Abstract
Background:
Although there are several consensus definitions of sarcopenia, their association with health care utilization has not been studied.
Methods:
We included women from the prospective Study of Osteoporotic Fractures with complete assessment of sarcopenia by several definitions at the Study of Osteoporotic Fractures Year 10 (Y10) exam (1997–1998) who also had available data from Medicare Fee- For-Service Claims (N = 566) or Kaiser Encounter data (N = 194). Sarcopenia definitions evaluated were: International Working Group, European Working Group for Sarcopenia in Older Persons, Foundation for the NIH Sarcopenia Project, Baumgartner, and Newman. Hurdle models and logistic regression were used to assess the relation between sarcopenia status (the summary definition and the components of slowness, weakness and/or lean mass) and outcomes that included hospitalizations, cumulative inpatient days/year, short-term (part A paid) skilled nursing facility stay in the 3 years following the Y10 visit.
Results:
None of the consensus definitions, nor the definition components of weakness or low lean mass, was associated with increased risk of hospitalization or greater likelihood of short-term skilled nursing facility stay. Women with slowness by any criterion definition were about 50% more likely to be hospitalized; had a greater rate of hospitalization days amongst those hospitalized; and had 1.8 to 2.1 times greater likelihood of a short-term skilled nursing facility stay than women without slowness. There was the suggestion of a protective association of low lean mass by the various criterion definitions on short-term skilled nursing facility stay.
Conclusion:
Estimated effects of sarcopenia on health care utilization were negligible. However, slowness was associated with greater health care utilization.
Keywords: Epidemiology, Fee for service, Women, Walking speed
Sarcopenia has been conceptualized as the loss of muscle mass and accompanying decline in physical performance seen with advancing age (1,2). Early operational definitions of sarcopenia included only appendicular lean mass (ALM) from dual energy x-ray absorptiometry (3). More recently, consensus groups have proposed clinical definitions of sarcopenia that also include muscle strength and/or physical performance in addition to lean body mass (2,4,5).
Previous reports have investigated the possible link between sarcopenia and various health outcomes including falls, fractures, and mortality (6,7), but there are little data regarding health care utilization or cost associated with the various sarcopenia definitions in community dwelling adults. A paper by Janssen estimated the costs associated with sarcopenia (defined by low ALM/height2 alone) as $18.5 billion in 2000 (8). However, this study has a number of limitations. First, the authors did not use participant level cost estimates. Second, they utilized a number of assumptions which may have over- or underestimated the true costs of sarcopenia. Finally, unlike the three more recently proposed definitions of sarcopenia, the authors did not include measures of strength or physical performance in the definition of sarcopenia. Some components of the sarcopenia definitions, namely walking speed and strength, have been associated with increased risk of hospitalization in older adults (9,10), but it is unknown whether all of the various sarcopenia definitions are similarly related to hospitalizations or post-hospital skilled nursing facility (SNF) stays intended for rehabilitation. There are at least two compelling reasons to describe the relation between sarcopenia and health care utilization. First, such analyses will allow us to test the general hypothesis that those with sarcopenia are less robust than those without, and are therefore more likely to have higher risks of a variety of health outcomes including greater health care use. Second, increased health care cost and burden are often cited as one reason for developing interventions to thwart sarcopenia (11), and data to support or refute such an association would provide guidance about the potential utility of such interventions.
Using Medicare Fee-For-Service (FFS) claims data and Kaiser Encounter data linked to cohort data from the Study of Osteoporotic Fractures (SOF), a prospective cohort study of older women, we aimed to determine whether sarcopenia status as delineated by a variety of definitions (and the individual components of these definitions) was associated with future health care utilization, including hospitalization, and short-term stay in SNF.
Methods
In 1986–1988, 9,704 ambulatory Caucasian women were recruited to participate in the SOF at four academic U.S. clinical centers (12). Black women were initially excluded due to their low incidence of fractures; at the Year 10 SOF visit (1997–1998), 662 black women were recruited, bringing total enrollment to 10,366 women. Participants returned to the clinical centers approximately every 2 years for assessment.
Medicare Data Linkage
Linkage of the SOF cohort to Medicare claims data was completed as previously described (13,14). Of the 10,366 women enrolled in the SOF, 9,986 were alive as of January 1, 1991, and of these women, 9,381 (93.9%) were determined to be valid linkages to Medicare claims data. Participants at the SOF Portland site were originally recruited into the study through membership in the Kaiser Permanente Northwest health plan, and thus there was a high rate (96%) of Medicare Advantage enrollment (Part C plan) at this site. Thus, SOF Portland participants were also linked to Kaiser Permanente Northwest Encounter records as of January 1, 1991. In combining Medicare claims and Kaiser Permanente Northwest encounter records, 9,381 SOF participants (93.9% of surviving SOF participants as of January 1, 1991) were linked to claims data.
