Abstract
Context:
Adrenal production of dehydroepiandrosterone sulfate (DHEA-S) increases throughout childhood owing to expansion of the zona reticularis (ZR). ZR features cells with a steroidogenic phenotype distinct from that of the adjacent zona fasciculata, with higher expression of cytochrome b5 type A (CYB5A) and steroid sulfotransferase type 2A1 but decreased 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2). In addition to DHEA-S, three adrenal Δ5-steroid sulfates could provide additional tools to define adrenal maturation.
Objective:
This study sought to simultaneously measure serum levels of four adrenal Δ5-steroid sulfates, pregnenolone sulfate (Preg-S), 17α-hydroxypregnenolone sulfate (17OHPreg-S), DHEA-S, and 5-androstenediol-3-sulfate (Adiol-S) as a function of age and relate their production to the age-dependent adrenal localization of CYB5A.
Participants and Methods:
Δ5-steroid sulfates were quantified by liquid chromatography–tandem mass spectrometry in sera from 247 normal children (129 males,118 females) age 1.5–18 y and 42 adults (20 males, 22 females). Immunofluorescence localized HSD3B2 and CYB5A in normal adrenal glands from subjects age 2–35 y. Finally, HAC15 adrenocortical cells were transduced with lentiviral short hairpin RNA to suppress CYB5A expression.
Results:
Of the Δ5-steroid sulfates quantified, DHEA-S was most abundant. Adiol-S increased in parallel with DHEA-S. Steroid ratios (17OHPreg-S/DHEA-S) suggested increases in 17,20-lyase activity during childhood. Immunofluorescence analysis showed age-related increases in ZR CYB5A immunoreactivity. Furthermore, silencing CYB5A in HAC15 adrenocortical cells significantly reduced DHEA-S and Adiol-S production.
Conclusion:
Adiol-S shows a similar age-related increase to that of DHEA-S. This likely results from the childhood expansion of CYB5A-expressing ZR, which enhances 17,20-lyase activity and the production of DHEA-S and Adiol-S.
The adrenal steroids 5-androstenediol-3-sulfate and dehydroepiandrosterone sulfate showed similar age-related increases parallel to the expression of cytochrome b5 in the adrenal zona reticularis.
The human fetal adrenal gland secretes large amounts of the 19-carbon (C19) steroid, dehydroepiandrosterone sulfate (DHEA-S), but DHEA-S levels decrease drastically after birth and remain low for the first few years of life. The expansion and differentiation of the zona reticularis (ZR) in the definitive adrenal cortex yields resurgence in dehydroepiandrosterone (DHEA) and DHEA-S production during childhood (1–4). Neither DHEA nor DHEA-S is a potent androgen, but both are precursors of bioactive androgens via metabolism in peripheral tissues (4–7). Adrenarche is the clinical manifestation of enhanced DHEA-S concentrations in childhood and is reflected by the onset of body odor, the appearance of mild acne, and the development of axillary and/or pubic hair in boys and girls (4, 8–11). The levels of DHEA-S gradually peak in the second decade of life after which there is a gradual decline with further aging. The physiologic and/or endocrine signals that cause the variation in circulating DHEA-S levels during childhood remain to be defined.
The initiation of production of DHEA and DHEA-S has been attributed to a series of intra-adrenal changes in the expression of steroidogenic enzymes needed to synthesize these C19 steroids. The progression of this phenomenon relies on the differential expression of 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) in the zona fasciculata (ZF), and that of cytochrome b5 type A (CYB5A) and sulfotransferase type 2A1 (SULT2A1) in the ZR. HSD3B2 competes with CYP17A1 (17α-hydroxylase/17, 20-lyase) and SULT2A1 for pregnenolone (Preg) and 17α-hydroxypregnenolone (17OHPreg) and diverts steroidogenesis toward mineralo- and glucocorticoid pathways in the zona glomerulosa and ZF, respectively (Supplemental Figure 1). CYB5A is an allosteric regulator of CYP17A1 that enhances its 17,20-lyase activity, which is required for DHEA synthesis in the ZR (Supplemental Figure 1). SULT2A1 is a cytoplasmic enzyme expressed in the cells of the adrenal ZR and has broad steroid substrate specificity, which includes DHEA, Preg, and 17OHPreg (12, 13). SULT2A1 is necessary for the synthesis of the sulfated form of DHEA, which is the most abundant circulating steroid in human serum (14). Thus, the relative absence of HSD3B2 expression/activity combined with the augmenting expression of CYB5A and SULT2A1 in the expanding ZR facilitates DHEA-S synthesis through the postnatal period to adult life (15–17).
Circulating DHEA-S has a much lower rate of clearance than DHEA (18), and as a result of its extended half-life, exhibits little diurnal rhythm. Serum DHEA-S is therefore a general reflection of adrenal cortex function and ZR development. DHEA-S levels have been assessed in humans from birth to adulthood using RIA, chemiluminescence methods, and liquid chromatography–tandem mass spectrometry (LC-MS/MS) (19–27). In this study, LC-MS/MS was used to simultaneously track the changes in the serum concentrations of four Δ5-steroid sulfates: pregnenolone sulfate (Preg-S), 17-hydroxypregnenolone sulfate (17OHPreg-S), DHEA-S, and 5-androstenediol-3-sulfate (Adiol-S) (Supplemental Figure 1), as a function of age. In addition, our adrenal tissue and cell-based studies suggest that age-related alterations in the expression of CYB5A are instrumental in the increased production of DHEA-S and Adiol-S.
Materials and Methods
An expanded Supplemental Materials and Methods section with statistical analyses is provided.
Patient sera
After approval from the Institutional Review Board at the University of Michigan and University of Texas Southwestern Medical Center, serum was obtained from 247 children (129 males, 118 females) between the ages of 1.5 and 18 years (y) and from 42 adults (20 males, 22 females) between ages 20 and 40 years. Subjects presented to pediatric and adult outpatient clinics for minor health conditions and routine visits. Criteria of exclusion were major comorbidities, acute or debilitating chronic illnesses, and steroid therapy.
Quantitation of Δ5-steroid sulfates
Serum Δ5-steroid sulfates were measured using LC-MS/MS. (Refer to Supplemental Materials and Methods and Supplemental Tables 1–3.)
