ABSTRACT
Knowledge regarding the genomic structure of Enterobacter spp., the second most prevalent carbapenemase-producing Enterobacteriaceae, remains limited. Here we sequenced 97 clinical Enterobacter species isolates that were both carbapenem susceptible and resistant from various geographic regions to decipher the molecular origins of carbapenem resistance and to understand the changing phylogeny of these emerging and drug-resistant pathogens. Of the carbapenem-resistant isolates, 30 possessed blaKPC-2, 40 had blaKPC-3, 2 had blaKPC-4, and 2 had blaNDM-1. Twenty-three isolates were carbapenem susceptible. Six genomes were sequenced to completion, and their sizes ranged from 4.6 to 5.1 Mbp. Phylogenomic analysis placed 96 of these genomes, 351 additional Enterobacter genomes downloaded from NCBI GenBank, and six newly sequenced type strains into 19 phylogenomic groups—18 groups (A to R) in the Enterobacter cloacae complex and Enterobacter aerogenes. Diverse mechanisms underlying the molecular evolutionary trajectory of these drug-resistant Enterobacter spp. were revealed, including the acquisition of an antibiotic resistance plasmid, followed by clonal spread, horizontal transfer of blaKPC-harboring plasmids between different phylogenomic groups, and repeated transposition of the blaKPC gene among different plasmid backbones. Group A, which comprises multilocus sequence type 171 (ST171), was the most commonly identified (23% of isolates). Genomic analysis showed that ST171 isolates evolved from a common ancestor and formed two different major clusters; each acquiring unique blaKPC-harboring plasmids, followed by clonal expansion. The data presented here represent the first comprehensive study of phylogenomic interrogation and the relationship between antibiotic resistance and plasmid discrimination among carbapenem-resistant Enterobacter spp., demonstrating the genetic diversity and complexity of the molecular mechanisms driving antibiotic resistance in this genus.
IMPORTANCE
Enterobacter spp., especially carbapenemase-producing Enterobacter spp., have emerged as a clinically significant cause of nosocomial infections. However, only limited information is available on the distribution of carbapenem resistance across this genus. Augmenting this problem is an erroneous identification of Enterobacter strains because of ambiguous typing methods and imprecise taxonomy. In this study, we used a whole-genome-based comparative phylogenetic approach to (i) revisit and redefine the genus Enterobacter and (ii) unravel the emergence and evolution of the Klebsiella pneumoniae carbapenemase-harboring Enterobacter spp. Using genomic analysis of 447 sequenced strains, we developed an improved understanding of the species designations within this complex genus and identified the diverse mechanisms driving the molecular evolution of carbapenem resistance. The findings in this study provide a solid genomic framework that will serve as an important resource in the future development of molecular diagnostics and in supporting drug discovery programs.
INTRODUCTION
In the last decade, the emergence of carbapenem resistance in Enterobacteriaceae became a significant public health concern, as carbapenems are regarded as being among the few antibiotics that can be used to treat severe infection in this family of common bacterial pathogens. Dissemination of carbapenemase-producing Enterobacteriaceae (CPE) in the United States has largely been associated with the class A β-lactamase Klebsiella pneumoniae carbapenemase (KPC). The resistance gene, blaKPC, is typically plasmid borne and can move between genera of Enterobacteriaceae, thereby facilitating its dissemination (1).
The first KPC-producing strain identified in the United States was a K. pneumoniae isolate collected in a North Carolina hospital in 1996 (2). Since then, KPC-producing isolates have spread globally and into various Gram-negative species (predominantly in K. pneumoniae); with KPC outbreaks reported in New Jersey and New York City hospitals (2–7). Much like the emergence and epidemic spread of carbapenem-resistant K. pneumoniae in the New York-New Jersey region, blaKPC-harboring isolates among other members of the family Enterobacteriaceae, especially Enterobacter spp., were frequently identified in clinical settings, representing a major infection control and therapeutic challenge. Overall, Enterobacter spp. are the sixth leading cause of health care-associated infections globally (8).
