Abstract
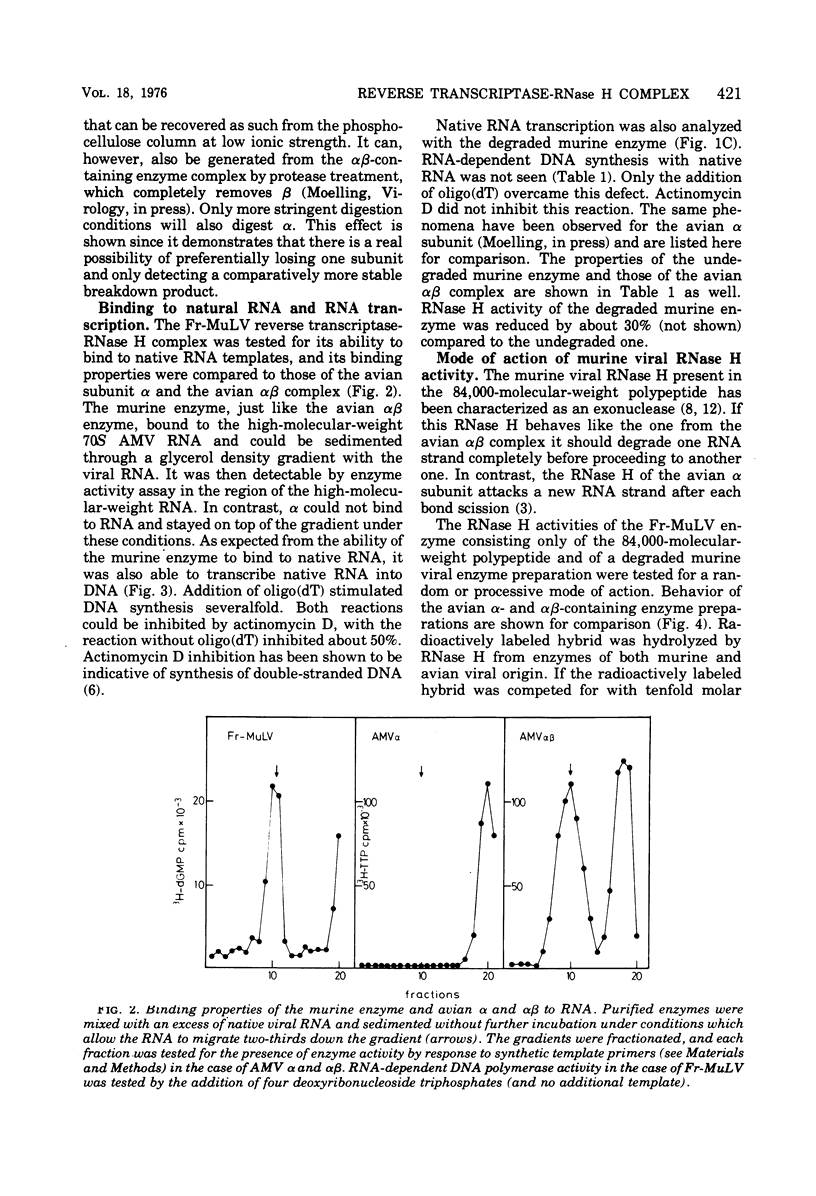
The purified reverse transcriptase-RNase H complex from Friend murine leukemia virus consists of a single polypeptide of 84,000 molecular weight, which after mild protease treatment in vitro or after intentional degradation during the purification procedure allows the generation of several additional polypeptides. Degradation destroys the RNA-dependent DNA polymerase activity with native RNA templates and reduces RNase H but does not affect response to synthetic template primers such as poly (rA)-Oligo (dT). The properties of the intact murine enzyme consisting of a single polypeptide of 84,000 molecular weight are compared to those of the avian alpha subunit and the avian alpha beta enzyme complex. The intact murine enzyme resembles the avian beta-containing enzyme complex and is different from alpha in the following respects: (i) it binds to native RNA templates; (ii) it transcribes native RNA templates into DNA, a reaction which can be inhibited by actinomycin D; (iii) RNase H activity behaves like a processive exonuclease; and (iv) analysis of the RNase H digestion products reveals oligonucleotides approximately four bases in length.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrell J. W., Gallo R. C. Purification, characterization, and comparison of the DNA polymerases from two primate RNA tumor viruses. J Virol. 1973 Sep;12(3):431–439. doi: 10.1128/jvi.12.3.431-439.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard G. F., Grandgenett D. P. Purification and characterization of the DNA polymerase and RNase H activities in Moloney murine sarcoma-leukemia virus. J Virol. 1975 Apr;15(4):785–797. doi: 10.1128/jvi.15.4.785-797.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Green M. Different mode of action of ribonuclease H in purified alpha and alpha beta ribonucleic acid-directed deoxyribonucleic acid polymerase from avian myeloblastosis virus. J Biol Chem. 1974 Aug 25;249(16):5148–5152. [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- McDonnell J. P., Garapin A. C., Levinson W. E., Quintrell N., Fanshier L., Bishop J. M. DNA polymerases of Rous sarcoma virus: delineation of two reactions with actinomycin. Nature. 1970 Oct 31;228(5270):433–435. doi: 10.1038/228433a0. [DOI] [PubMed] [Google Scholar]
- Moelling K. Characterization of reverse transcriptase and RNase H from friend-murine leukemia virus. Virology. 1974 Nov;62(1):46–59. doi: 10.1016/0042-6822(74)90302-x. [DOI] [PubMed] [Google Scholar]
- Moelling K., Gelderblom H., Pauli G., Friis R. A comparative study of the avian reticuloendotheliosis virus: relationship to murine leukemia virus and viruses of the avian sarcoma-leukosis complex. Virology. 1975 Jun;65(2):546–557. doi: 10.1016/0042-6822(75)90059-8. [DOI] [PubMed] [Google Scholar]
- Moelling K. Reverse transcriptase and RNase H: present in a murine virus and in both subunits of an avian virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):969–973. doi: 10.1101/sqb.1974.039.01.111. [DOI] [PubMed] [Google Scholar]
- Schäfer W., Seifert E. Production of a potent complement-fixing murine leukemia virus-antiserum from the rabbit and its reactions with various types of tissue culture cells. Virology. 1968 Jun;35(2):323–328. doi: 10.1016/0042-6822(68)90273-0. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Fan H., Baltimore D. Hamster leukemia virus: lack of endogenous DNA synthesis and unique structure of its DNA polymerase. J Virol. 1974 May;13(5):1075–1082. doi: 10.1128/jvi.13.5.1075-1082.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M. Studies on reverse transcriptase of RNA tumor viruses III. Properties of purified Moloney murine leukemia virus DNA polymerase and associated RNase H. J Virol. 1975 Apr;15(4):843–854. doi: 10.1128/jvi.15.4.843-854.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H. DNA polymerase of murine sarcoma-leukemia virus: lack of detectable RNase H and low activity with viral RNA and natural DNA templates. J Virol. 1973 Dec;12(6):1512–1521. doi: 10.1128/jvi.12.6.1512-1521.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann B. J., Schmidt J., Wolfrum D. I. RNA-dependent DNA polymerase and ribonuclease H from Friend virions. FEBS Lett. 1974 Jul 1;43(1):37–44. doi: 10.1016/0014-5793(74)81100-2. [DOI] [PubMed] [Google Scholar]


