Abstract
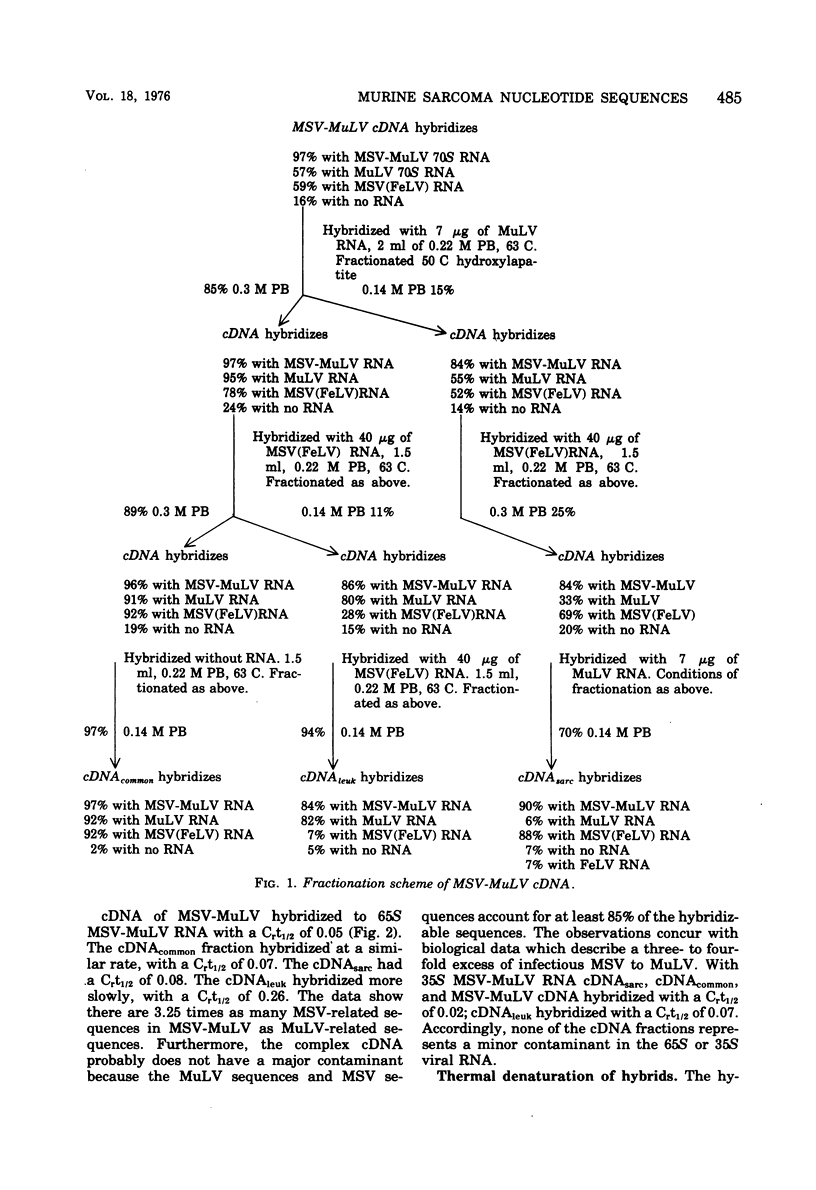
Radioactive DNA complementary to nucleotide sequences in Moloney murine sarcoma virus (MSV) and Moloney leukemia virus (M-MuLV) complex was made by the endogenous reverse transcriptase reaction. These virus stocks contained a threefold excess of MSV over M-MuLV as measured by biological assay. The complementary DNA was an accurate copy of the viral RNA in that 86% of 35S viral RNA hybridized with complementary (cDNA) DNA at a 1.5 to 1 cDNA-RNA molar ratio. The complementary DNA, of a 4-6S size, was fractionated by sequential absorptions with MulV and the feline leukemia virus pseudotype of MSV, [MSV(FeLV)] RNA. In this manner three sets of nucleotide sequences whichrepresent different portions of the MSV viral complex were obtained: a sarcoma virus-specific fraction (cDNAsarc) with sequences that had no homology to M-MuLV RNA but which hybridized to MSV (FeLV) RNA, a sarcoma-leukemia fraction (cDNA common) with sequences common to MSV as well as M-MuLV viral RNA, and a cDNAleuk representing those nucleotide sequences found only in M-MuLV. Hybridization of MSV-MuLV viral 35S RNA with a threefold molar excess of cDNA's revealed that approximately 20% was hybridized with cDNAsarc, whereas approximately 75% was hybridized with cDNAcommon. M-MuLV 35S RNA alone did not hybridize with cDNAsarc but did hybridize 40 and 50% with cDNAleuk and cDNAcommon, respectively. The cDNAsarc represents about 25% of the total MSV sequences, whereas the cDNAcommon represents the remainder of the MSV virus genome. Some cDNAcommon sequences were shared by two other sarcoma viruses and several distinctly different isolates of MulV. In contrast, the MSV "sarc" sequences had little or no homology with two other murine sarcoma virus isolates.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Colcher D., Drohan W., Schlom Mason-Pfizer virus RNA genome: relationship to the RNA of morphologically similar isolates and other oncornaviruses. J Virol. 1976 Mar;17(3):705–712. doi: 10.1128/jvi.17.3.705-712.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanshier L., Garapin A. C., McDonnell J., Faras A., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase associated with avian tumor viruses: secondary structure of the deoxyribonucleic acid product. J Virol. 1971 Jan;7(1):77–86. doi: 10.1128/jvi.7.1.77-86.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Moore C. O., O'Connor T. E. Isolation and identification of a helper virus found in the Moloney sarcoma-leukemia virus complex. J Natl Cancer Inst. 1969 Apr;42(4):605–622. [PubMed] [Google Scholar]
- Fischinger P. J., O'Conner T. E. Viral infection across species barriers: reversible alteration of murine sarcoma virus for growth in cat cells. Science. 1969 Aug 15;165(3894):714–716. doi: 10.1126/science.165.3894.714. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Schaefer W., Seifert E. Detection of some murine leukemia virus antigens in virus particles derived from 3T3 cells transformed only by murine sarcoma virus. Virology. 1972 Jan;47(1):229–235. doi: 10.1016/0042-6822(72)90254-1. [DOI] [PubMed] [Google Scholar]