The first strain of an Enterobacter species carrying plasmid-encoded blaKPC-2 was obtained from a patient with sepsis at a Boston hospital in 2001 (9). Since its initial identification, sporadic cases with KPC-harboring Enterobacter spp. have been described and several outbreaks have been reported worldwide (10–15). According to two surveillance studies (in 2006 and 2009), a total of 758 KPC-positive Gram-negative isolates were collected, revealing that Enterobacter spp. were second to K. pneumoniae in harboring the blaKPC gene (16). In a study in Detroit between September 2008 and September 2009, blaKPC-harboring Enterobacter spp. accounted for ~15% of the carbapenem-resistant Enterobacteriaceae isolates in an urban health care system (17). Other studies have reported the spread of carbapenem resistance plasmids among related yet polyclonal strains of phenotypically similar Enterobacter spp. (11, 18), while another report describes the clonal dissemination of an outbreak strain between hospitals (19).
Despite the clinical significance of KPC-harboring Enterobacter spp., little attention has been focused on understanding the evolution and spread of blaKPC in Enterobacter spp. The molecular epidemiology of these strains in terms of genetic background, blaKPC type, and the structure of Tn4401 and nature of the plasmids harboring these resistant transposons, remains unknown. With this in mind, we sequenced to completion the genomes of six different KPC-producing Enterobacter spp. clinical isolates and then performed comparative whole-genome sequencing of 91 additional genomes of diverse Enterobacter clinical isolates obtained from the United States, South America, and the Mediterranean region to define the genetic structure of these drug-resistant emerging pathogens. These sequences were placed in a broader phylogenetic context by including 351 publicly available Enterobacter genome sequences in our analysis, including 77 that carry the blaKPC gene, and by performing complete genome sequencing of an additional six Enterobacter type strains.
RESULTS
Complete sequencing of six clinical isolates of carbapenem-resistant Enterobacter spp.
In this study, we sequenced to closure six blaKPC-harboring Enterobacter spp. clinical isolates by using Pacific Biosciences (PacBio) single-molecule real-time sequencing technology. The six isolates were obtained between 2011 and 2012 from patients at different health care institutions in New York, Florida, and Illinois (Table 1). Their genome sizes ranged from 4.6 to 5.1 Mbp (Fig. 1), similar in length to other completely sequenced Enterobacter spp. genomes (range, 4.5 to 5.4 Mbp) (20–23). In silico multilocus sequence typing analysis of seven target genes (dnaA, fusA, gyrB, leuS, pyrG, rplB, and rpoB) developed by Miyoshi-Akiyama et al. (24) assigned the six isolates to multiple sequence types (STs), ST113, ST114, ST171, ST269, ST594 and ST595. These isolates contained 4,275 to 4,559 coding genes and harbored between three and nine prophages (Table 1). The average GC content of the chromosomes was 55.41%, with a minimum and a maximum of 55.1% for BK34978 and 55.8% for BK35734. Moreover, these isolates harbored 83 or 84 tRNA genes and 25 rRNA genes.
