Abstract
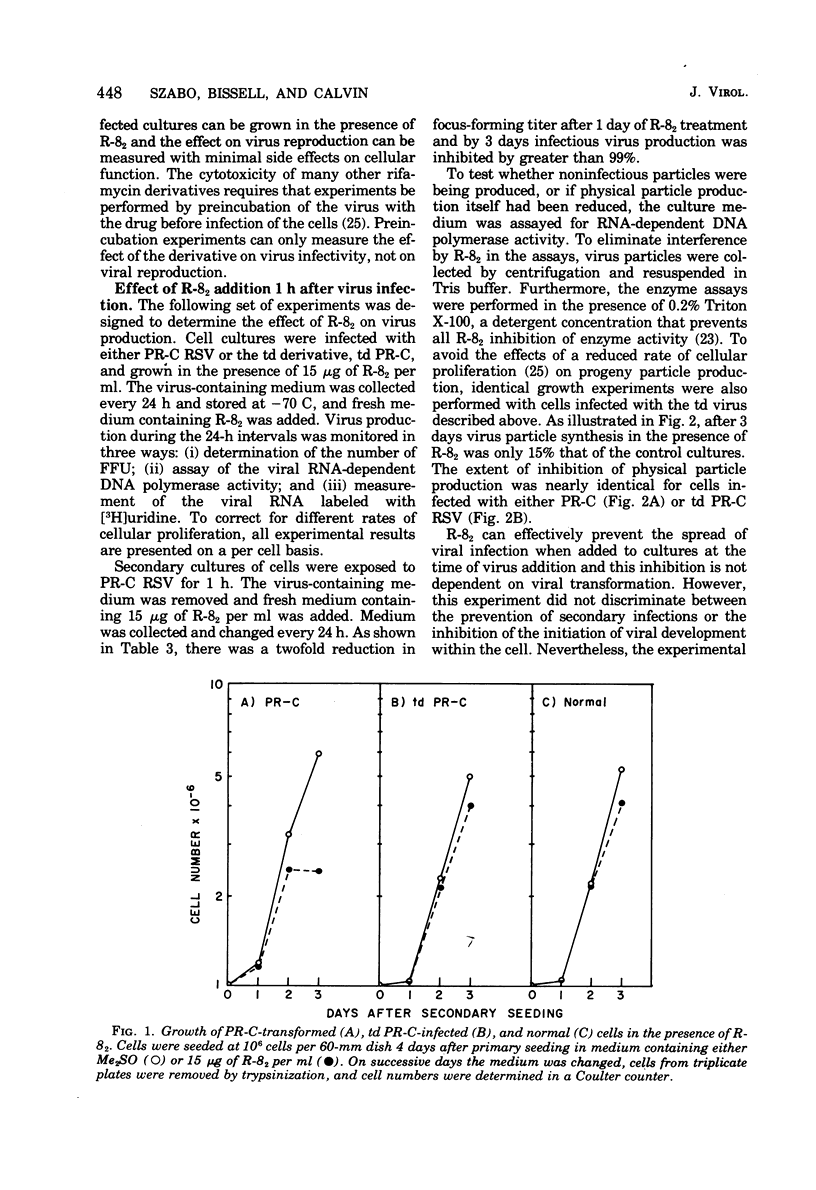
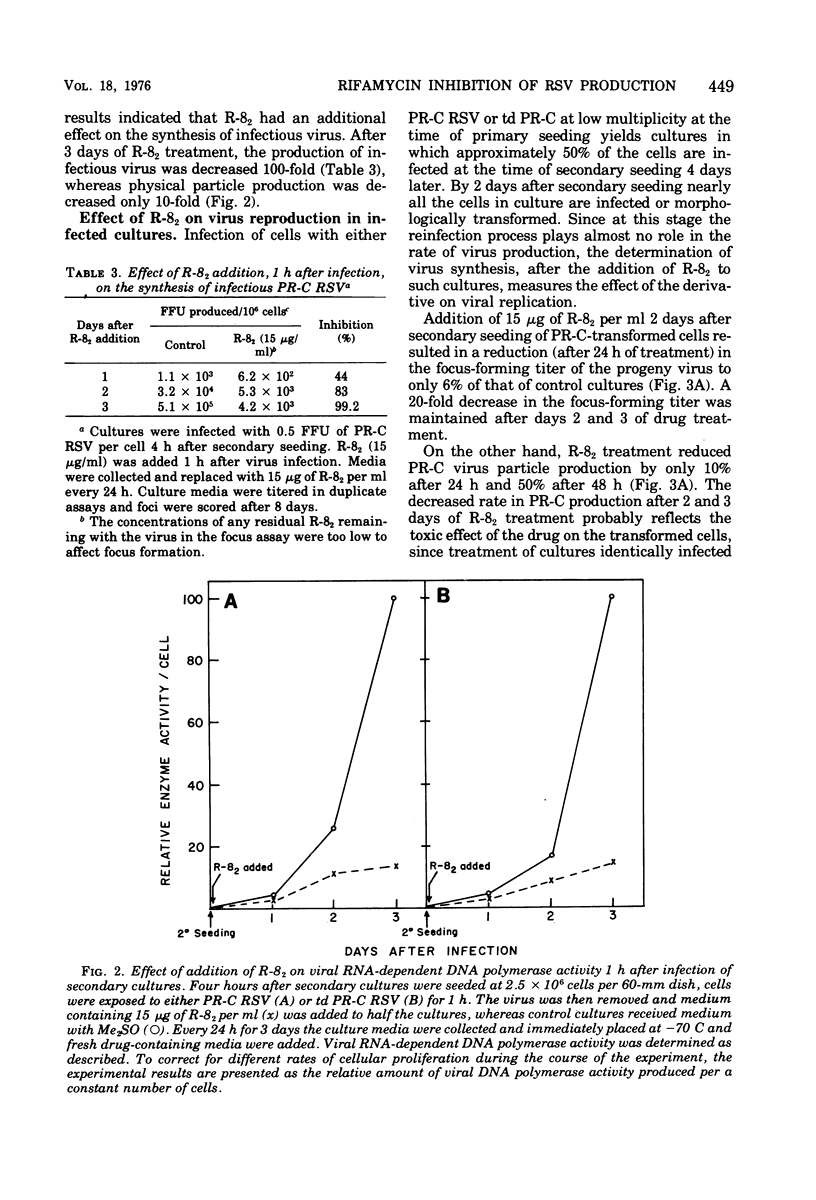
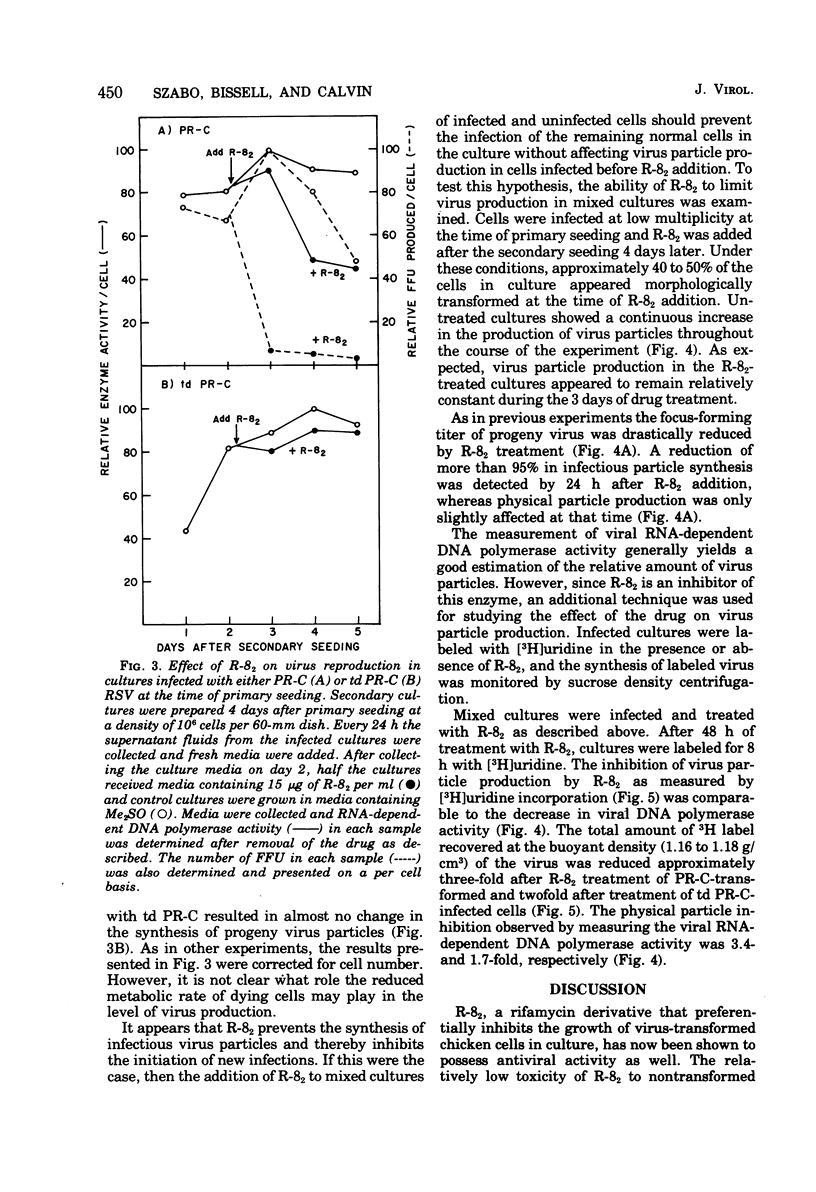
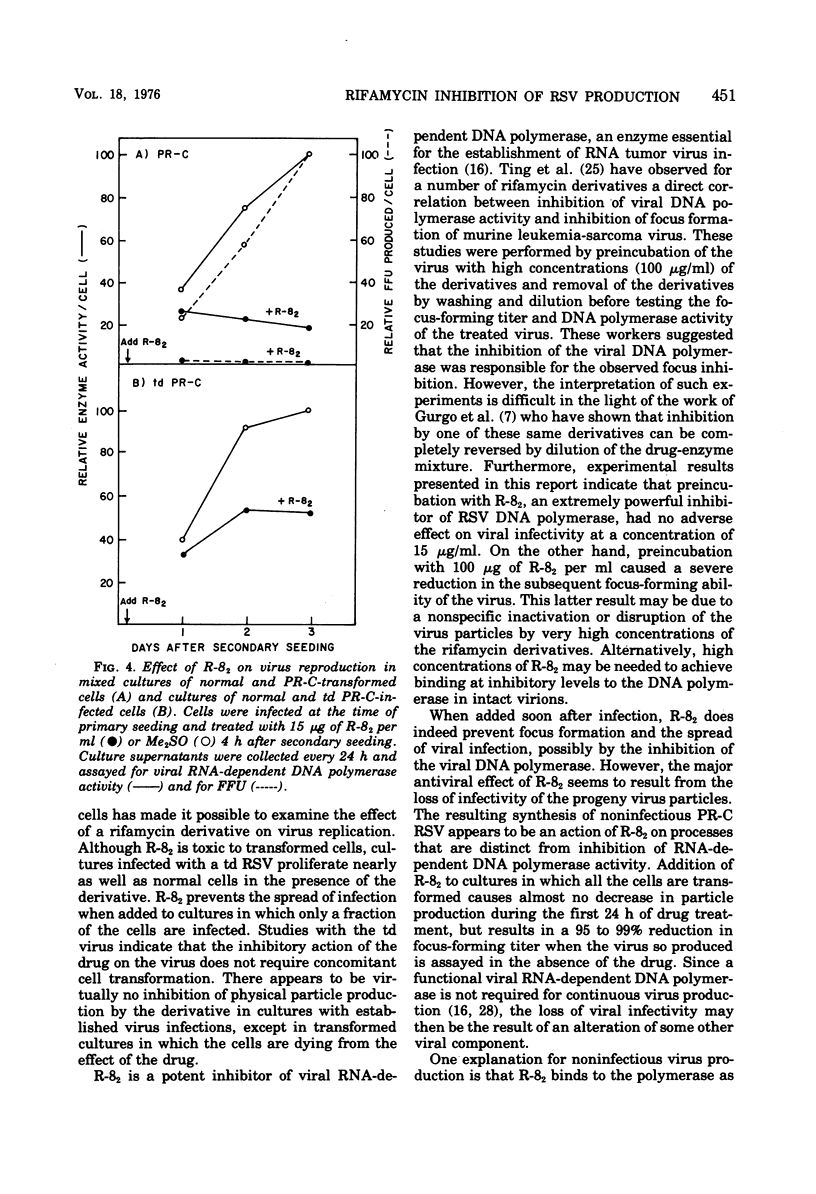
A new rifamycin derivative, rifazone-82 (R-82), an inhibitor of viral RNA-dependent DNA polymerase, is selectively toxic to transformed chicken cells in culture. R-82 has now been shown to possess antiviral activity as well. The relatively nontoxic properties of R-82 to nontransformed cells have permitted the execution of experiments examining the effect of a rifamycin derivative on virus reproduction. Addition of low concentrations of R-82 (15 mug/ml) to cultures soon after Rous sarcoma virus infection prevents the spread of infection thoroughout the culture. This inhibition is not dependent on concomitant cellular transformation as identical results were obtained with cells infected with a transformation-defective Rous sarcoma virus. Addition of R-82 to cultures in which all the cells are infected does not substantially affect the yield of physical particles as measured by RNA-dependent DNA polymerase activity and by (3H) uridine incorporation into viral RNA. However, the infectivity of the progeny virus, as measured by focus-forming ability, is decrreased 95 to 99% by R-82 treatment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ishai Z., Heller E., Goldblum N., Becker Y. Rifampicin and poxvirus replication. Nature. 1969 Oct 4;224(5214):29–32. doi: 10.1038/224029a0. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Hatie C., Tischler A. N., Calvin M. Preferential inhibition of the growth of virus-transformed cells in culture by rifazone-82, a new rifamycin derivative. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2520–2524. doi: 10.1073/pnas.71.6.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., White R. C., Hatie C., Bassham J. A. Dynamics of metabolism of normal and virus-transformed chick cells in culture. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2951–2955. doi: 10.1073/pnas.70.10.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P., Helm K. V., Canaani E. Comparative properties of RNA and DNA templates for the DNA polymerase of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2505–2509. doi: 10.1073/pnas.68.10.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P., Helm K. V., Canaani E. Properties of a soluble DNA polymerase isolated from Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):747–751. doi: 10.1073/pnas.68.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Bragdon J., Rankin A. 3-Cyclic amine derivatives of rifamycin: strong inhibitors of the DNA polymerase activity of RNA tumor viruses. Proc Natl Acad Sci U S A. 1972 May;69(5):1294–1298. doi: 10.1073/pnas.69.5.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgo C., Grandgenett D. P., Gerad G. F., Green M. Interaction of purified ribonucleic acid directed deoxyribonucleic acid polymerase of avian myeloblastosis virus and murine sarcoma-leukemia virus with a rifamycin SV derivative. Biochemistry. 1974 Feb 12;13(4):708–713. doi: 10.1021/bi00701a012. [DOI] [PubMed] [Google Scholar]
- Gurgo C., Ray R. K., Thiry L., Green M. Inhibitors of the RNA and DNA dependent polymerase activities of RNA tumour viruses. Nat New Biol. 1971 Jan 27;229(4):111–114. doi: 10.1038/newbio229111a0. [DOI] [PubMed] [Google Scholar]