Clinic Measures
Whole-body dual energy x-ray absorptiometry scans were completed at the Year 10 visit on Hologic QDR-2000 scanners. Women walked at their usual pace over a 6-meter course; the average speed of two trials in meters/second was calculated. Grip strength was measured twice on each hand using handheld Jamar dynamometers; maximum strength was analyzed. Ability and time to rise from a chair five times was assessed. Participants self-reported functional limitations (any difficulty with activities of daily living or instrumental activities of daily living: walking 2–3 blocks, climbing up 10 steps, preparing meal, heavy housework, shopping); alcohol use (any vs none); smoking status (current vs past or never); history of fracture since age 50; physician diagnosis of medical conditions (see footnote, Table 2). Height was measured with stadiometers and weight using balance beam scales; body mass index (BMI) was calculated as weight (kg)/height2 (m2).
Table 2.
Characteristics of Participants by Hospitalization Status During Follow-up
| No Hospitalization During Follow-up (N = 418) | At Least One Hospitalization During Follow-up (N = 342) | |
|---|---|---|
| Age (y) | 76.9±4.6 | 78.0±5.1 |
| White race | 220 (52.6) | 168 (49.1) |
| Weight (kg) | 69.6±13.8 | 70.5±15.3 |
| Height (m) | 158.4±5.8 | 158.1±6.4 |
| BMI (kg/m2) | 27.7±5.3 | 28.2±5.9 |
| ALM/ht2 (kg/m2) | 6.4±1.0 | 6.5±1.1 |
| ALM (kg) | 16.1±2.8 | 16.3±3.0 |
| ALM/BMI | 0.59±0.08 | 0.59±0.09 |
| Residual of ALM: actual ALM − predicted ALM from equation† (kg) | 0.21±2.15 | 0.26±2.30 |
| Grip strength (kg) | 18.5±4.8 | 18.2±4.6 |
| Walking speed (m/s) | 0.93±0.19 | 0.83±0.23 |
| Chair stands time (s) | 13.8±5.4 | 15.5±7.1 |
| Inability to rise from chair | 39 (9.3) | 60 (17.5) |
| Functional limitations | 142 (34.0) | 200 (58.5) |
| History of fracture since age 50 | 149 (35.7) | 134 (39.2) |
| Smoking | 29 (6.9) | 27 (7.9) |
| Alcohol | 169 (40.4) | 110 (32.3) |
| Comborbidity* | ||
| 0 | 85 (20.3) | 35 (10.2) |
| 1–2 | 261 (62.4) | 181 (52.9) |
| 3+ | 72 (17.2) | 126 (36.8) |
Notes: Bold text indicates p < .05.
*Rheumatoid arthritis, osteoarthritis, diabetes, chronic obstructive pulmonary disease (COPD), angina, hypertension, congestive heart failure, heart attack, hyperthyroid, Parkinson’s disease and stroke.
†From the Newman sarcopenia definition: ALM (kg) = −13.19+14.75 × height (m) + 0.23 × total fat mass (kg) as derived for women in the Health ABC study (17); the cut-point for the residual was ≤ −1.53kg/m2.
ALM = appendicular lean mass; BMI = body mass index.
Hospitalization and Short-term SNF Stay
Days of hospitalization were derived for participants enrolled in Medicare FFS using their MEDPAR inpatient claims. For women at the SOF Portland site, we used Kaiser Encounter data to determine hospitalization and cumulative inpatient days.
For women enrolled in FFS, short-term stays in SNF designed to provide skilled nursing and rehabilitation were determined as the Medicare Part A covered benefit that followed an inpatient stay. Women with Kaiser Encounter data were excluded from the short-term SNF stays analyses.
Enrollment in Medicare and Kaiser fluctuated over time, thus the follow-up time was limited to 3 years after the Year 10 clinic visit.