TABLE 1 .
Key features of six clinical Enterobacter isolates sequenced to closure
| Characteristic | 34399 | 34977 | 34978 | 34983 | 34998 | 35734 |
|---|---|---|---|---|---|---|
| Species or subspecies | E. xiangfangensis | E. hormaechei subsp. steigerwaltii | E. xiangfangensis | E. hormaechei subsp. hormaechei | E. hormaechei subsp. steigerwaltii | E. cloacae complex Hoffmann cluster IV |
| ST | 114 | 594 | 171 | 269 | 113 | 595 |
| Location | Illinois | New York City | New York City | New York City | New York City | Florida |
| Phylogenetic group | A | B | A | E | B | M |
| Resistance gene(s) | ||||||
| Chromosome | blaACT-25 | blaACT-42, aph(3′)-Ia | blaACT-45, aph(3′)-Ia | blaACT-37 | blaACT-32 | blaMIR-20, qnrB19, qnrB19 |
| Plasmids | qnrS1, blaKPC-3, blaTEM-1A | blaKPC-2, blaTEM-1B, blaSHV-12, strB, strA, aadA2, aac(6.0′)-Iic, aph(3′)-Ia, qnrB2, ere(A), sul1, sul1, sul1, sul2, dfrA18 | blaKPC-3, blaOXA-9, blaTEM-1A, aac(6.0′)-Ib, aadA1, strB, strA, aac(6.0′)-Ib-cr, sul2, dfrA14 | blaKPC-2, blaTEM-1B, qnrB2, sul1, sul1, dfrB3 | blaKPC-4, blaTEM-1A, blaOXA-1, aadA1, aac(3)-via, aph(3′)-Ic, aac(6.0′)-Ib-cr, aac(6.0′)-Ib-cr, mph(A), catB3, arr-3, sul1, sul1, strA, strB, blaTEM-1B, sul2, tet(D), dfrA14, qnrS1 | blaKPC-3, blaKPC-3, blaOXA-9, blaTEM-1A, aac(6.0′)-Ib, aadA1, aac(6.0′)-Ib-cr, qnrB19 |
| Genome size (bp) | 4,784,288 | 4,897,485 | 4,930,963 | 4,632,074 | 4,688,189 | 5,017,289 |
| % G+C | 55.3 | 55.4 | 55.1 | 55.2 | 55.7 | 55.8 |
| No. of coding sequences | 4458 | 4495 | 4559 | 4275 | 4310 | 4493 |
| No. of plasmids | 3 | 3 | 6 | 3 | 5 | 3 |
| No. of prophages | 7 | 3 | 9 | 5 | 6 | 8 |
| No. of IS elements | 6 | 12 | 17 | 35 | 5 | 31 |
| No. of tRNA genes | 84 | 83 | 83 | 84 | 84 | 83 |
| No. of rRNA genes | 25 | 25 | 25 | 25 | 25 | 25 |
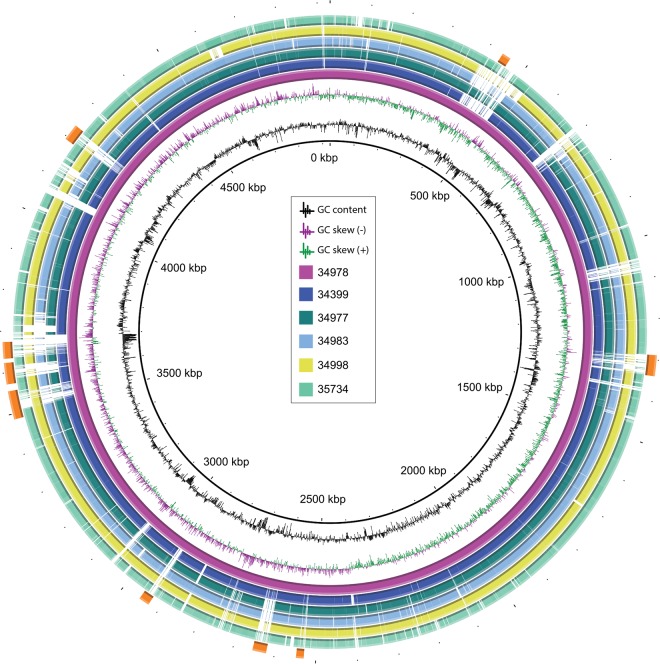
FIG 1 .
Enterobacter sp. genomes. Five completely sequenced Enterobacter genomes were compared with the E. hormaechei ST171 strain 34978 genome (pink inner circle). Genes that are present in 34978 but not in the other genomes are shown as blank spaces in the rings representing the five genomes. The GC content and GC skew of strain 34978 are plotted, and prophage locations are indicated by orange rectangles.
All six strains were resistant to β-lactam antibiotics (including carbapenem antibiotics), except BK34983, which has intermediate susceptibility to imipenem and meropenem but was susceptible to doripenem (see Data Set S1 in the supplemental material). Moreover, they were resistant to β-lactamase inhibitors (e.g., clavulanate, sulbactam, and tazobactam) and showed variable resistance to quinolones (ciprofloxacin, moxifloxacin, and levofloxacin), aminoglycosides (amikacin, gentamicin, and tobramycin), and tetracycline (tetracycline, doxycycline, and minocycline) (see Data Set S1). The majority of the antibiotic resistance determinants described above were located on plasmids (Table 1; see Table S1 in the supplemental material). These strains contained three to six plasmids, varying in size from 2.7 to 328 kbp, that collectively encode resistance to multiple classes of antibiotics (see Table S1).