Immunolocalization of HSD3B2 and CYB5A in human adrenals
Ten percent formalin-fixed and paraffin-embedded adrenal glands from individuals between ages 2 and 35 years were obtained from deceased renal transplant donors and autopsy cases without any adrenal pathology under Institutional Review Board approval from University of Michigan and Tohoku University Hospital. Quantitative dual immunofluorescence was performed for CYB5A (antihuman mouse monoclonal) and HSD3B2 (antihuman rabbit polyclonal) (also see the representative images shown in Supplemental Figure 2). The ratio of CYB5A-expressing area/HSD3B2-expressing area was calculated and compared in different age groups.
Cell culture and knockdown of CYB5A
The expression of CYB5A was silenced in HAC15 adrenocortical cells (28) using GIPZ lentiviral short hairpin RNA (shRNA) technology (Supplemental File). In short, lentiviral particles containing scrambled (control) and CYB5A shRNAs were transduced into HAC15 cells with 6 μg/mL of polybrene. After antibiotic selection with puromycin, HAC15 cells with the CYB5A shRNA were plated at cloning density and the cell clone with the lowest expression of CYB5A (CYB5A-KO) was used for this study. The scrambled and CYB5A-KO cells were plated at a density of 200 000 cells/well (24-well dish) in growth medium and grown to 60–70% confluence, then cultured in low serum medium for 18 hours prior to treatment with or without forskolin for 48 hours. At the end of each treatment, the medium was collected from each well and stored at −20°C for quantification of Δ5-steroid sulfates using LC-MS/MS, whereas cells were frozen at −80°C for protein assay, or RNA isolation, with subsequent qPCR analysis.
For protein assay, cells were lysed in 100 μL mammalian protein extraction reagent, and the protein content was estimated by the manufacturer's protocol (ThermoFisher Scientific). RNA extraction and gene expression assays for Peptidylprolyl isomerase A (PPIA, Cyclophilin A, control gene) and CYB5A were performed as described previously (29).
Immunocytochemistry
Scrambled and CYB5A-KO cells were grown on microscope slides and subsequently fixed with methanol at −20°C (Supplemental File). Immunocytochemistry was performed for CYB5A with a secondary Alexa fluor 594-conjugated goat antimouse antibody.
Data analysis and statistical methods
Steroid metabolite levels across age groups for boys and girls were compared by one-way ANOVA followed by the Tukey honest significance difference post-hoc test. To correct for heteroscedasticity, skewness, and non-normal distribution of the residuals, we performed the box-cox transformation on the raw data (30) (Supplemental File for details). The smoothed steroid concentrations-for-age and steroid ratios-for-age centile curves were modeled with the generalized additive model for location, scale, and shape (GAMLSS) package for R (31) (Supplemental File). Immunofluorescence and cell culture data were analyzed by One-Way ANOVA using Holm-Sidak method for multiple comparisons and t test for comparison between two groups. Significance was accepted at P < .05.
Results
Association of Δ5-steroid sulfates with age and sex
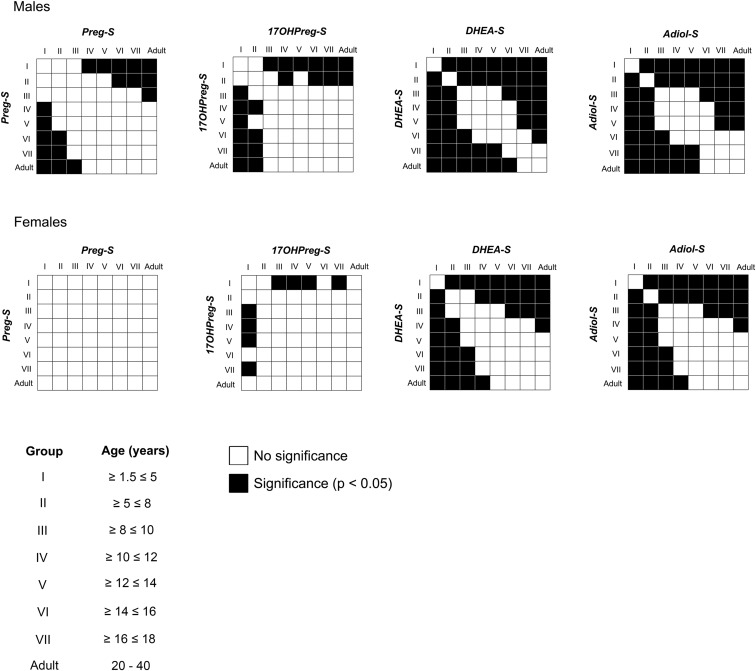
Subjects were grouped by chronological age and sex and their steroid concentrations were assessed (Table 1 and Supplemental Table 4). Age centiles of four Δ5-steroid sulfates were constructed as shown in Figure 1. DHEA-S was the most abundant Δ5-steroid sulfate across the targeted age spectrum in both sexes at all ages. Along with DHEA-S, serum levels of Adiol-S showed age-dependent increases in both the sexes after the first 5 years of life. Both DHEA-S and Adiol-S levels were low until age 5 years and then increased gradually until adulthood (Figures 1 and 2). The first significant increase in the levels of DHEA-S and Adiol-S was seen in Group II (5–8 y) in both the sexes (Table 1 and Figure 2; P < .05). Serum concentrations of Preg-S and 17OHPreg-S remained low for the first decade of life, after which a slow increase in Preg-S was observed for males only and in 17OHPreg-S for both males and females. None of the Δ5-steroid sulfates differed between males and females between 1.5 and 18 years; however, DHEA-S and Adiol-S were significantly higher in males than females for adults (P < .05).
Table 1.
