Abstract
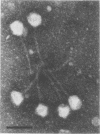
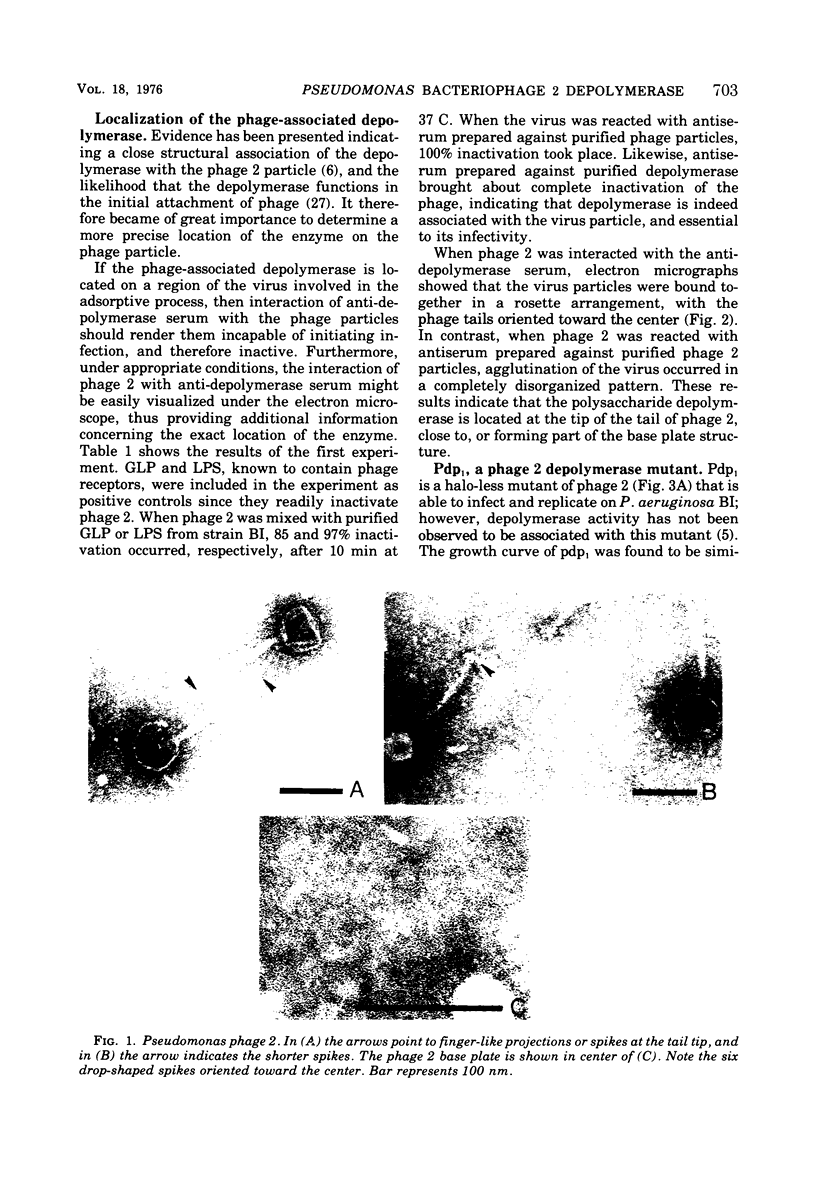
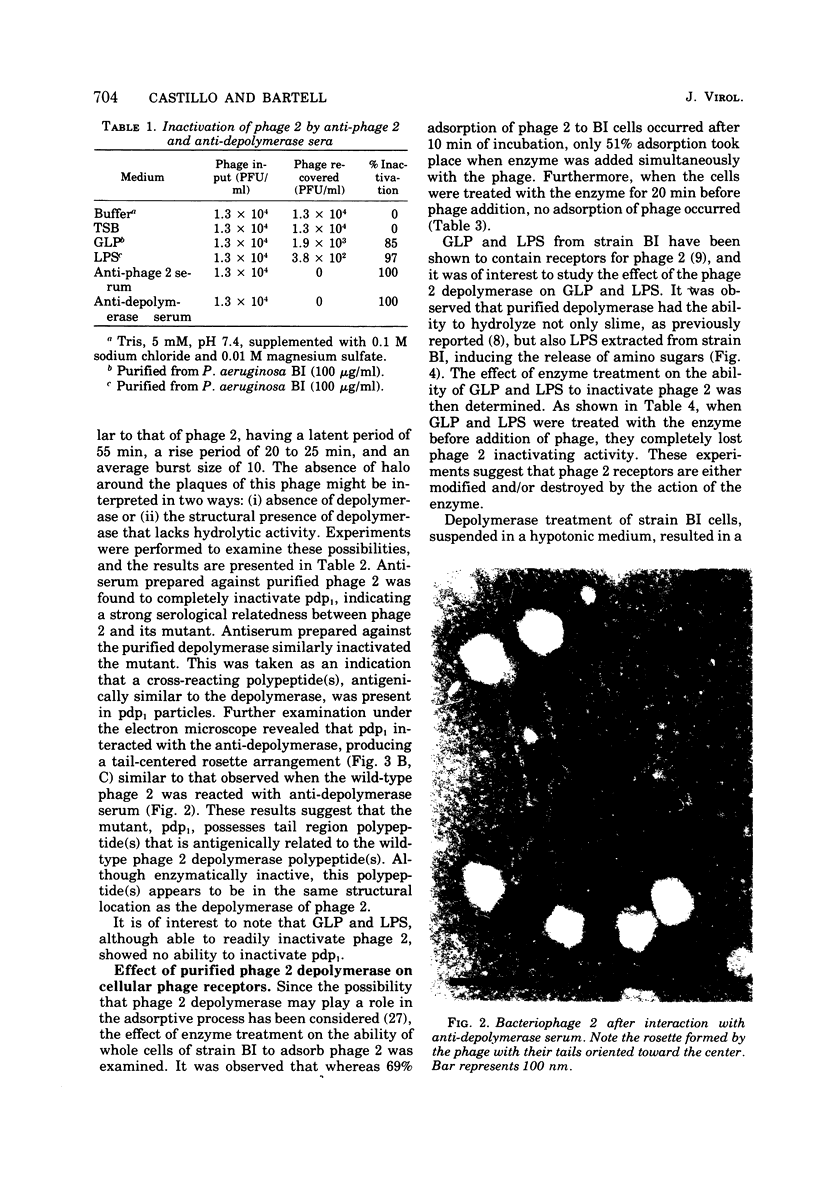
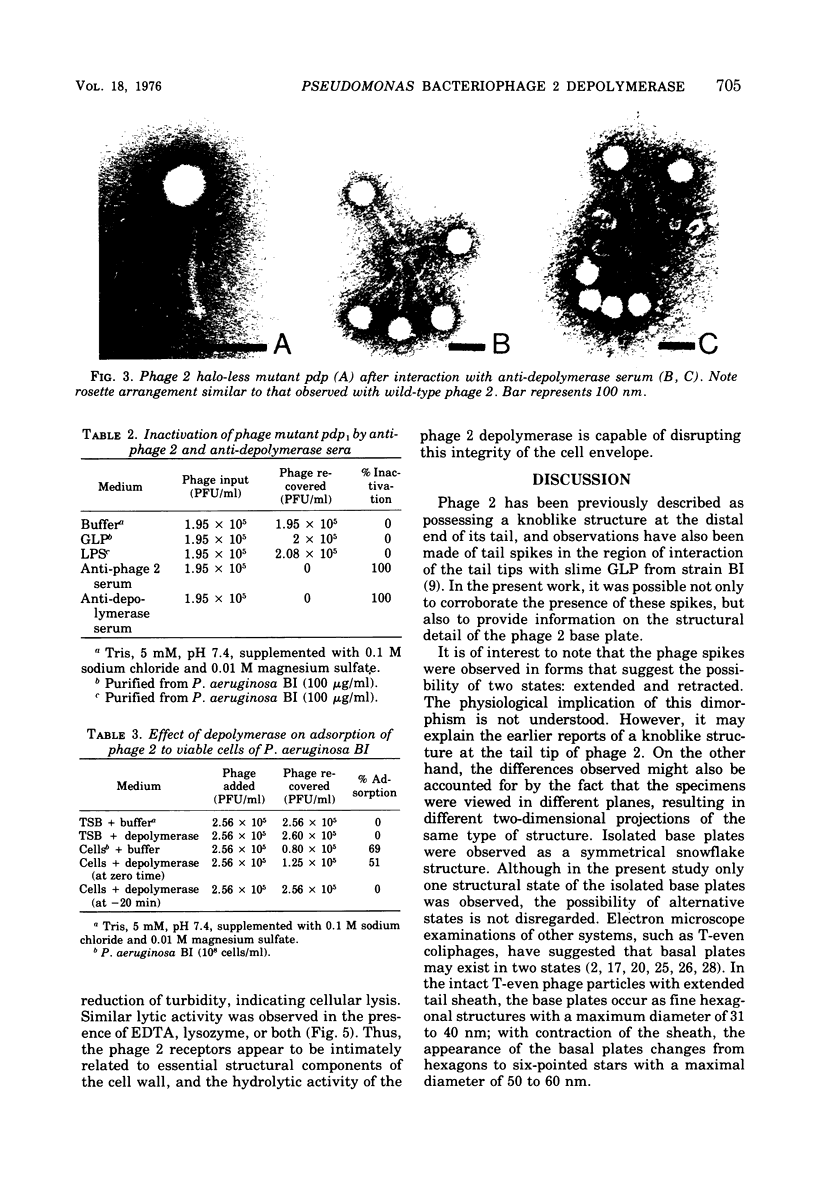
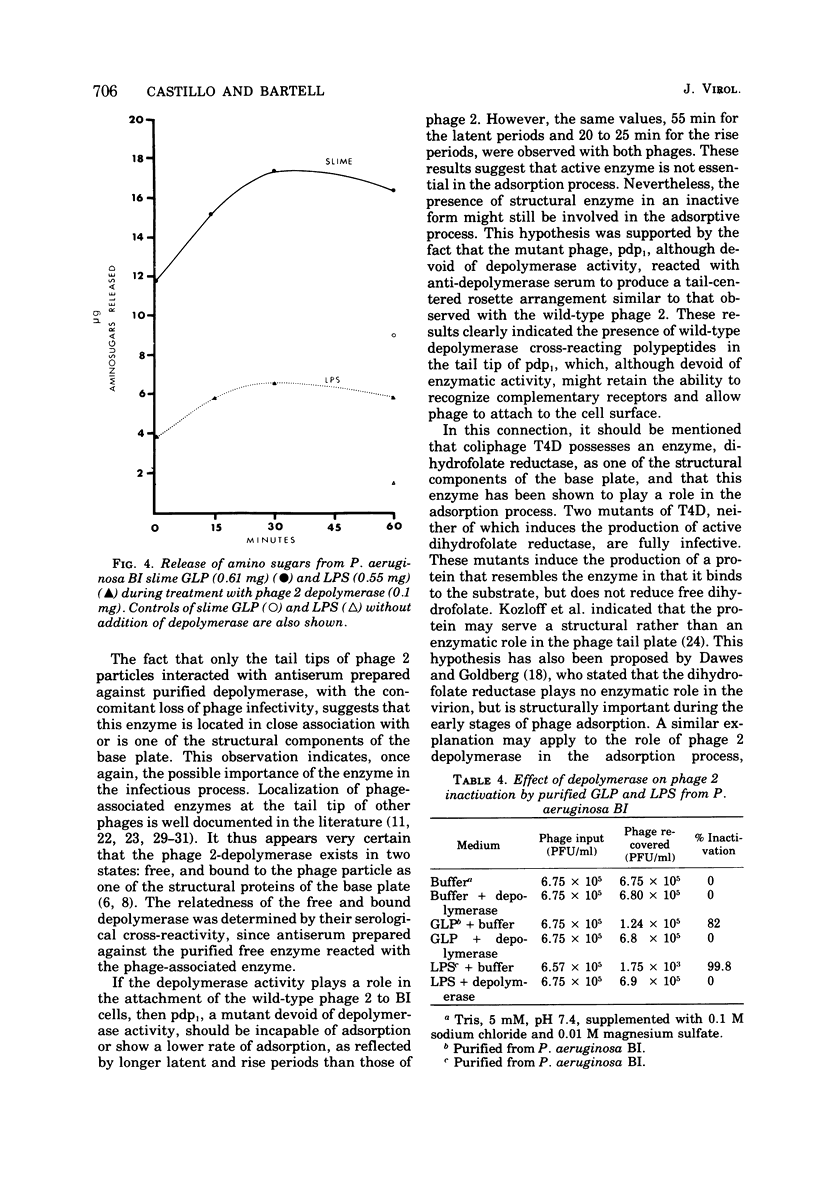
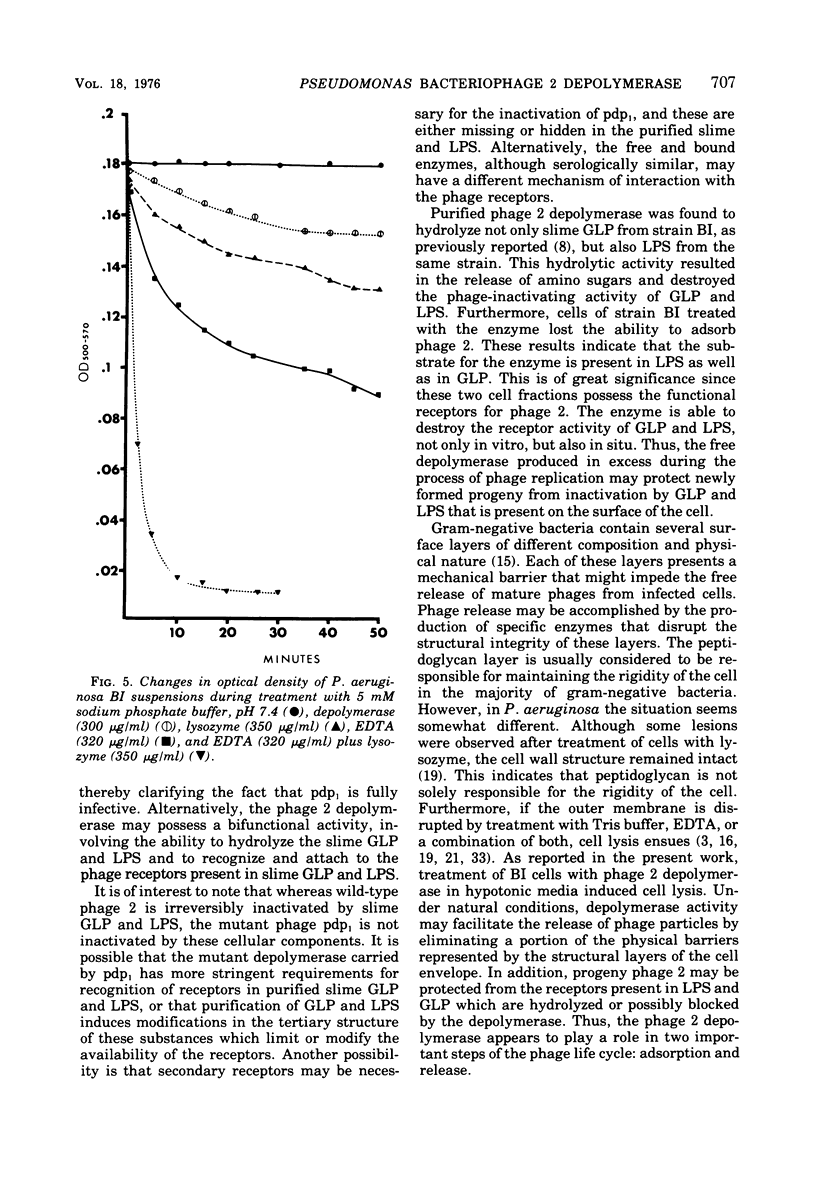
The adsorption apparatus of phage 2 consits of a symmetrical base plate of snowflake appearance, composed of six droplike spikes 7.0 to 7.5 nm in length with a maximum diameter of 4.5 to 5.0 nm. The spikes are attached by their narrow ends to a central ring 7.0 to 7.5 nm in diameter. Phage 2 deopolymerase, a phage 2-induced hydrolytic enzyme, was found to be a structural protein of phage 2 or in close association with the base plate. Pdp1, a phage 2 mutant, possesses a polypeptide that is antigenically similar to the depolymerase, but devoid of hydrolytic activity. This polypeptide was found to be located in the region of the base plate of pdp1. Treatment of intact cells of strain BI with purified phage 2 depolymerase inhibited the adsorption of phage 2. When phage receptor-containing fractions of slime glycolipoprotein and lipopolysaccharide were hydrolyzed by the depolymerase, amino sugars were