In four of the six strains, blaKPC-harboring plasmids (p34977-B, p34983-A, p34399-A, and p35734-B) were identical or highly similar to conjugative plasmid pKPC_UVA01, initially described from K. pneumoniae isolates recovered from patients in the University of Virginia Health System and long-term acute care hospital (see Fig. S1 in the supplemental material) (25). blaKPC-harboring Tn4401 in this plasmid was inserted within the tnpA gene of a Tn2-like element. Identical to pKPC_UVA01, plasmids p34977-B and p34983-A were 43,621 bp in size, carried a novel replicon (nontypeable by current incompatibility [Inc] group typing) (26), and harbored blaKPC-2 on a Tn4401b-Tn2-like transposon structure. In comparison to pKPC_UVA01, the blaKPC-3-harboring Tn4401b-Tn2-like structure in p34399-A was incorporated at a distinct location between genes encoding a hypothetical protein and a chaperonin protein, demonstrating independent acquisition of blaKPC in the same plasmid backbone. Moreover, a similar pKPC_UVA01-like plasmid (pKPC-f91) was found in strain ECNIH2, isolated from a sink drain at the National Institutes of Health (NIH) Clinical Center (see Fig. S1) (21). Interestingly, p35734-B is similar to p34399-A with an insertion of another plasmid sequence related to plasmid pENT-c88 from strain ECNIH4 (21). Plasmid p35734-B harbored two copies of blaKPC-3 on two separate Tn4401b elements; however, blaKPC was not located within the Tn4401-Tn2-like structure as described above. The potential functional significance of this duplication appears to be supported by the elevated MICs of carbapenem antibiotics for strain BK35734 (see Data Set S1).
Plasmid p34978-F is identical to IncFIA plasmid pBK30683 from K. pneumoniae (27), which is one of the most predominant blaKPC-harboring plasmids circulating in K. pneumoniae strains in New York-New Jersey hospitals (see Table S1). Of note, pBK30683 is a cointegrate plasmid that has an ~70-kb IncFIA plasmid backbone nearly identical to that of pBK30661 and the integration of the second IncF plasmid harboring the tra operon for plasmid transfer (27). Therefore, IncFIA pBK30683-like plasmids have two different forms, the nonconjugative pBK30661 type and the cointegrate pBK30683 type (pBK30661 containing a second IncF plasmid carrying the functional tra operon) (27).
Interestingly, strain BK34998 has a blaKPC-4-harboring IncA/C plasmid, p34998-E. p34998-E carries a plasmid backbone similar to that of the other IncA/C plasmids pRA1 (28) (IncA/C reference plasmid isolated in 1971 from Aeromonas hydrophila) and p35734-C (isolated from strain BK35734) (see Fig. S2 in the supplemental material). However, p34998-E acquired an ~66-kb region encoding genes for β-lactam resistance, aminoglycoside resistance, quinolone resistance, phenicol resistance, macrolide resistance, sulfonamide resistance, and rifampin resistance (see Fig. S2). The region surrounding blaKPC-4 showed homology to the blaKPC-4 region from IncN plasmid pBK31551 from K. pneumoniae that we described previously (29).
In addition to the blaKPC-harboring plasmids, the six PacBio sequenced strains harbored an additional two to five plasmids. These plasmids ranged in size from 2,725 to 328,905 bp, and most of them did not carry any antimicrobial resistance genes (see Table S1). Among them, p34977-A; p34978-A, -B, -C, and -D; p34998-A; and p35734-A belonged to the ColE superfamily. The two IncF plasmids p34399-B (from 34399, ST114) and p34998-C (from 34998, ST113) were nearly identical and both harbored the quinolone resistance gene qnrS1 (see Table S1). In contrast, plasmids p34983-B and p34998-B belonged to the same incompatibility group, IncN3, but share only 51% homology with 97% identity. Plasmid p34977-C is an IncHI2 plasmid and harbors genes for resistance to β-lactams (blaSHV-12), aminoglycosides [strB, strA, aadA2, aac(6′)-IIc, aph(3′)-Ia], quinolones (qnrB2), sulfonamide (sul1, sul2), trimethoprim (dfrA18), and macrolides [ere(A)]. Plasmid p34983-C belongs to the IncHI1 group but does not harbor any antimicrobial resistance genes. Lastly, plasmid p35734-C belongs to the IncA/C group and shares homology with blaKPC-4-carrying plasmid p34998-E, as described above. However, p35734-C does not harbor any resistance genes (see Fig. S2).