Serum Concentrations of Δ5-Steroid Sulfates in 129 Males and 118 Females Between Ages 1.5 and 18 Years
| Group | Age, y | n | Preg-S, μg/dL, Mean (95% CI) | 17OHPreg-S, μg/dL, Mean (95% CI) | DHEA-S, μg/dL, Mean (95% CI) | Adiol-S, μg/dL, Mean (95% CI) |
|---|---|---|---|---|---|---|
| Males | ||||||
| I | ≥1.5 ≤ 5 | 23 | 0.96 (0.65–1.37) | 0.44 (0.31–0.60) | 3.92 (2.11–6.67) | 0.33 (0.22–0.50) |
| II | >5 ≤ 8 | 17 | 1.06 (0.72–1.50) | 0.52 (0.37–0.72) | 15.4 (9.31–24.1) | 0.95 (0.63–1.41) |
| III | >8 ≤ 10 | 20 | 1.50 (1.14–1.93) | 0.77 (0.59–0.97) | 54.6 (38.3–75.6) | 3.25 (2.14–4.85) |
| IV | >10 ≤ 12 | 22 | 2.05 (1.65–2.51) | 1.03 (0.86–1.22) | 72.1 (53.8–94.8) | 4.88 (3.35–7.01) |
| V | >12 ≤ 14 | 13 | 2.00 (1.16–3.23) | 0.92 (0.58–1.36) | 83.3 (45.7–140.2) | 7.42 (4.03–13.2) |
| VI | >14 ≤ 16 | 19 | 2.31 (1.58–3.27) | 1.05 (0.84–1.29) | 117.2 (87.2–154.1) | 9.97 (6.56–14.9) |
| VII | >16 ≤ 18 | 15 | 2.32 (1.84–2.88) | 1.07 (0.88–1.29) | 164.1 (124.1–213.0) | 18.0 (12.4–25.7) |
| Adults | 20–40 | 20 | 2.72 (2.18–3.34) | 1.18 (0.96–1.43) | 212.2 (174.9–255.3) | 20.1 (16.5–24.4) |
| Females | ||||||
| I | ≥1.5 ≤ 5 | 18 | 1.00 (0.79–1.25) | 0.45 (0.34–0.60) | 6.12 (3.91–9.22) | 0.43 (0.21–0.61) |
| II | >5 ≤ 8 | 20 | 1.09 (0.87–1.37) | 0.57 (0.47–0.70) | 20.5 (12.5–32.0) | 1.34 (0.83–2.10) |
| III | >8 ≤ 10 | 15 | 1.78 (1.30–2.38) | 0.84 (0.60–1.19) | 48.1 (30.5–72.7) | 3.48 (1.86–6.26) |
| IV | >10 ≤ 12 | 17 | 1.69 (1.22–2.30) | 0.86 (0.65–1.14) | 65.2 (49.9–83.9) | 4.69 (3.27–6.64) |
| V | >12 ≤ 14 | 16 | 1.71 (1.07–2.65) | 0.91 (0.71–1.17) | 88.8 (54.7–137.7) | 5.51 (3.47–8.56) |
| VI | >14 ≤ 16 | 18 | 1.55 (1.10–2.14) | 0.76 (0.54–1.09) | 122.4 (88.7–165.2) | 8.35 (5.78–11.9) |
| VII | >16 ≤ 18 | 14 | 1.91 (1.39–2.58) | 0.83 (0.61–1.14) | 116.9 (88.3–152.2) | 8.47 (5.49–12.8) |
| Adults | 20–40 | 22 | 1.80 (1.33–2.39) | 0.73 (0.57–0.95) | 130.0 (96.8–171.6) | 10.4 (7.70–14.0) |
Abbreviation: CI, confidence interval.
Data are expressed as mean with corresponding 95% CIs calculated on transformed values. Statistical significance was determined by one-way ANOVA followed by the Tukey post-hoc test on transformed data (Figure 2).
Figure 1.
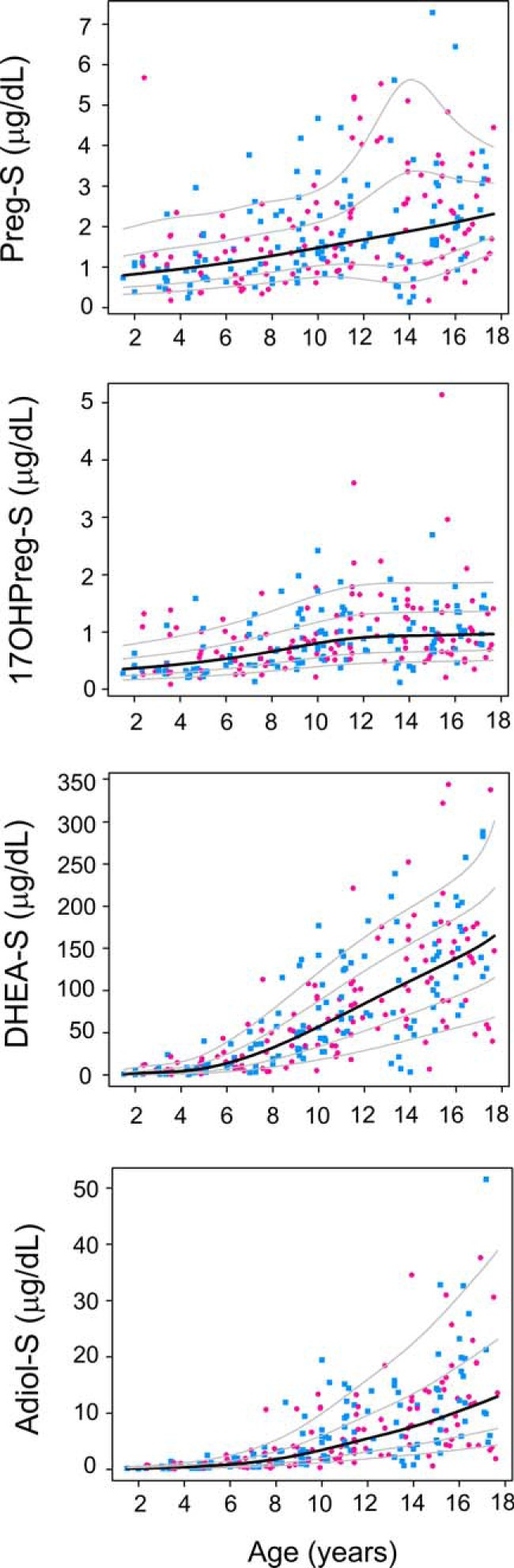
Serum concentrations of Δ5-steroid sulfates across ages. LC-MS/MS was used to quantify serum concentrations of Δ5-steroid sulfates in the serum of males (n = 129, indicated by blue squares) and females (n = 118, indicated by pink circles) between ages 1.5 and 18 y. Age-centile curves were constructed from the raw data. Median is denoted by the black line and the 10th, 25th, 75th, and 90th percentiles are denoted by gray lines.