- Gurgo C., Ray R., Green M. Rifamycin derivatives strongly inhibiting RNA leads to DNA polymerase (reverse transcriptase) of murine sarcoma viruses. J Natl Cancer Inst. 1972 Jul;49(1):61–79. [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Noninfectious RSV deficient in DNA polymerase. Virology. 1971 Jan;43(1):313–316. doi: 10.1016/0042-6822(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Hartmann G., Honikel K. O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- KILBOURNE E. D. Inhibition of influenza virus multiplication with a glucose antimetabolite (2-deoxy-D-glucose). Nature. 1959 Jan 24;183(4656):271–272. doi: 10.1038/183271b0. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Scholtissek C., Rott R. Inhibition of the multiplication of enveloped RNA-viruses by glucosamine and 2-deoxy-D-glucose. J Gen Virol. 1972 Mar;14(3):251–259. doi: 10.1099/0022-1317-14-3-251. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Scholtissek C., Rott R. Inhibition of glycoprotein biosynthesis of influenza virus by D-glucosamine and 2-deoxy-D-glucose. Virology. 1972 Sep;49(3):723–734. doi: 10.1016/0042-6822(72)90529-6. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H., Horst J., Vogt P. K. Avian tumor virus RNA: a comparison of three sarcoma viruses and their transformation-defective derivatives by oligonucleotide fingerprinting and DNA-RNA hybridization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2266–2270. doi: 10.1073/pnas.70.8.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Mason W. S. Characterization of two conditional early mutants of Rous sarcoma virus. Virology. 1973 May;53(1):258–273. doi: 10.1016/0042-6822(73)90484-4. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Katz E., Grimley P. M. Rifampicin: a specific inhibitor of vaccinia virus assembly. Nature. 1969 Dec 27;224(5226):1280–1284. doi: 10.1038/2241280a0. [DOI] [PubMed] [Google Scholar]