Genome sequencing of geographically diverse Enterobacter spp. clinical isolates.
To gain a better understanding of the emergence and evolution of the blaKPC-harboring Enterobacter spp. and to perform a whole-genome-based phylogenetic classification of these isolates, we next performed whole-genome sequencing of 91 additional Enterobacter clinical isolates (see Data Set S1). Among them, 66 possessed blaKPC, 2 had blaNDM, and 23 were carbapenem susceptible. Isolates were selected on the basis of their geographic and temporal distributions, KPC variants, and Tn4401 patterns. Isolates were obtained from health care facilities at diverse geographic locations in the United States (Florida, Illinois, Michigan, Ohio, Pennsylvania, New Jersey, New York, and Texas), Gaza, and Colombia between 2006 and 2014. Strain 40874 was identified as Pluralibacter gergoviae (formerly E. gergoviae) and was not analyzed further.
Phylogenetic structure of Enterobacter species.
To estimate the genetic relationships among Enterobacter strains, the 97 genomes sequenced in this study were compared with 351 Enterobacter genomes publicly available in GenBank by using both average nucleotide identity (ANI) and a single-nucleotide polymorphism (SNP)-based phylogeny. SNPs were identified from the combined set of genome sequences by using kSNP (30). Nucleotide positions present in at least 80% of all genomes were used to build a phylogenetic tree with RAxML (31).
Strains were clustered into 19 phylogenetic groups. One group corresponded to E. aerogenes, and the remaining 18 groups belong to the E. cloacae complex (groups A to R in Fig. 2; Table 2; see Fig. S4 and Data Sets S1 and S2 in the supplemental material) (32–34). The 18 E. cloacae complex groups typically had mean within-group ANI values >99% but always >96.5%. Mean ANI values between groups were always ≤95%, except among E. hormaechei subspecies groups A to E, G, and H (Table 2). Groups J and K may turn out to be subspecies, since they have 95% ANI. ANI and SNP phylogeny were concordant in clustering the genomes into phylogenetic groups. Additional details regarding the recommended ANI threshold to define species groups are described in Text S1 in the supplemental material.
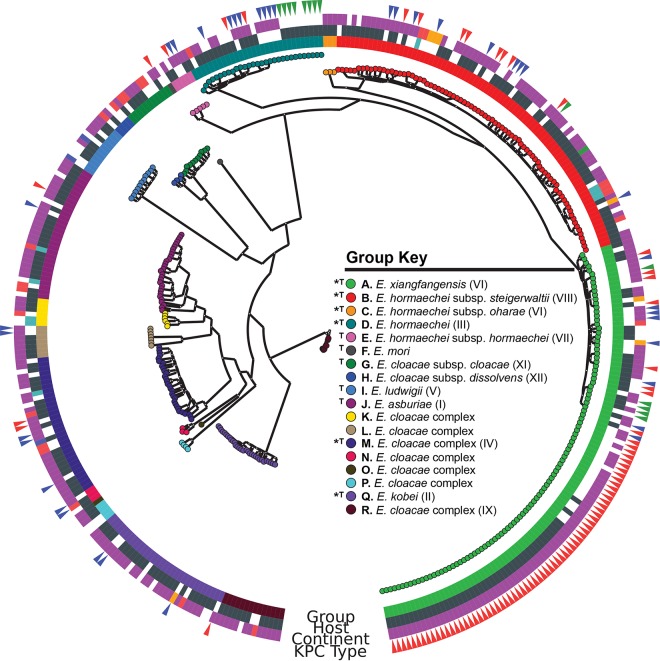
FIG 2 .