Figure 2.
Statistical comparison of serum steroid levels between different age groups. Statistical significance was determined by one-way ANOVA followed by the Tukey post-hoc test on transformed data and the comparison between all pairs of age groups was plotted as a correlation matrix. Black boxes indicate statistical significance between the two groups (P < .05) and white boxes indicate no significant difference.
Ratios of serum steroid metabolites
Subjects were grouped by age and sex (Supplemental Table 5), and age centiles for ratios of serum 17OHPreg-S/Preg-S, DHEA-S/17OHPreg-S, and Adiol-S/DHEA-S were plotted (Supplemental Figure 3). Although 17OHPreg-S/Preg-S remained stable throughout the age spectrum, DHEA-S/17OHPreg-S increased with age in both sexes (Supplemental Figure 3). The augmentation in the DHEA-S/17OHPreg-S ratio paralleled that for circulating DHEA-S levels shown in Figure 1 (Supplemental Figure 3), with the first significant increase occurring in Group II (5–8 y) in both the sexes (Supplemental Table 5 and Supplemental Figure 4; P < .05). In contrast, the serum Adiol-S/DHEA-S ratio was slightly, but significantly higher between 1.5–5 years compared with 5–12 years in males (Supplemental Table 5 and Supplemental Figure 4; P < .05). In females, we observed the first significant increase in the serum Adiol-S/DHEA-S ratio only in adulthood (Supplemental Table 5 and Supplemental Figure 4; P < .05).
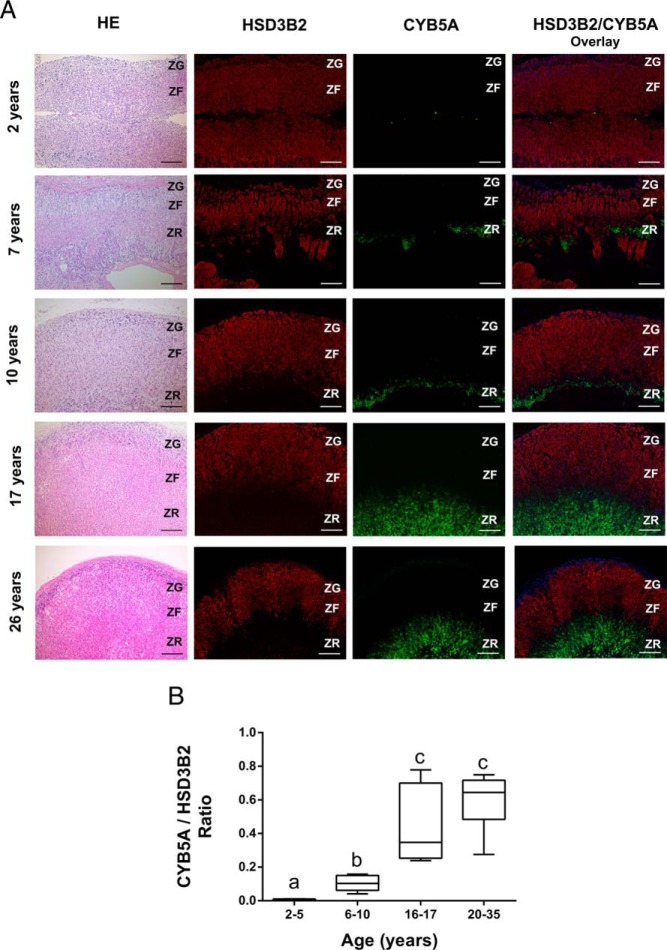
Adrenal zonal expression of HSD3B2 and CYB5A across chronological age
The age-related increase in production of DHEA-S and Adiol-S along with the DHEA-S/17OHPreg-S ratio suggested a corresponding expansion of the ZR. Double immunofluorescence analysis was performed for enzymes and cofactor proteins most highly expressed in the ZF and ZR (HSD3B2 and CYB5A, respectively) in normal adrenal glands of males and females in the following age groups: Group A (2–5 y), Group B (6–10 y), Group C (16–17 y), and Group D (20–35 y) (n ≥ 4, each age group). Representative images are shown in Figure 3. There were no significant differences for the area showing immunoreactivity for HSD3B2 among different age groups (Figure 3A); however, CYB5A immunoreactivity (including the ratio of CYB5A/HSD3B2) showed a significant augmentation with age (Figure 3, A and B; P < .05).
Figure 3.
Immunolocalization of HSD3B2 and CYB5A in the human adrenal. A, Representative images of double immunofluorescence of HSD3B2 (red) and CYB5A (green) in adrenal glands from following age groups: 2–5 y (2 y male), 6–10 y (7 and 10 y males), 16–17 y (17 y female) and 20–35 y (26 y female). HSD3B2 and CYB5A are sharply segregated to the zona glomerulosa–ZF and the ZR, respectively. Nuclei are counterstained in DAPI (blue). Scale bars, 200 μm. B, Dual immunofluorescence was performed for HSD3B2 and CYB5A in the adrenal glands of the following age groups: 2–5 y (n = 4), 6–10 y (n = 6), 16–17 y (n = 6), and 20–35 y (n = 6). The areas expressing CYB5A (green) and HSD3B2 (red) were quantified using image analysis, and ratios of areas with CYB5A expression/HSD3B2 expression were then calculated and compared in different age groups. Data are represented as mean ± SEM. Statistical significance was determined using a one-way ANOVA followed by a Holm-Sidak test. Different letters above the bars indicate statistically significant differences (P < .05) between the different age groups.