- HARVEY J. J. AN UNIDENTIFIED VIRUS WHICH CAUSES THE RAPID PRODUCTION OF TUMOURS IN MICE. Nature. 1964 Dec 12;204:1104–1105. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Recombination between endogenous and exogenous RNA tumor virus genes as analyzed by nucleic acid hybridization. J Virol. 1975 Jun;15(6):1367–1377. doi: 10.1128/jvi.15.6.1367-1377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Hartley J. W., Rowe W. P., Lane W. T., Capps W. I. Rescue of the defective genome of Moloney sarcoma virus from a noninfectious hamster tumor and the production of pseudotype sarcoma viruses with various murine leukemia viruses. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1164–1169. doi: 10.1073/pnas.56.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney J. B. A virus-induced rhabdomyosarcoma of mice. Natl Cancer Inst Monogr. 1966 Sep;22:139–142. [PubMed] [Google Scholar]
- Nomura S., Fischinger P. J., Mattern C. F., Peebles P. T., Bassin R. H., Friedman G. P. Revertants of mouse cells transformed by murine sarcoma virus. I. Characterization of flat and transformed sublines without a rescuable murine sarcoma virus. Virology. 1972 Oct;50(1):51–64. doi: 10.1016/0042-6822(72)90345-5. [DOI] [PubMed] [Google Scholar]
- Oskarsson M. K., Robey W. G., Harris C. L., Fischinger P. J., Haapala D. K., Vande Woude G. F. A p60 polypeptide in the feline leukemia virus pseudotype of Moloney sarcoma virus with murine leukemia virus p30 antigenic determinants. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2380–2384. doi: 10.1073/pnas.72.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles P. T., Bassin R. H., Haapala D. K., Phillips L. A., Nomura S., Fischinger P. J. Rescue of murine sarcoma virus from a sarcoma-positive leukemia-negative cell line: requirement for replicating leukemia virus. J Virol. 1971 Nov;8(5):690–694. doi: 10.1128/jvi.8.5.690-694.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles P. T., Fischinger P. J., Bassin R. H., Papageorge A. G. Isolation of human amnion cells transformed by rescuable murine sarcoma virus. Nat New Biol. 1973 Mar 28;242(117):98–101. doi: 10.1038/newbio242098a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Howk R. S., Anisowicz A., Peebles P. T., Scher C. D., Parks W. P. Separation of sarcoma virus-specific and leukemia virus-specific genetic sequences of Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4650–4654. doi: 10.1073/pnas.72.11.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P. Harvey sarcoma virus: a second murine type C sarcoma virus with rat genetic information. J Virol. 1974 Jun;13(6):1211–1219. doi: 10.1128/jvi.13.6.1211-1219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Rands E., Williams D., Parks W. P. Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. J Virol. 1973 Sep;12(3):458–463. doi: 10.1128/jvi.12.3.458-463.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. P., Theilen G. H. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. doi: 10.1038/2211074a0. [DOI] [PubMed] [Google Scholar]
- Solymosy F., Fedorcsák I., Gulyás A., Farkas G. L., Ehrenberg L. A new method based on the use of diethyl pyrocarbonate as a nuclease inhibitor for the extraction of undegraded nucleic acid from plant tissues. Eur J Biochem. 1968 Sep 24;5(4):520–527. doi: 10.1111/j.1432-1033.1968.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Wolfe L. G., Deinhardt F., Theilen G. H., Rabin H., Kawakami T., Bustad L. K. Induction of tumors in marmoset monkeys by simian sarcoma virus, type 1 (Lagothrix): a preliminary report. J Natl Cancer Inst. 1971 Nov;47(5):1115–1120. [PubMed] [Google Scholar]


