neinast MD, Sun K, et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2:227–242. doi:10.1016/j.molmet.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Andersson T, Soderstrom I, Simonyte K, Olsson T. Estrogen reduces 11beta-hydroxysteroid dehydrogenase type 1 in liver and visceral, but not subcutaneous, adipose tissue in rats. Obesity. 2010;18:470–475. doi:10.1038/oby.2009.294 [DOI] [PubMed] [Google Scholar]
- 79. Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J Mol Med. 2011;89:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S4–S9. doi:10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 81. Moiseeva O, Deschenes-Simard X, St-Germain E, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell. 2013;12:489–498. [DOI] [PubMed] [Google Scholar]
- 82. Laberge RM, Sun Y, Orjalo AV, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nature Cell Biol. 2015;17:1049–1061. doi:10.1038/ncb3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lumeng CN, Liu J, Geletka L, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–6216. doi:10.4049/jimmunol.1102188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Morley JE, Sinclair A. The metabolic syndrome in older persons: a loosely defined constellation of symptoms or a distinct entity? Age Ageing. 2009;38:494–497. doi:10.1093/ageing/afp105 [DOI] [PubMed] [Google Scholar]
- 85. Perry RJ, Camporez JP, Kursawe R, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. doi:10.1016/j.cell.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sheedfar F, Di B, iase S, Koonen D, Vinciguerra M. Liver diseases and aging: friends or foes? Aging Cell. 2013;12:950–954. doi:10.1111/acel.12128 [DOI] [PubMed] [Google Scholar]
- 87. Stratakis CA, Vottero A, Brodie A, et al. The aromatase excess syndrome is associated with feminization of both sexes and autosomal dominant transmission of aberrant P450 aromatase gene transcription. J Clin Endocrinol Metab. 1998;83:1348–1357. doi:10.1210/jcem.83.4.4697 [DOI] [PubMed] [Google Scholar]
- 88. Howard BV, Rossouw JE. Estrogens and cardiovascular disease risk revisited: the Women’s Health Initiative. Curr Opin Lipidol. 2013;24:493–499. doi:10.1097/MOL.0000000000000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Abbott RD, Launer LJ, Rodriguez BL, et al. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68:563–568. doi:10.1212/01.wnl.0000254473.88647.ca [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.