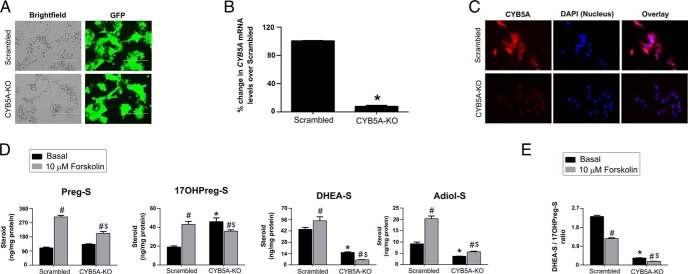
Effect of CYB5A knockdown on adrenocortical cell biosynthesis of Δ5-steroid sulfates
Selective shRNA for CYB5A was used to further examine the role of CYB5A in the synthesis of DHEA-S and Adiol-S in the HAC15 adrenocortical cell line. After antibiotic selection, the scrambled and CYB5A-KO cells expressed the transduction marker, green fluorescent protein (Figure 4A). Successful knockdown of CYB5A in the CYB5A-KO cells was confirmed by qPCR for mRNA (95% reduction compared with scrambled) (Figure 4B) and immunocytochemistry for protein levels (Figure 4C). DHEA-S and Adiol-S production was decreased in CYB5A-KO cells compared with scrambled cells under both basal and forskolin-stimulated conditions (Figure 4D; P < .05). In contrast, the CYB5A-KO showed increased basal synthesis of 17OHPreg-S (Figure 4D; P < .05) and a reduced DHEA-S/17OHPreg-S ratio under both basal and forskolin-stimulated conditions (Figure 4E; P < .05).
Figure 4.
Adrenocortical cell knockdown of CYB5A. A, Expression of green fluorescent protein in HAC15 adrenocortical cells transduced with lentiviral shRNA. Scale bars, 200 μm. B, Knockdown of CYB5A mRNA in scrambled and CYB5A-KO cells. Data are shown as the percentage change compared with scrambled. Results are given as mean ± SEM. Statistical analyses were performed using the t test, *P < .001 vs scrambled. C, Immunocytochemistry denoting expression of CYB5A protein (red) in scrambled and knockdown cells. Nuclei are counterstained with DAPI (blue). D, Effect of CYB5A knockdown on HAC15 cell production of Δ5-steroid sulfates. Scrambled and CYB5A-KO cells were treated with the 10 μM forskolin for 48 h. The Δ5-steroid sulfate content of media was determined using LC-MS/MS and normalized with cellular protein content. Results represent the mean ± SEM from three independent experiments performed in triplicate. Statistical significance was determined using a one-way ANOVA followed by Holm-Sidak test. *, P < .05 vs scrambled under basal conditions; $, P < .05 vs scrambled under forskolin-stimulated conditions; #, P < .05 vs basal in the same cell type. E, Ratios of DHEA-S/17OHPreg-S in scrambled and CYB5A-KO. Scrambled and CYB5A-KO cells were treated with the 10 μM forskolin for 48 h. The Δ5-steroid sulfate content of media was determined using LC-MS/MS and normalized with cellular protein content. Ratios of DHEA-S/17OHPreg-S were calculated and compared in different conditions. Results represent the mean ± SEM from three independent experiments performed in triplicate. Statistical significance was determined using a one-way ANOVA followed by Holm-Sidak test. *, P < .05 vs scrambled under basal conditions; $, P < .05 vs scrambled under forskolin-stimulated conditions; #, P < .05 vs basal in the same cell type.
Discussion
The increased production of DHEA-S during childhood is a direct consequence of the genesis of the adrenal ZR, which manifests clinically as adrenarche. To our knowledge, this is the first report on the simultaneous quantification of serum DHEA-S along with three other Δ5-steroid sulfates in a large population of normal children between ages 1.5 to 18 years using LC-MS/MS. Our study also demonstrates for the first time, a parallel increase in the levels of Adiol-S in accordance with the characteristic adrenarche steroid marker DHEA-S and CYB5A, the allosteric protein that augments the 17,20-lyase activity of CYP17A1 (32).
DHEA-S has been extensively studied across the entire age spectrum by RIA, demonstrating that the serum levels rapidly decline in the first 2 years of life, stay low for the next several years, and then dramatically increase around 6 years of age (8, 22–25). In agreement with these studies, as well as more recent analyses employing chemiluminescence immunoassay and LC-MS/MS (19, 27, 33), our studies showed that the first discernible increase in the serum DHEA-S levels occurs between 5 and 8 years of age followed by a continuous increase until adulthood in both sexes. We and others have observed marginally higher concentrations of serum DHEA-S between ages 2 and 5 years in girls compared with boys (21–23, 34). In accordance with other studies, our data also suggest that between 8 and 18 years, serum DHEA-S levels were modestly higher in males, and this difference became significant in adulthood (19, 22–24, 34). Studies employing radio- and chemiluminescence immunoassays found comparable but slightly higher DHEA-S levels compared with our observations. The difference in absolute values could relate to the possible cross-reactivity of immunoassays (19, 23, 24). The age-related patterns of DHEA-S observed in our study are similar to the most recent study tracking DHEA-S levels from birth to adulthood used LC-MS/MS in combination with Tanner classification of subjects (27).
Previous studies using RIAs suggested that serum Preg-S is secreted at very low levels through the first decade of life followed by a progressive increase until adulthood (35–37). We have confirmed similar observations in males; however, we did not find an age-related increase in Preg-S levels in females. RIA studies by Shimozawa et al (38) suggested that serum 17OHPreg-S is present at low levels between 3 and 6 years of age and a gradually increases from 7 years until adulthood. Our study demonstrated similar data in both sexes.