Sarcopenia Definitions
Published operational definitions for sarcopenia include: Baumgartner (3); Newman (15); the International Working Group (IWG) (5); the European Working Group on Sarcopenia Older Persons (EWGSOP) (4); European Society for Clinical Nutrition and Metabolism Special Interest Group on cachexia-anorexia in chronic wasting diseases (ESPEN) (16); the Society of Sarcopenia, Cachexia, and Wasting Disorders (SCWD) (17); and the Foundation for the NIH Sarcopenia Project (FNIH Sarcopenia Project) (2) (Table 1). The ESPEN and SCWD recommendations were similar to EWGSOP and IWG, respectively, and therefore were not analyzed separately. All of the consensus definitions of sarcopenia are similar in that all combine lean mass assessed by dual energy x-ray absorptiometry with a strength and/or a physical performance component as part of a summary index; the Newman and Baumgartner definitions rely on lean mass estimates without strength or walking speed.
Table 1.
Criteria and Prevalence for Consensus Definitions of Sarcopenia in the Study of Osteoporotic Fractures (SOF)
| Slowness | Weakness | Low Lean Mass | Summary Definition | |||||
|---|---|---|---|---|---|---|---|---|
| Definition | Prevalence | Definition | Prevalence | Definition | Prevalence | Definition | Prevalence | |
| International Working Group (IWG) | Gait speed <1.0 m/s | 70.4% | Not included | N/A | ALM/ht2 ≤ 5.67kg/m2 | 22.6% | Sarcopenia: both slowness and weakness | 16.3% |
| European Working Group on Sarcopenia Older Persons (EWGSOP) | Gait speed ≤0.8 m/s | 30.4% | Grip strength <20 kg | 62.1% | ALM/ht2 ≤ 5.67kg/m2 | 22.6% | (a) Sarcopenia: low lean mass plus either slowness or weakness | Sarcopenia: 19.0% |
| Severe sarcopenia: 6.1% | ||||||||
| (b) Severe sarcopenia: all three criteria | ||||||||
| Foundation for the NIH (FNIH) Sarcopenia Project primary definition | Gait speed ≤0.8 m/s | 30.4% | Grip strength <16 kg | 32.6% | ALM/BMI < 0.512 | 19.1% | (a) weakness and low lean mass | Weakness and low lean mass: 8.3% |
| (b) slowness with weakness and low lean mass* | Slowness with weakness and low lean mass: 4.3% | |||||||
| FNIH Sarcopenia Project alternative definition | Gait speed ≤0.8 m/s | 30.4% | Grip strength <16 kg | 32.6% | ALM < 15.02 | 36.3% | (a) weakness and low lean mass | Weakness and low lean mass: 18.3% |
| (b) slowness with weakness and low lean mass* | Slowness with weakness and low lean mass: 7.6% | |||||||
| Baumgartner | Not included | N/A | Not included | N/A | ALM/ht2 < 5.45kg/m2 | 15.3% | Presence of low lean mass | 15.3% |
| Newman | Not included | N/A | Not included | N/A | Residual of actual ALM − predicted ALM from equation† ≤ −1.53kg/m2 | 20.0% | Presence of low lean mass | 20.0% |
Notes: *Since prevalence of EWGSOP severe sarcopenia was low, both sarcopenia and severe sarcopenia were analyzed together. The prevalence of FNIH “slowness with weakness and low lean mass” was also uncommon to it was not analyzed separately.
†The equation used to calculate residuals was ALM (kg) = −13.19+14.75 × height (m) + 0.23 × total fat mass (kg) as derived for women in the Health ABC study (17).
ALM = appendicular lean mass.
