Abstract
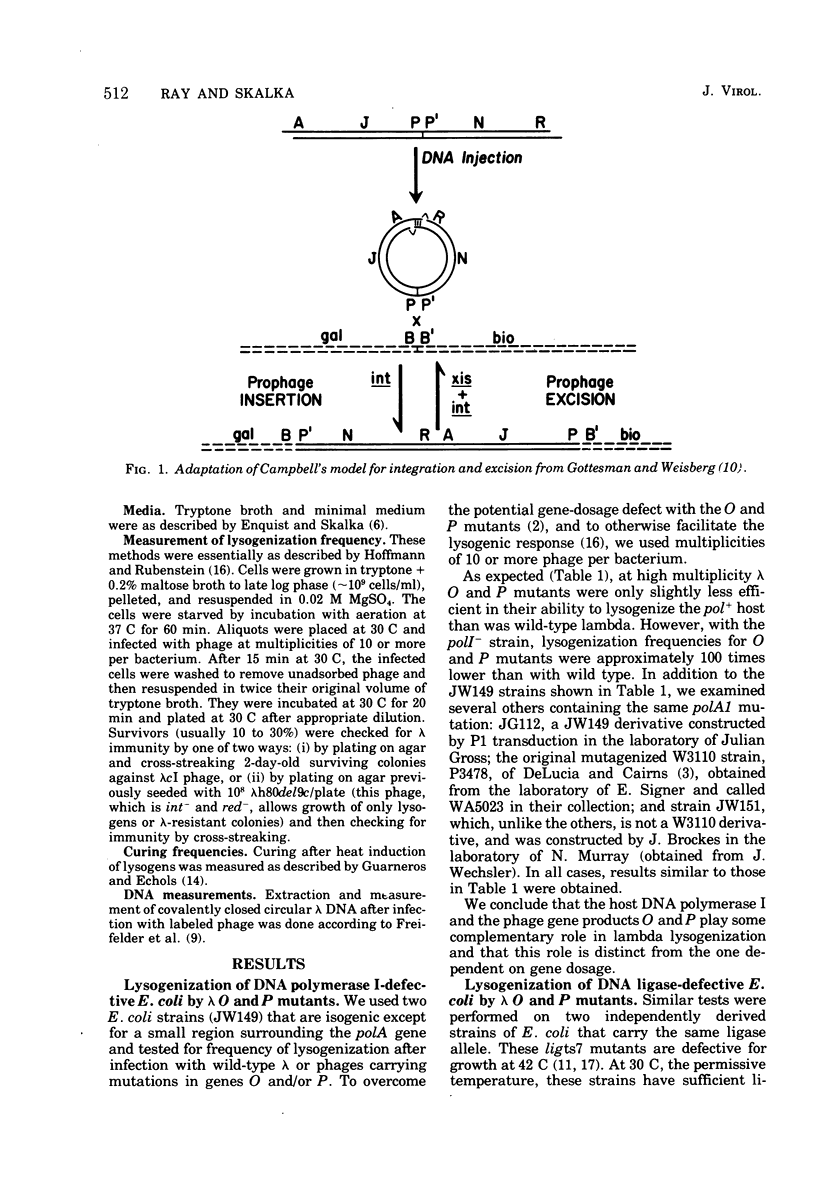
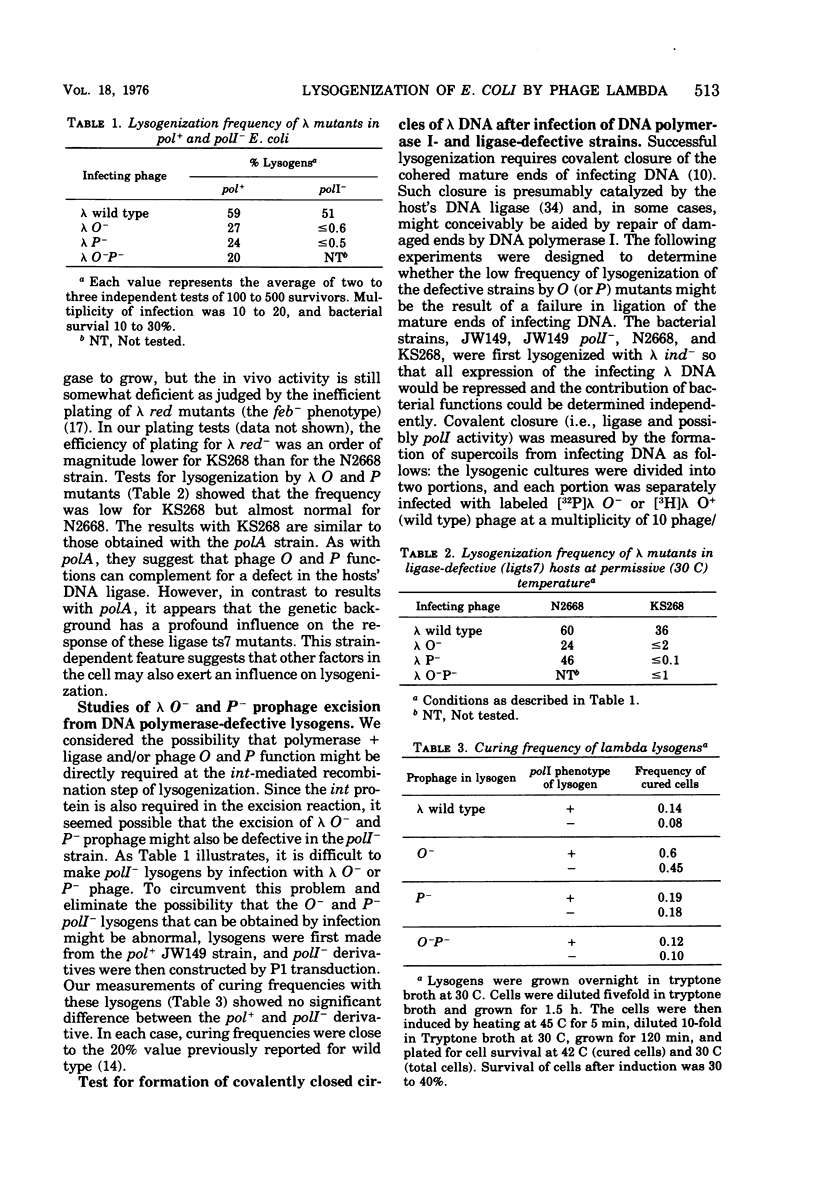
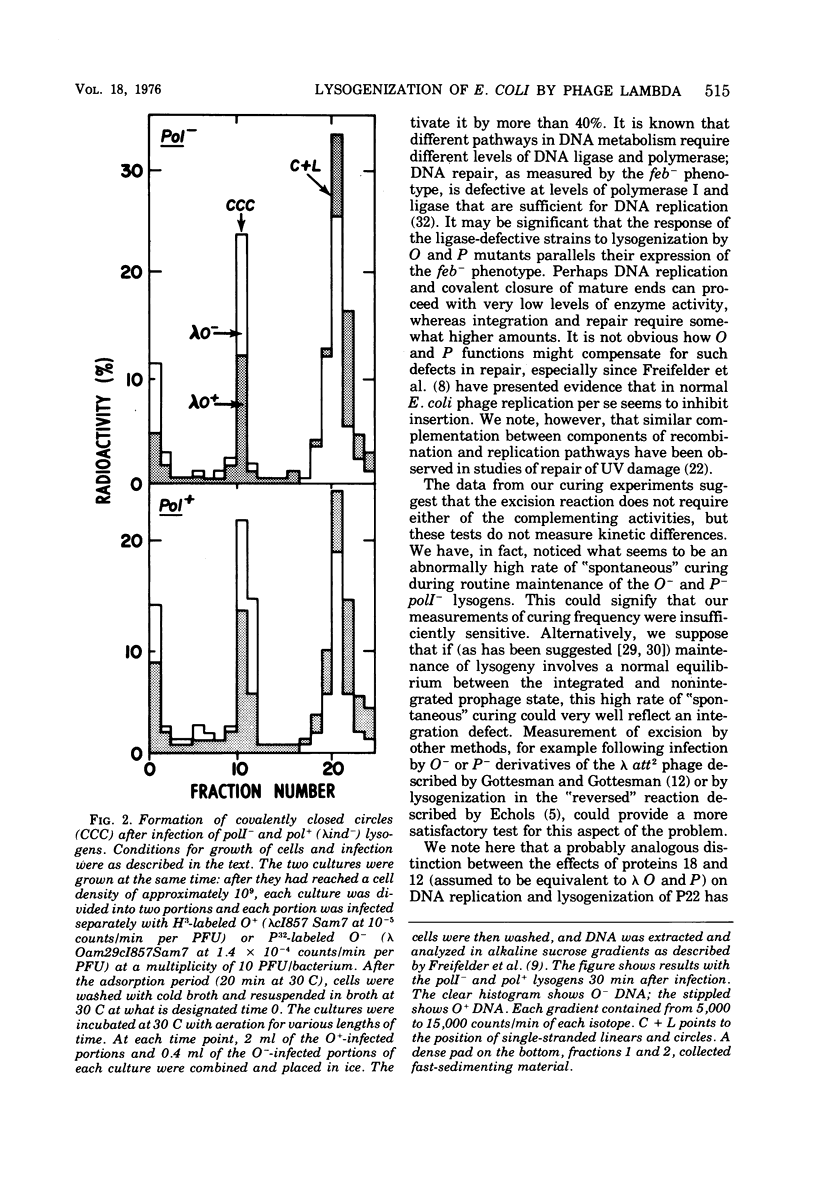
When bacteriophage lambda DNA replication is blocked by mutation in phage genes O or P, the efficiency of lysogenization drops to a very low value unless high multiplicities of infecting phage are used. Our results show that even at high multiplicity, lambda O or P mutants cannot efficiently lysogenize some hosts that are defective in either DNA polymerase I or DNA ligase. Covalent closure of infecting DNA molecules, a preliminary step for insertion according to Campbell's model and an obvious candidate for this lysogenization defect, appears to occur normally under our conditions. In addition, prophage excision as measured by the frequency of curing O- and P- lysogens seemed normal when tested in the poll- strain. These results suggest that the Escherichia coli enzymes DNA polymerase I and ligase, and phage proteins O and P, are able to provide some complementary activity whose function is required specifically for prophage integration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS K. STUDIES IN THE PHYSIOLOGICAL GENETICS OF SOME SUPPRESSOR-SENSITIVE MUTANTS OF BACTERIOPHAGE LAMBDA. Virology. 1965 Jul;26:489–499. doi: 10.1016/0042-6822(65)90011-5. [DOI] [PubMed] [Google Scholar]
- Becker A., Gold M. Isolation of the bacteriophage lambda A-gene protein. Proc Natl Acad Sci U S A. 1975 Feb;72(2):581–585. doi: 10.1073/pnas.72.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Echols H. Constitutive integrative recombination by bacteriophage lambda. Virology. 1975 Apr;64(2):557–559. doi: 10.1016/0042-6822(75)90133-6. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Skalka A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J Mol Biol. 1973 Apr 5;75(2):185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Folkmanis A., Freifelder D. Studies on lysogeny in Escherichia coli with bacteriophage lambda. Physical observation of the insertion process. J Mol Biol. 1972 Mar 14;65(1):63–73. doi: 10.1016/0022-2836(72)90492-5. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Baran N., Chud L., Folkmanis A., Levine E. E. Requirements for insertion of bacteriophase lambda DNA into the DNA of Escherichia coli. J Mol Biol. 1975 Feb 5;91(4):401–408. doi: 10.1016/0022-2836(75)90268-5. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Folkmanis A., Kirschner I. Studies on Escherichia coli sex factors: evidence that covalent circles exist within cells and the general problem of isolation of covalent circles. J Bacteriol. 1971 Mar;105(3):722–727. doi: 10.1128/jb.105.3.722-727.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Hicks M. L., Gellert M. Genetics and function of DNA ligase in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):531–547. doi: 10.1016/0022-2836(73)90221-0. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Gottesman M. E. Elements involved in site-specific recombination in bacteriophage lambda. J Mol Biol. 1975 Feb 5;91(4):489–499. doi: 10.1016/0022-2836(75)90275-2. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Gottesman M. Excision of prophage lambda in a cell-free system. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2188–2192. doi: 10.1073/pnas.72.6.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneros G., Echols H. New mutants of bacteriophage lambda with a specific defect in excision from the host chromosome. J Mol Biol. 1970 Feb 14;47(3):565–574. doi: 10.1016/0022-2836(70)90323-2. [DOI] [PubMed] [Google Scholar]