There are limited data demonstrating the relationship between age and Adiol-S. Only one report has quantified serum Adiol-S in a small cohort of 14 normal prepubertal girls between ages 5–7 years (39). Their data are consistent with the Adiol-S concentration observed in girls between 5–8 years in our study. Interestingly, the parallel increase of Adiol-S with DHEA-S that we observed between 5–8 years suggests that Adiol-S could represent a surrogate measure of adrenarche. The increase in Adiol-S between 5–8 years could be a consequence of the discernible expression of the enzyme 17β-hydroxysteroid dehydrogenase type 5 (AKR1C3) in the ZR detected around that period (16). AKR1C3 metabolizes DHEA to 5-androstenediol (5-Adiol) (14), which is in turn sulfated by SULT2A1 in the developing ZR to form Adiol-S. A pathway that involves the direct conversion of, DHEA-S to Adiol-S has yet to be confirmed. Adiol-S can be desulfated to act as a precursor for the production of more potent androgens, including T, in peripheral tissues like skin and liver. In human skin, 5-Adiol is transformed to a greater extent than DHEA to T and 5α-dihydrotestosterone (DHT) (40). Indeed, Remer et al (41) have identified urinary 5-Adiol conjugates as a marker of adrenarche. They demonstrated a logarithmic increase in the urinary excretion of 5-Adiol between 3–18 years in both sexes in a cohort of 400 healthy children, with the first significant increase occurring as early as 7–8 years of age. Their study used gas chromatography/mass spectrometry to profile urinary steroids after deconjugation of steroid sulfates and glucuronides; consequently, the proportion of urinary 5-Adiol derived from Adiol-S is not known. Further investigation to characterize the increase of both 5-Adiol and its sulfate during adrenarche will provide additional insight to the development of the zona reticularis in the human adrenal. 5-Adiol also functions as an estrogen receptor beta agonist (42–44). Circulating 5-Adiol levels surge during menopausal transition in women (45–47). The perimenopausal increase in 5-Adiol levels has been thought to provide an estrogenic source as ovarian estradiol production decreases at menopause.
The pathway leading to the synthesis of DHEA-S in the ZR requires three steroidogenic enzymes, namely cytochrome P450 cholesterol side-chain cleavage (CYP11A1), CYP17A1, and SULT2A1, plus the hemoprotein CYB5A, which enhances the 17,20-lyase activity of CYP17A1 (32, 48, 49). In addition, the lack of the competing enzyme HSD3B2 in the ZR ensures that Preg is almost exclusively metabolized via the Δ5-pathway to DHEA-S (50, 51). The relationship between the adrenal zonal expression of these steroidogenic enzymes and age has been investigated in detail (16, 17). CYP11A1 is expressed in all zones of the adrenal, and the expression pattern within the ZR does not change during adrenarche (17). CYP17A1 protein seemed to increase in both ZF and ZR around age 5 years, and therefore the regulation of CYP17A1 expression does not seem to be a cause of adrenarche (4). The increased prepubertal secretion of DHEA-S during adrenarche has been attributed to the compartmentalized expression of HSD3B2 in the ZF and of CYB5A and SULT2A1 in the developing ZR at approximately age 6 years (4, 16, 17). The increased conversion of 17OHPreg to DHEA due to the elevated 17,20-lyase activity of CYP17A1 in the ZR is pivotal to understanding the mechanisms underlying adrenarche. Our data suggested that the serum 17OHPreg-S/Preg-S ratio did not change in either sex with age, suggesting that the adrenal expression of CYP17A1 and its 17α-hydroxylase activity is uniform through the age spectrum of our study. 17OHPreg-S can be synthesized by sulfation of 17OHPreg, which is generated by the 17α-hydroxylation of Preg (52). In addition, 17α-hydroxylation of Preg-S was also recently identified as a mechanism for synthesis of 17OHPreg-S (53).
In comparison, the serum DHEA-S/17OHPreg-S ratio showed a clear age-associated augmentation starting between 5 and 8 years. This result suggests an age-related increase in the 17,20-lyase activity of CYP17A1, leading to accumulation of the DHEA, the unconjugated steroid precursor for DHEA-S. These findings support the concept that the adrenal expression of CYP17A1 is uniform through the age spectrum as opposed to that of CYB5A, which starts increasing in the ZR between 5 and 8 years. We corroborated these observations by performing dual immunofluorescence studies (HSD3B2 and CYB5A) in a series of human adrenals between 2 and 35 years. We demonstrated that HSD3B2 expression was segregated to the ZF and did not increase with age, whereas expression of CYB5A was ZR-specific, became discernible at approximately 6–7 years, and increased as age progressed. In addition, we also found an age-related increase in the adrenal CYB5A/HSD3B2 area ratio, which is reflective of the increase in serum DHEA-S levels and the serum DHEA-S/17OHPreg-S ratio between 5 and 8 years.
Consistent with the idea that changes in CYB5A expression are largely responsible for the hormonal changes of adrenarche, silencing of CYB5A expression in HAC15 adrenocortical cells inhibited production of DHEA-S and Adiol-S under basal and forskolin-stimulated conditions. This finding implies that the synthesis of DHEA-S as well as Adiol-S requires the 17,20-lyase activity of CYP17A1, albeit indirectly. The decrease in DHEA-S and Adiol-S levels could be attributed to the decrement in their unconjugated precursors (DHEA and 5-Adiol, respectively), whose production essentially relies on the 17,20-lyase activity, and thereby CYB5A. The marked increase in 17OHPreg-S in CYB5A-KO under basal conditions could be a result of the sulfation of the accumulated 17OHPreg formed as a result of the block in the 17,20-lyase activity due to abrogation of CYB5A.
In summary, we have simultaneously profiled the levels of four Δ5-steroid sulfates between ages 1.5 and 18 years in both the sexes using LC-MS/MS. The study confirms that DHEA-S is the most abundant steroid sulfate in circulation, followed by Adiol-S, Preg-S, and 17OHPreg-S. We have demonstrated that Adiol-S levels show a concomitant age-related increase with the conventional adrenarche marker DHEA-S and could represent a surrogate measure of this process. Collectively, our immunohistochemical and cell culture experiments suggest that ZR is likely the site of the biosynthesis of both DHEA-S and Adiol-S, given that their production relies on the 17,20-lyase activity of CYP17A1, which is high only in the presence of CYB5A. Adrenarche represents onset of pubic and axillary hair, body odor, and/or acne, individually or collectively or in varying degrees of combinations. To date, DHEA-S provides us with a unidimensional assessment of a wide range of clinical expression of adrenal androgens. Expanding the profile and the quality of markers of ZR could allow clinicians to define adrenarche with a more nuanced stratification. The childhood increase in Adiol-S could provide a novel source for peripheral tissue production of active sex steroids with potential implications in adrenarche manifestations and progression.