Analysis Sample
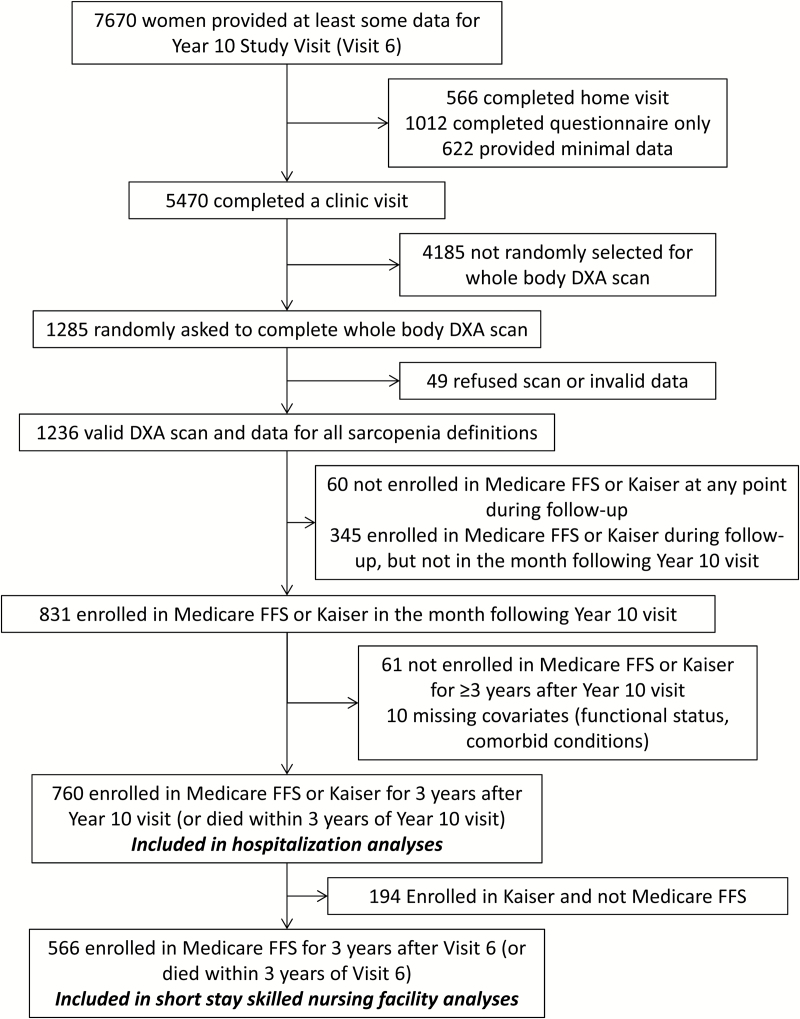
At the Year 10 visit, 7,670 women provided at least some data (5,470 had a clinic visit, 566 had a home visit, 1,634 completed questionnaires). Of those who completed a clinic visit, a random subset (N = 1,285) also completed a whole-body dual energy x-ray absorptiometry scan. Valid data for ALM, total fat, height, weight, grip strength, and gait speed were available for 1,236 women. Of these, 1,176 women were enrolled in Medicare or Kaiser at any point following their Year 10 clinic visit, and 831 were enrolled in FFS or Kaiser in at least the month following their Year 10 clinic visit. Of these, 760 women were enrolled in FFS or Kaiser for at least 3 years following Year 10 (or died within 3 years following Year 10) and had completed data for covariates and were included in the hospitalization analyses. Of these, 566 were enrolled in FFS and were included in the short-term SNF stay analyses (Figure 1).
Figure 1.
Inclusion of participants in analyses.
Statistical Analyses
We used two-part models (“hurdle” models) (18) with bootstrapping to estimate the likelihood of hospitalization, the rate ratio of inpatient hospital days amongst those hospitalized, and the mean number of inpatient days among all participants according to sarcopenia status, for each definition or component separately. The two-part hurdle mode estimates the odds of being hospitalized (yes/no) using a logit function, and then among those who are hospitalized, the counts of inpatient data were estimated using log-link functions. We used logistic regression to estimate the odds ratios of short-term SNF stays associated with sarcopenia status. All models were adjusted for covariates (see footnote, Table 3). Women were censored at time of death, outcome or end of follow-up period (3 years after the clinic visit).
Table 3.