released, and the phage-inactivating activities of these fractions were lost. The depolymerase was also observed to induce the lysis of strain BI cells in hypotenic medium. The phage 2 depolymerase appears to play a role in adsorption and release of phage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. F., Stephens R. Decomposition of T6 bacteriophage in alkaline solutions. Virology. 1964 May;23(1):113–117. doi: 10.1016/s0042-6822(64)80017-9. [DOI] [PubMed] [Google Scholar]
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Lam G. K., Orr T. E. Purification and properties of polysaccharide depolymerase associated with phage-infected Pseudomonas aeruginosa. J Biol Chem. 1968 May 10;243(9):2077–2080. [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Chudio B. Purification and Chemical Composition of the Protective Slime Antigen of Pseudomonas aeruginosa. Infect Immun. 1970 Nov;2(5):543–548. doi: 10.1128/iai.2.5.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E. Distinct slime polysaccharide depolymerases of bacteriophage-infected Pseudomonas aeruginosa: evidence of close association with the structured bacteriophage particle. J Virol. 1969 Nov;4(5):580–584. doi: 10.1128/jvi.4.5.580-584.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Lam G. K. Polysaccharide depolymerase associated with bacteriophage infection. J Bacteriol. 1966 Jul;92(1):56–62. doi: 10.1128/jb.92.1.56-62.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E. Origin of polysaccharide depolymerase associated with bacteriophage infection. J Virol. 1969 Mar;3(3):290–296. doi: 10.1128/jvi.3.3.290-296.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Reese J. F., Imaeda T. Interaction of Pseudomonas bacteriophage 2 with the slime polysaccharide and lipopolysaccharide of Pseudomonas aeruginosa strain B1. J Virol. 1971 Sep;8(3):311–317. doi: 10.1128/jvi.8.3.311-317.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessler W., Freund-Mölbert E., Knüfermann H., Rduolph C., Thurow H., Stirm S. A bacteriophage-induced depolymerase active on Klebsiella K11 capsular polysaccharide. Virology. 1973 Nov;56(1):134–151. doi: 10.1016/0042-6822(73)90293-6. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo F. J., Bartell P. F. Studies on the bacteriophage 2 receptors of Pseudomonas aeruginosa. J Virol. 