Acknowledgments
We thank Dr C. R. Parker (University of Alabama–Birmingham) for the gift of HSD3B2 antibody. We also thank Dr Sunil K. Upadhyay (University of Michigan–Ann Arbor) for the synthesis of Preg-S, 17OHPreg-S, and Adiol-S, and Mr. Robert Chomic (Michigan Metabolomics and Obesity Center, University of Michigan) for technical assistance with liquid chromatography–tandem mass spectrometry.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK069950 and R01DK43140 to W.E.R., R01GM086596 to R.J.A; J.R is supported by the Postdoctoral Translational Scholars Program fellowship award 2UL1TR000433 from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). Mass spectrometry used core services supported by Grant DK089503 from the NIH to the University of Michigan under the Michigan Nutrition Obesity Research Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 17OHPreg
- 17α-hydroxypregnenolone
- Adiol-S
- 5-androstenediol-3-sulfate
- CYP11A1
- cytochrome P450 cholesterol side-chain cleavage
- CYP17A1
- 17α-hydroxylase/17, 20-lyase
- CYB5A
- cytochrome b5 type A
- DHEA-S
- dehydroepiandrosterone sulfate
- HSD3B2
- decreased 3β-hydroxysteroid dehydrogenase type 2
- LC-MS/MS
- liquid chromatography–tandem mass spectrometry
- Preg
- Pregnenolone
- Prog
- Progesterone
- Preg-S
- Pregnenolone sulfate
- shRNA
- short hairpin RNA
- SULT2A1
- sulfotransferase type 2A1
- ZF
- zona fasciculata
- ZR
- zona reticularis.
Reference
- 1. Auchus RJ, Rainey WE. Adrenarche-physiology, biochemistry and human disease. Clin Endocrinol (Oxf). 2004;60:288–296. [DOI] [PubMed] [Google Scholar]
- 2. Cutler GB, Jr, Loriaux DL. Andrenarche and its relationship to the onset of puberty. Fed Proc. 1980;39:2384–2390. [PubMed] [Google Scholar]
- 3. Miller WL. Androgen synthesis in adrenarche. Rev Endocr Metab Disord. 2009;10:3–17. [DOI] [PubMed] [Google Scholar]
- 4. Rege J, Rainey WE. The steroid metabolome of adrenarche. J Endocrinol. 2012;214:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaufman FR, Stanczyk FZ, Matteri RK, Gentzschein E, Delgado C, Lobo RA. Dehydroepiandrosterone and dehydroepiandrosterone sulfate metabolism in human genital skin. Fertil Steril. 1990;54:251–254. [PubMed] [Google Scholar]
- 6. Pelletier G. Expression of steroidogenic enzymes and sex-steroid receptors in human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:223–228. [DOI] [PubMed] [Google Scholar]
- 7. Rosenfield RL. Hirsutism and the variable response of the pilosebaceous unit to androgen. J Investig Dermatol Symp Proc. 2005;10:205–208. [DOI] [PubMed] [Google Scholar]
- 8. Ducharme JR, Forest MG, De Peretti E, Sempé M, Collu R, Bertrand J. Plasma adrenal and gonadal sex steroids in human pubertal development. J Clin Endocrinol Metab. 1976;42:468–476. [DOI] [PubMed] [Google Scholar]
- 9. Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: Adrenarche. Semin Reprod Med. 2004;22:337–347. [DOI] [PubMed] [Google Scholar]
- 10. Rosenfield RL, Lucky AW. Acne, hirsutism, and alopecia in adolescent girls. Clinical expressions of androgen excess. Endocrinol Metab Clin North Am. 1993;22:507–532. [PubMed] [Google Scholar]
- 11. Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39:85–95. [DOI] [PubMed] [Google Scholar]
- 12. Luu-The V, Bernier F, Dufort I. Steroid sulfotransferases. J Endocrinol. 1996;150 Suppl:S87–S97. [PubMed] [Google Scholar]
- 13. Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23:703–732. [DOI] [PubMed] [Google Scholar]
- 14. Rege J, Nakamura Y, Satoh F, et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98:1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gell JS, Carr BR, Sasano H, et al. Adrenarche results from development of a 3beta-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab. 1998;83:3695–3701. [DOI] [PubMed] [Google Scholar]
- 16. Hui XG, Akahira J, Suzuki T, et al. Development of the human adrenal zona reticularis: Morphometric and immunohistochemical studies from birth to adolescence. J Endocrinol. 2009;203:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki T, Sasano H, Takeyama J, et al. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: Immunohistochemical studies. Clin Endocrinol (Oxf). 2000;53:739–747. [DOI] [PubMed] [Google Scholar]
- 18. Longcope C. Dehydroepiandrosterone metabolism. J Endocrinol. 1996;150 Suppl:S125–S127. [PubMed] [Google Scholar]
- 19. Guran T, Firat I, Yildiz F, et al. Reference values for serum dehydroepiandrosterone-sulphate in healthy children and adolescents with emphasis on the age of adrenarche and pubarche. Clin Endocrinol (Oxf). 2015;82:712–718. [DOI] [PubMed] [Google Scholar]
- 20. Dhom G. The prepuberal and puberal growth of the adrenal (adrenarche). Beitr Pathol. 1973;150:357–377. [DOI] [PubMed] [Google Scholar]
- 21. Reiter EO, Fuldauer VG, Root AW. Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J Pediatr. 1977;90:766–770. [DOI] [PubMed] [Google Scholar]
- 22. Sulcová J, Hill M, Hampl R, Stárka L. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. J Endocrinol. 1997;154:57–62. [DOI] [PubMed] [Google Scholar]
- 23. Tung YC, Lee JS, Tsai WY, Hsiao PH. Physiological changes of adrenal androgens in childhood. J Formos Med Assoc. 2004;103:921–924. [PubMed] [Google Scholar]
- 24. de Peretti E, Forest MG. Pattern of plasma dehydroepiandrosterone sulfate levels in humans from birth to adulthood: Evidence for testicular production. J Clin Endocrinol Metab. 1978;47:572–577. [DOI] [PubMed] [Google Scholar]
- 25. Korth-Schutz S, Levine LS, New MI. Dehydroepiandrosterone sulfate (DS) levels, a rapid test for abnormal adrenal androgen secretion. J Clin Endocrinol Metab. 1976;42:1005–1013. [DOI] [PubMed] [Google Scholar]