Association Between Various Definitions of Sarcopenia and Hospitalization (N = 760) and Short-term Nursing Facility (SNF) Stay (N = 566) Over 3 Years in Older Women
| Likelihood of Hospitalization* OR (95% CI) | Rate Ratio of Inpatient Days Among Those Hospitalized* (95% CI) | Mean Rate of Inpatient Days Among All Women (d/y)* | Likelihood of SNF Stay† (OR, 95% CI) | |
|---|---|---|---|---|
| IWG summary definition | ||||
| Sarcopenia | 1.19 (0.78–1.83) | 0.89 (0.72–1.16) | 1.36 (0.96–1.78) | 0.72 (0.34–1.54) |
| No sarcopenia | 1.00 (referent) | 1.00 (referent) | 1.39 (1.17–1.58) | 1.00 (referent) |
| IWG definition components | ||||
| Slowness | 1.55 (1.08–2.21) | 1.06 (0.80–1.44) | 1.50 (1.23–1.76) | 1.81 (0.87–3.77) |
| No slowness | 1.00 (referent) | 1.00 (referent) | 1.10 (0.79–1.46) | 1.00 (referent) |
| Low lean mass | 0.94 (0.64–1.37) | 0.88 (0.71–1.11) | 1.22 (0.89–1.59) | 0.52 (0.25–1.09) |
| No low lean mass | 1.00 (referent) | 1.00 (referent) | 1.43 (1.21–1.65) | 1.00 (referent) |
| European Working Group for Sarcopenia in Older Persons (EWGSOP) summary definition | ||||
| Sarcopenia | 0.96 (0.64–1.44) | 0.92 (0.75–1.17) | 1.27 (0.91–1.70) | 0.47 (0.21–1.06) |
| No sarcopenia | 1.00 (referent) | 1.00 (referent) | 1.41 (1.19–1.61) | 1.00 (referent) |
| EWGSOP definition components | ||||
| Weakness | 0.87 (0.63–1.22) | 1.26 (0.93–1.70) | 1.47 (1.18–1.73) | 1.19 (0.67–2.13) |
| No weakness | 1.00 (referent) | 1.00 (referent) | 1.26 (0.94–1.57) | 1.00 (referent) |
| Slowness | 1.43 (1.00–2.04) | 1.27 (0.99–1.65) | 1.84 (1.37–2.36) | 2.14 (1.22–3.73) |
| No slowness | 1.00 (referent) | 1.00 (referent) | 1.20 (0.98–1.37) | 1.00 (referent) |
| Low lean mass | 0.94 (0.64–1.37) | 0.88 (0.71–1.11) | 1.22 (0.90–1.56) | 0.52 (0.25–1.09) |
| No low lean mass | 1.00 (referent) | 1.00 (referent) | 1.43 (1.19–1.65) | 1.00 (referent) |
| Foundation for the NIH (FNIH) Sarcopenia Project primary summary definition | ||||
| Weakness with low lean mass | 0.82 (0.47–1.43) | 0.93 (0.69–1.27) | 1.17 (0.72–1.69) | 0.47 (0.17–1.29) |
| No sarcopenia | 1.00 (referent) | 1.00 (referent) | 1.40 (1.19–1.59) | 1.00 (referent) |
| FNIH primary definition components | ||||
| Weakness | 0.97 (0.69–1.38) | 1.31 (1.04–1.59) | 1.64 (1.25–2.02) | 0.83 (0.47–1.49) |
| No weakness | 1.00 (referent) | 1.00 (referent) | 1.28 (1.05–1.48) | 1.00 (referent) |
| Slowness | 1.43 (1.00–2.04) | 1.27 (1.00–1.67) | 1.84 (1.37–2.36) | 2.14 (1.22–3.73) |
| No slowness | 1.00 (referent) | 1.00 (referent) | 1.20 (0.99–1.39) | 1.00 (referent) |
| Low lean mass | 0.99 (0.67–1.46) | 1.00 (0.80–1.27) | 1.37 (0.99–1.80) | 0.47 (0.23–0.96) |
| No low lean mass | 1.00 (referent) | 1.00 (referent) | 1.39 (1.15–1.60) | 1.00 (referent) |
| FNIH alternative summary definition | ||||
| Weakness with low lean mass | 0.92 (0.60–1.39) | 1.06 (0.83–1.39) | 1.40 (0.96–1.89) | 0.36 (0.16–0.80) |
| No sarcopenia | 1.00 (referent) | 1.00 (referent) | 1.38 (1.16–1.55) | 1.00 (referent) |
| FNIH alternative definition components | ||||
| Weakness | 0.97 (0.69–1.38) | 1.31 (1.04–1.65) | 1.64 (1.25–2.06) | 0.83 (0.47–1.49) |
| No weakness | 1.00 (referent) | 1.00 (referent) | 1.28 (1.05–1.48) | 1.00 (referent) |
| Slowness | 1.43 (1.00–2.04) | 1.27 (0.99–1.66) | 1.84 (1.41–2.37) | 2.14 (1.22–3.73) |
| No slowness | 1.00 (referent) | 1.00 (referent) | 1.20 (0.97–1.37) | 1.00 (referent) |
| Low lean mass | 0.86 (0.62–1.20) | 1.00 (0.80–1.30) | 1.31 (0.98–1.66) | 0.37 (0.20–0.69) |
| No low lean mass | 1.00 (referent) | 1.00 (referent) | 1.42 (1.16–1.64) | 1.00 (referent) |
| Baumgartner definition | ||||
| Sarcopenia | 1.09 (0.70–1.68) | 0.87 (0.68–1.14) | 1.27 (0.90–1.70) | 0.58 (0.25–1.36) |
| No sarcopenia | 1.00 (referent) | 1.00 (referent) | 1.40 (1.18–1.61) | 1.00 (referent) |
| Newman definition | ||||
| Sarcopenia | 1.10 (0.75–1.61) | 0.80 (0.65–0.98) | 1.21 (0.91–1.50) | 0.57 (0.28–1.19) |
| No sarcopenia | 1.00 (referent) | 1.00 (referent) | 1.43 (1.19–1.67) | 1.00 (referent) |