1974 Oct;14(4):904–909. doi: 10.1128/jvi.14.4.904-909.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S. T., Jr, Eagon R. G. Action of ethylenediaminetetraacetic acid, tris(hydroxymethyl)-aminomethane, and lysozyme on cell walls of Pseudomonas aeruginosa. Can J Microbiol. 1968 Aug;14(8):913–922. doi: 10.1139/m68-153. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S., Kusy A. R., Stone K. R. Characterization of T-even bacteriophage substructures. II. Tail plates. J Virol. 1970 Oct;6(4):545–555. doi: 10.1128/jvi.6.4.545-555.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes J., Goldberg E. B. Functions of baseplate components in bacteriophage T4 infection. I. Dihydrofolate reductase and dihydropteroylhexaglutamate. Virology. 1973 Oct;55(2):380–390. doi: 10.1016/0042-6822(73)90178-5. [DOI] [PubMed] [Google Scholar]
- EAGON R. G., CARSON K. J. LYSIS OF CELL WALLS AND INTACT CELLS OF PSEUDOMONAS AERUGINOSA BY ETHYLENEDIAMINE TETRAACETIC ACID AND BY LYSOZYME. Can J Microbiol. 1965 Apr;11:193–201. doi: 10.1139/m65-025. [DOI] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- Iwashita S., Kanegasaki S. Smooth specific phage adsorption: endorhamnosidase activity of tail parts of P22. Biochem Biophys Res Commun. 