- 26. Ilondo MM, Vanderschueren-Lodeweyckx M, Vlietinck R, et al. Plasma androgens in children and adolescents. Part I: Control subjects. Horm Res. 1982;16:61–77. [DOI] [PubMed] [Google Scholar]
- 27. Søeborg T, Frederiksen H, Mouritsen A, et al. Sex, age, pubertal development and use of oral contraceptives in relation to serum concentrations of DHEA, DHEAS, 17α-hydroxyprogesterone, δ4-androstenedione, testosterone and their ratios in children, adolescents and young adults. Clin Chim Acta. 2014;437:6–13. [DOI] [PubMed] [Google Scholar]
- 28. Wang T, Rainey WE. Human adrenocortical carcinoma cell lines. Mol Cell Endocrinol. 2012;351:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nanba K, Chen A, Nishimoto K, Rainey WE. Role of Ca(2+)/calmodulin-dependent protein kinase kinase in adrenal aldosterone production. Endocrinology. 2015;156:1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Box G, Cox D. An analysis of transformations (with discussion). J R Stat Soc Ser B. 1964;26:211–252. [Google Scholar]
- 31. Rigby R, Stasinopoulos D. Generalized additive models for location, scale and shape. Applied Statistics. 2005;54:507–554. [Google Scholar]
- 32. Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. [DOI] [PubMed] [Google Scholar]
- 33. Søeborg T, Frederiksen H, Fruekilde P, Johannsen TH, Juul A, Andersson AM. Serum concentrations of DHEA, DHEAS, 17α-hydroxyprogesterone, Δ4-androstenedione and testosterone in children determined by TurboFlow-LC-MS/MS. Clin Chim Acta. 2013;419:95–101. [DOI] [PubMed] [Google Scholar]
- 34. Babalola AA, Ellis G. Serum dehydroepiandrosterone sulfate in a normal pediatric population. Clin Biochem. 1985;18:184–189. [DOI] [PubMed] [Google Scholar]
- 35. de Peretti E, Forest MG, Loras B, et al. Usefulness of plasma pregnenolone sulfate in testing pituitary-adrenal function in children. Acta Endocrinol Suppl (Copenh). 1986;279:259–263. [DOI] [PubMed] [Google Scholar]
- 36. de Peretti E, Mappus E. Pattern of plasma pregnenolone sulfate levels in humans from birth to adulthood. J Clin Endocrinol Metab. 1983;57:550–556. [DOI] [PubMed] [Google Scholar]
- 37. Meloun M, Hill M, Vceláková-Havlíková H. Minimizing the effects of multicollinearity in the polynomial regression of age relationships and sex differences in serum levels of pregnenolone sulfate in healthy subjects. Clin Chem Lab Med. 2009;47:464–470. [DOI] [PubMed] [Google Scholar]
- 38. Shimozawa K, Saisho S, Yata J, Kambegawa A. Age-related changes in serum 17-hydroxypregnenolone and 17-hydroxypregnenolone sulfate concentrations in human infancy and childhood. Endocrinol Jpn. 1988;35:189–195. [DOI] [PubMed] [Google Scholar]
- 39. Montalto J, Yong AB, Funder JW, Connelly JF. Serum 5-androstene-3 beta,17 beta-diol sulphate, sex hormone binding globulin and free androgen index in girls with premature adrenarche. J Steroid Biochem. 1989;33:1149–1154. [DOI] [PubMed] [Google Scholar]
- 40. Faredin I, Tóth I. The metabolism of [4–14C]5-androstene-3 beta, 17 beta-diol by normal human skin in vitro. Acta Med Acad Sci Hung. 1975;32:139–152. [PubMed] [Google Scholar]
- 41. Remer T, Boye KR, Hartmann MF, Wudy SA. Urinary markers of adrenarche: Reference values in healthy subjects, aged 3–18 years. J Clin Endocrinol Metab. 2005;90:2015–2021. [DOI] [PubMed] [Google Scholar]
- 42. Chen J, Wang WQ, Lin SX. Interaction of Androst-5-ene-3β,17β-diol and 5α-androstane-3β,17β-diol with estrogen and androgen receptors: a combined binding and cell study. J Steroid Biochem Mol Biol. 2013;137:316–321. [DOI] [PubMed] [Google Scholar]
- 43. Miller KK, Al-Rayyan N, Ivanova MM, et al. DHEA metabolites activate estrogen receptors alpha and beta. Steroids. 2013;78:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baker ME, Uh KY, Chandsawangbhuwana C. 3D models of human ERα and ERβ complexed with 5-androsten-3β,17β-diol. Steroids. 2012;77:1192–1197. [DOI] [PubMed] [Google Scholar]
- 45. Lasley BL, Chen J, Stanczyk FZ, et al. Androstenediol complements estrogenic bioactivity during the menopausal transition. Menopause. 2012;19:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McConnell DS, Stanczyk FZ, Sowers MR, Randolph JF, Jr, Lasley BL. Menopausal transition stage-specific changes in circulating adrenal androgens. Menopause. 2012;19:658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lasley BL, Crawford SL, McConnell DS. Ovarian adrenal interactions during the menopausal transition. Minerva Ginecol. 2013;65:641–651. [PMC free article] [PubMed] [Google Scholar]
- 48. Rege J, Nakamura Y, Wang T, Merchen TD, Sasano H, Rainey WE. Transcriptome profiling reveals differentially expressed transcripts between the human adrenal zona fasciculata and zona reticularis. J Clin Endocrinol Metab. 2014;99:E518–E527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rainey WE, Nakamura Y. Regulation of the adrenal androgen biosynthesis. J Steroid Biochem Mol Biol. 2008;108:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1996;81:3558–3565. [DOI] [PubMed] [Google Scholar]
- 51. Wang W, Yang L, Suwa T, Casson PR, Hornsby PJ. Differentially expressed genes in zona reticularis cells of the human adrenal cortex. Mol Cell Endocrinol. 2001;173:127–134. [DOI] [PubMed] [Google Scholar]
- 52. Brock BJ, Waterman MR. Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry. 1999;38:1598–1606. [DOI] [PubMed] [Google Scholar]
- 53. Neunzig J, Sanchez-Guijo A, Mosa A, et al. A steroidogenic pathway for sulfonated steroids: The metabolism of pregnenolone sulfate. J Steroid Biochem Mol Biol. 2014;144 Pt B:324–333. [DOI] [PubMed] [Google Scholar]