Notes: Models adjusted for age, clinical center, functional limitations (any difficulty with activities of daily living or instrumental activities of daily living: walking 2–3 blocks, climbing up 10 steps, preparing meal, heavy housework, shopping), and comorbidity score (none, 1, or 2 or more from the list of prevalent fracture, arthritis, diabetes, chronic obstructive pulmonary disease [COPD], angina, hypertension, congestive heart failure [CHF], MI, Parkinson’s, stroke, thyroid problem). IWG: presence of slowness (gait < 1.0 m/s) and low lean mass (ALM/ht2 ≤ 5.67kg/m2). EWGSOP: presence of slowness (gait ≤0.8 m/s) plus low lean mass (ALM/ht2 ≤ 5.67kg/m2) or weakness (grip < 20kg). FNIH primary definition: Presence of both weakness (grip < 16kg) and low lean mass (ALM/BMI < 0.512); slowness defined as gait ≤0.8 m/s. FNIH alternative definition: Presence of both weakness (grip < 16kg) and low lean mass (ALM<0.15.02); slowness defined as gait ≤0.8 m/s. Baumgartner: ALM/ht2 < 5.45kg/m2. Newman: Residual of actual ALM − predicted ALM from prediction equation ≤ −1.53kg/m2.
*Calculated using Logit-Poisson Hurdle model, bootstrapped CI’s presented for annualized rate of inpatient days. Analyses limited to women with Medicare Fee-for-Service claims (all clinics) or Kaiser Encounter data (Portland clinic only). N = 760.
†Calculated using logistic regression. Analyses limited to women with Medicare Fee-for-Service claims (all clinics). N = 566.
ALM = appendicular lean mass; BMI = body mass index; CI = confidence interval; EWGSOP = European Working Group for Sarcopenia in Older Persons; FNIH = Foundation for NIH Sarcopenia Project; IWG = International Working Group; MI = myocardial infarction; NIH = National Institute of Health.
Results
Characteristics of participants by hospitalization status are presented in Table 2. Women who were hospitalized during follow-up were older, had worse physical performance, reported more functional limitations, were less likely to consume alcohol and had a greater comorbidity burden than women who were not hospitalized. There were no differences by hospital status in race, lean mass (by any definition), grip strength, body size, fracture history, or smoking status. Characteristics of participants by presence or absence of sarcopenia for each definition are presented in Supplementary Table 1. Women with sarcopenia by any definition were older than those without, except for the Newman definition where age was similar. Women classified as having sarcopenia (by any definition) were weaker and had lower lean mass than those classified as not having sarcopenia. Associations between sarcopenia classification and other covariates varied by the definition utilized.
Sarcopenia and Hospitalization Status
In the 3 years after the sarcopenia assessment, of the 760 women in the analysis, 342 (45.0%) experienced at least one hospitalization. Estimated effects of sarcopenia status on health care utilization were negligible and many had confidence intervals that excluded clinically important effect sizes (Table 3). Slowness, by either the FNIH or EWGSOP definition (≤0.8 m/s), or IWG definition (<1.0 m/s), was associated with an increased likelihood of hospitalization. None of the other components of sarcopenia were associated with likelihood of hospitalization. Only the slowness component of the FNIH or EWGSOP definitions (≤0.8 m/s) had a borderline association with the rate ratio of inpatient days once hospitalized; none of the other definitions or their components was associated with an increased rate of inpatient days once hospitalized. The mean rate of inpatient days among all women was significantly higher in those who met the slowness definition by IWG (<1.0 m/s) or FNIH/EWGSOP (≤0.8 m/s), but did not differ for the other summary definitions or their components.
Sarcopenia and Likelihood of Short-term SNF Stay
In the 3 years after the sarcopenia assessment, of the women with available data, 75 (13.3%) women had at least one short-term SNF stay. Slowness by the IWG definition (walking speed <1.0 m/s) or the FNIH definition/EWGSOP definition (≤0.8 m/s) was associated with 1.8- to 2.1-fold-increased likelihood of short-term SNF stay (Table 3). Low lean mass by the FNIH primary definition (ALM/BMI<0.512) and FNIH alternative definition (ALM < 15.02kg) were associated with a lower likelihood of SNF stay, and the association between low lean mass by other definitions (IWG or EWGSOP or Baumgartner, ALM/ht2 ≤ 5.67kg/m2 and Newman, residual ≤ −1.53kg/m2) suggested a lower likelihood of short-term SNF stay, however, the associations were not statistically significant. None of the other definitions or their components was significantly associated with short-term SNF stay.