- Prochownik E. V., Panem S., Kirsten W. H. Biological and physical modifications of a murine oncornavirus by 2-deoxy-D-glucose. J Virol. 1975 Jun;15(6):1323–1331. doi: 10.1128/jvi.15.6.1323-1331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Rubin H. Effects of local cell concentrations upon the growth of chick embryo cells in tissue culture. Exp Cell Res. 1968 Mar;49(3):666–678. doi: 10.1016/0014-4827(68)90213-9. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe J. H., Timbury M. C., Williams J. F. Rifampicin inhibits the growth of some mammalian viruses. Nature. 1969 Apr 26;222(5191):341–345. doi: 10.1038/222341a0. [DOI] [PubMed] [Google Scholar]
- Thompson F. M., Libertini L. J., Joss U. R., Calvin M. Detergent effects on a reverse transcriptase activity and on inhibition by rifamycin derivatives. Science. 1972 Nov 3;178(4060):505–507. doi: 10.1126/science.178.4060.505. [DOI] [PubMed] [Google Scholar]
- Thompson F. M., Tischler A. N., Adams J., Calvin M. Inhibition of three nucleotide polymerases by rifamycin derivatives. Proc Natl Acad Sci U S A. 1974 Jan;71(1):107–109. doi: 10.1073/pnas.71.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting R. C., Yang S. S., Gallo R. C. Reverse transcriptase, RNA tumour virus transformation and derivatives of rifamycin SV. Nat New Biol. 1972 Apr 12;236(67):163–166. doi: 10.1038/newbio236163a0. [DOI] [PubMed] [Google Scholar]