- Guarneros G., Echols H. Thermal asymmetry of site-specific recombination by bacteriophage lambda. Virology. 1973 Mar;52(1):30–38. doi: 10.1016/0042-6822(73)90395-4. [DOI] [PubMed] [Google Scholar]
- Hoffman D. B., Jr, Rubenstein I. Physical studies of lysogeny. I. Properties of intracellular parental bacteriophage DNA from lambda-infected sensitive bacteria. J Mol Biol. 1968 Aug 14;35(3):375–399. doi: 10.1016/s0022-2836(68)80001-4. [DOI] [PubMed] [Google Scholar]
- Konrad E. B., Modrich P., Lehman I. R. Genetic and enzymatic characterization of a conditional lethal mutant of Escherichia coli K12 with a temperature-sensitive DNA ligase. J Mol Biol. 1973 Jul 15;77(4):519–529. doi: 10.1016/0022-2836(73)90220-9. [DOI] [PubMed] [Google Scholar]
- Kourilsky P. Lysogenization by bacteriophage lambda and the regulation of lambda repressor synthesis. Virology. 1971 Sep;45(3):853–857. doi: 10.1016/0042-6822(71)90213-3. [DOI] [PubMed] [Google Scholar]
- Levine M., Schott C. Mutations of phage P22 affecting phage DNA synthesis and lysogenization. J Mol Biol. 1971 Nov 28;62(1):53–64. doi: 10.1016/0022-2836(71)90130-6. [DOI] [PubMed] [Google Scholar]
- Mandal N. C., Crochet M., Lieb M. Properties of polylysogens containing derepressed lambdaN- prophages. 3. A large number of intergrated lambda prophages. Virology. 1974 Jan;57(1):85–92. doi: 10.1016/0042-6822(74)90110-x. [DOI] [PubMed] [Google Scholar]