Discussion
In this cohort of community dwelling older women, none of the consensus definitions of sarcopenia evaluated was associated with measures of health care utilization, specifically hospitalization and short-term SNF stay. Weakness (low grip strength) was unrelated to these health care utilization outcomes, regardless of the specific criterion definition used. Low lean mass was not associated with hospitalization, although there was the suggestion of a protective effect of low lean mass (by the various definition criteria) on risk of short-term SNF stay. On the other hand, women with slowness compared to those without slowness were more likely to be hospitalized, had a greater rate of hospitalization and were more likely to have a short-term SNF stay.
Our results differ somewhat from a previous study of the relation between sarcopenia and hospitalization in older Chinese men and women in Hong Kong (6). In that study, sarcopenia (by a variety of definitions) was generally associated with greater likelihood of having more than 20 days of hospitalization over 7 years in men; in women, the associations were of borderline significance but power was limited as few participants experienced the outcome. The differences between these studies may be due to study design (such as the length of follow-up or the definition of the outcome). On the other hand, these results may indicate that sarcopenia definitions have different associations with various outcomes in different populations; further research using similar analysis techniques and identical outcome definitions will more directly address this issue.
The associations we observed between slowness and health care utilization, rather than weakness or low lean mass, may reflect the multifactorial nature of the determinants of walking speed and slowness. Many disparate factors are associated with impaired walking speed, including arthritis (19), cardiopulmonary disease and deficits (20), poor cognitive function (21), and many other conditions (22). Thus, given the multifactorial nature of deficits in walking speed, slowness may be a better measure of overall health than weakness or low lean mass, and this may explain why we observed that slowness was more strongly related to acute inpatient and SNF utilization than measures of lean mass or weakness.
The suggestion of a protective effect of low lean mass on short-term SNF stay warrants further discussion, as this finding does not support the hypothesis that low lean mass is detrimental to health outcomes. One explanation for this association may be that there is a U-shaped relationship between lean mass and short-term SNF stay, whereby women with the either highest or lowest lean mass have the highest risk of short-term SNF stay relative to women with intermediate levels of lean mass. It may be that in the SOF study of generally healthy women, there are few with particularly low lean mass and that the current low lean mass cut-points do not adequately identify those with extremely low lean mass that may be associated with poor health. In this scenario, we would also observe a higher risk of SNF stay among those with the very highest lean mass, as these individuals would have high lean mass due to obesity and very large body size rather than from greater fitness or physical activity. Therefore, under this theory, we observed a protective effect of low lean mass since there were too few low values to reflect a detrimental effect. It may be that a larger study in a population with lower lean mass values would demonstrate different associations. On the other hand, it may be that low lean mass is, in fact, protective for SNF stay, either directly, or through its correlation to greater body size and obesity.
Our results are similar to previous reports that demonstrate a strong association between measured walking speed and hospitalization risk (9,10). In addition, self-reported inability to walk ¼ mile has been associated with greater health care costs and more hospitalizations (23). Furthermore, in previous analyses in older men, we found that the strongest association between sarcopenia definitions (and their components) and clinical outcomes including falls, fractures, disability and mortality were between slowness and these outcomes, but with nonsignificant or less strong associations for the other components (weakness, low lean mass) or the summary definitions (article in press). Thus, we generally conclude that any associations between consensus definitions of sarcopenia and clinical outcomes are likely due to the slowness component of these definitions. These data provide further evidence that slowness, rather than sarcopenia per se, may be the most useful identifying individuals at greatest risk of adverse health outcomes.
In contrast to the findings of Janssen (8), we did not find a strong relationship between sarcopenia and health care utilization, although in this report we did not specifically estimate costs. However, health care utilization and cost should demonstrate similar relationships with sarcopenia, and it is unlikely that specific investigation of cost per se would significantly alter our overall conclusions.