1973 Nov 16;55(2):403–409. doi: 10.1016/0006-291x(73)91101-7. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S., Wright A. Studies on the mechanism of phage adsorption: interaction between phage epsilon15 and its cellular receptor. Virology. 1973 Mar;52(1):160–173. doi: 10.1016/0042-6822(73)90406-6. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Verses C., Lute M., Crosby L. K. Bacteriophage tail components. II. Dihydrofolate reductase in T4D bacteriophage. J Virol. 1970 Jun;5(6):740–753. doi: 10.1128/jvi.5.6.740-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poglazov B. F., Rodikova L. P., Sultanova R. A. Isolation and characterization of bacteriophage T4 base plates. J Virol. 1972 Oct;10(4):810–815. doi: 10.1128/jvi.10.4.810-815.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese J. F., Dimitracopoulos G., Bartell P. F. Factors influencing the adsorption of bacteriophage 2 to cells of Pseudomonas aeruginosa. J Virol. 1974 Jan;13(1):22–27. doi: 10.1128/jvi.13.1.22-27.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. II. Structure and function of the baseplate. Virology. 1967 Jun;32(2):298–305. doi: 10.1016/0042-6822(67)90278-4. [DOI] [PubMed] [Google Scholar]
- Stirm S., Bessler W., Fehmel F., Freund-Mölbert E. Bacteriophage particles with endo-glycosidase activity. J Virol. 1971 Sep;8(3):343–346. doi: 10.1128/jvi.8.3.343-346.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirm S., Bessler W., Fehmel F., Freund-Mölbert E., Thurow H. Isolation of spike-formed particles from bacteriophage lysates. Virology. 1971 Jul;45(1):303–308. doi: 10.1016/0042-6822(71)90138-3. [DOI] [PubMed] [Google Scholar]
- Stirm S., Bessler W., Fehmel F., Freund-Mölbert E., Thurow H. Uber eine Bakteriophagen-induzierte Colansäure-Depolymerase. Zentralbl Bakteriol Orig A. 1974 Feb;226(1):26–35. [PubMed] [Google Scholar]
- Takeda K., Uetake H. In vitro interaction between phage and receptor lipopolysaccharide: a novel glycosidase associated with Salmonella phage 15 . Virology. 1973 Mar;52(1):148–159. [PubMed] [Google Scholar]
- VOSS J. G. LYSOZYME LYSIS OF GRAM-NEGATIVE BACTERIA WITHOUT PRODUCTION OF SPHEROPLASTS. J Gen Microbiol. 1964 May;35:313–317. doi: 10.1099/00221287-35-2-313. [DOI] [PubMed] [Google Scholar]