- Tischler A. N., Joss U. R., Thompson F. M., Calvin M. Synthesis of some rifamycin derivatives as inhibitors of an RNA-instructed DNA polymerase function. J Med Chem. 1973 Oct;16(10):1071–1075. doi: 10.1021/jm00268a001. [DOI] [PubMed] [Google Scholar]
- Tischler A. N., Thomspon F. M., Libertini L. J., Calvin M. Rifamycin derivatives as inhibitors of a ribonucleic acid instructed deoxyribonucleic acid polymerase function. Effect of lipophilicity. J Med Chem. 1974 Sep;17(9):948–952. doi: 10.1021/jm00255a008. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Mason W. S., Drost S. D., Baltimore D. DNA polymerase activity from two temperature-sensitive mutants of Rous sarcoma virus is thermolabile. Nature. 1974 Sep 6;251(5470):27–31. doi: 10.1038/251027a0. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Spontaneous segregation of nontransforming viruses from cloned sarcoma viruses. Virology. 1971 Dec;46(3):939–946. doi: 10.1016/0042-6822(71)90092-4. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J. M., Oleinick N. L., Corcoran J. W. The inhibition of bacterial RNA synthesis by the rifamycin antibiotics. Biochim Biophys Acta. 1968 Aug 23;166(1):268–271. doi: 10.1016/0005-2787(68)90515-7. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Gallo R. C. Interaction between murine type-C virus RNA-directed DNA polymerases and rifamycin derivatives. Biochim Biophys Acta. 1974 Apr 10;340(4):419–436. doi: 10.1016/0005-2787(74)90063-x. [DOI] [PubMed] [Google Scholar]
- Yang S. S., Herrera F. M., Smith R. G., Reitz M. S., Lancini G., Ting R. C., Gallo R. C. Rifamycin antibiotics: inhibitors of Rauscher murine leukemia virus reverse transcriptase and of purified DNA polymerases from human normal and leukemic lymphoblasts. J Natl Cancer Inst. 1972 Jul;49(1):7–25. [PubMed] [Google Scholar]