Our study has several strengths. We used data from a very well characterized cohort of older community dwelling women and determined health care use through a unique linkage to both Medicare claims and Kaiser Encounter data. However, a number of important limitations must be noted. First, our study included mostly white older women who were healthy enough to return to our clinical centers, so generalizations to other populations such as younger adults, other races, men and the institutionalized may be limited. Second, the data were subset to women with data from Medicare FFS and/or Kaiser, which may also limit generalizability to other health care systems. Third, our sample size was relatively small limiting our power to detect small to moderate effects. However, given our point estimates and confidence intervals, any detrimental effect of sarcopenia on health care use that we would have missed would be of marginal clinical importance. Fourth, the prevalence of sarcopenia varies markedly by definition, and there is no gold standard definition of sarcopenia. With new and evolving definitions for sarcopenia currently under development, it is possible that a future definition of sarcopenia would identify women who would have greater health care utilization.
In summary, we found that slowness was associated with increased health care utilization in older women. In general we did not find evidence of increased health care utilization for those classified with sarcopenia, weakness, or low lean mass by a variety of proposed definitions. Thus, current sarcopenia definitions are unlikely to identify older women who are likely to have greater health care utilization.
Supplementary Material
Please visit the article online at http://biomedgerontology.oxfordjournals.org/ to view supplementary material.
Funding
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG02574, and R01 AG027576.
Conflict of Interest
P.M.C. reports consulting with Eli Lilly and Kinemed, and grants to her institution from Eli Lilly, GSK, IMS Health and Merck, all for work outside of this manuscript. All other authors report no conflicts.
Supplementary Material
References
- 1. Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19–21, 1988. Am J Clin Nutr. 1989;50(suppl 5):1121–1235. [PubMed] [Google Scholar]
- 2. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi:10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 4. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi:10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi:10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc. 2015;16:247–252. doi:10.1016/j.jamda.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 7. Cawthon PM, Blackwell TL, Cauley J, et al. Evaluation of the usefulness of consensus definitions of sarcopenia in older men: results from the observational osteoporotic fractures in men cohort study. J Am Geriatr Soc. 2015;63:2247–2259. doi:10.1111/jgs.13788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. [DOI] [PubMed] [Google Scholar]
- 9. Cawthon PM, Fox KM, Gandra SR, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–1419. doi:10.1111/j.1532-5415.2009.02366.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697. [DOI] [PubMed] [Google Scholar]
- 11. Camporez JG, Petersen MC, Abudukadier A, et al. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc Natl Acad Sci U S A. 2016;113:2212–2217. doi:10.1073/pnas.1525795113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. N Engl J Med. 1995;332:767–773. [DOI] [PubMed] [Google Scholar]
- 13. Schousboe JT, Gourlay M, Fink HA, et al. Cost-effectiveness of bone densitometry among Caucasian women and men without a prior fracture according to age and body weight. Osteoporos Int. 2013;24:163–177. doi:10.1007/s00198-012-1936-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schousboe JT, Paudel ML, Taylor BC, et al. Estimation of standardized hospital costs from Medicare claims that reflect resource requirements for care: impact for cohort studies linked to Medicare claims. Health Serv Res. 2014;49:929–949. doi:10.1111/1475-6773.12151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. [DOI] [PubMed] [Google Scholar]
- 16. Muscaritoli M, Anker SD, Argiles J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–159. doi:10.1016/j.clnu.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 17. Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12: 403–409. doi:10.1016/j.jamda.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mullahy J. Specification and testing of some modified count data models. J Economet. 1986;3:341–365. [Google Scholar]
- 19. Constantinou M, Barrett R, Brown M, Mills P. Spatial-temporal gait characteristics in individuals with hip osteoarthritis: a systematic literature review and meta-analysis. J Orthop Sports Phys Ther. 2014;44:291–B297. doi:10.2519/jospt.2014.4634 [DOI] [PubMed] [Google Scholar]
- 20. Elbaz A, Shipley MJ, Nabi H, Brunner EJ, Kivimaki M, Singh-Manoux A. Trajectories of the Framingham general cardiovascular risk profile in midlife and poor motor function later in life: the Whitehall II study. Int J Cardiol. 2014;172:96–102. doi:10.1016/j.ijcard.2013.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gale CR, Allerhand M, Sayer AA, Cooper C, Deary IJ. The dynamic relationship between cognitive function and walking speed: the English Longitudinal Study of Ageing. Age (Dordr). 2014;36:9682. doi:10.1007/s11357-014-9682-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cooper R, Kuh D, Cooper C, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40: 14–23. doi:10.1093/ageing/afq117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk ¼ mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26:130–135. doi:10.1007/s11606-010-1543-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.