- Masker W. E., Hanawalt P. C. Nucleoside triphosphate dependence of repair replication in toluenized Escherichia coli. J Mol Biol. 1974 Sep 5;88(1):13–23. doi: 10.1016/0022-2836(74)90292-7. [DOI] [PubMed] [Google Scholar]
- Menninger J. R., Wright M., Menninger L., Meselson M. Attachment and detachment of bacteriophage lambda DNA in lysogenization and induction. J Mol Biol. 1968 Mar 28;32(3):631–637. doi: 10.1016/0022-2836(68)90347-1. [DOI] [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash H. A. Integrative recombination in bacteriophage lambda: analysis of recombinant DNA. J Mol Biol. 1975 Feb 5;91(4):501–514. doi: 10.1016/0022-2836(75)90276-4. [DOI] [PubMed] [Google Scholar]
- Nash H. A. Integrative recombination of bacteriophage lambda DNA in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1072–1076. doi: 10.1073/pnas.72.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt L. F. Control of bacteriophage lambda repressor synthesis after phage infection: the role of the N, cII, cIII and cro products. J Mol Biol. 1975 Apr 5;93(2):267–288. doi: 10.1016/0022-2836(75)90132-1. [DOI] [PubMed] [Google Scholar]
- Shimada K., Campbell A. Int-constitutive mutants of bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Jan;71(1):237–241. doi: 10.1073/pnas.71.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Campbell A. Lysogenization and curing by int-constitutive mutants of phage lambda. Virology. 1974 Jul;60(1):157–165. doi: 10.1016/0042-6822(74)90373-0. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Syvanen M. In vitro genetic recombination of bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2496–2499. doi: 10.1073/pnas.71.6.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]