Phylogenetic SNP tree of E. cloacae complex genomes. A whole-genome core SNP tree was constructed for 379 E. cloacae complex genomes with kSNP (30) and RAxML (31) (see Materials and Methods). Groups identified by ANI (A to R) are noted as colored nodes, as well as the innermost circle (see group key). Hosts, continents of origin, and KPC type metadata are also plotted in concentric circles as noted. Host colors: human, dark gray; nonhuman, blue. Continent colors: North America, purple; South America, orange; Australia, green; Europe, blue; Asia, red. KPC type colors: 3, red; 2, blue; 4, green.
TABLE 2 .
Group labels for the E. cloacae complex
| Group | Hoffman cluster | Species, subspecies, or complex | Type strain | Accession no. | No. of strains |
|---|---|---|---|---|---|
| A | VI | E. xiangfangensis | LMG 27195a | NZ_CP017183.1 | 3 |
| B | VIII | E. hormaechei subsp. steigerwaltii | DSM 16691a | NZ_CP017179.1 | 83 |
| C | VI | E. hormaechei subsp. oharae | DSM 16687a | NZ_CP017180.1 | 104 |
| D | III | E. hormaechei | DSM 14563a | NZ_CP017186.1 | 30 |
| E | VII | E. hormaechei subsp. hormaechei | ATCC 49162 | GCA_000213995.1 | 5 |
| F | E. mori | LMG 25706 | AEXB00000000 | 1 | |
| G | XI | E. cloacae subsp. cloacae | ATCC 13047 | NC_014121.1 | 11 |
| H | XII | E. cloacae subsp. dissolvens | 4 | ||
| I | V | E. ludwigii | EN-119 | CP017279.1 | 11 |
| J | I | E. asburiae | ATCC 35953 | NZ_CP011863.1 | 30 |
| K | E. cloacae complex | 6 | |||
| L | E. cloacae complex | 7 | |||
| M | IV | E. cloacae complex | DSM 16690a | NZ_CP017184.1 | 31 |
| N | E. cloacae complex | 3 | |||
| O | E. cloacae complex | 1 | |||
| P | E. cloacae complex | 4 | |||
| Q | II | E. kobei | DSM 13645a | NZ_CP017181.1 | 31 |
| R | IX | E. cloacae complex | 14 |
Type strains sequenced in this study.
Identifying members of the E. cloacae complex to the subspecies level.
Before the advent of routine genome sequencing of type strains for validly published bacterial taxonomic species, there was quite a bit of confusion over what species make up the E. cloacae complex, with various species being moved to or from other genera or being reassigned as subspecies. Furthermore, many genomes have been submitted to GenBank as E. cloacae when they were in the E. cloacae complex but not the E. cloacae species, causing more confusion.
In a seminal work, Hoffmann and Roggenkamp (32) defined 12 genetic clusters (I to XII) based most exhaustively on hsp60 sequencing. Three of the clusters (cluster III, 58 strains; cluster VI, 28 strains; cluster VIII, 59 strains) accounted for 70% of the 206 strains studied. In a following study, Hoffmann et al. (35) named cluster VII E. hormaechei subsp. hormaechei, cluster VI E. hormaechei subsp. oharae, and cluster VIII E. hormaechei subsp. steigerwaltii. More recently, Gu et al. (36) defined Enterobacter xiangfangensis by using a phylogenetic tree based upon concatenated partial rpoB, atpD, gyrB, and infB gene sequences from a novel isolate and existing type strains where E. xiangfangensis was closest to E. hormaechei in the tree. Details of the previous taxonomic work are described in Text S1 in the supplemental material.
In order to clarify the proper naming of genomes in the E. cloacae complex, we sequenced the type strains for E. hormaechei subsp. steigerwaltii (B; DSM 16691), E. hormaechei subsp. oharae (C; DSM 16687), E. xiangfangensis (A; LMG 27195), Hoffmann cluster III (D; DSM 14563), Hoffmann cluster IV (M; DSM 16690), and E. kobei (Q; DSM 13645) (Table 2).
To link our ANI and kSNP trees to the hsp60 typing results, we performed “in silico” hsp60 typing of the genome sequences and included previously published hsp60 sequences. The hsp60 types for species type strains, species type strains with sequenced genomes, and ANI values within and between groups were used to assign names to groups A to R (Table 2). Genomes from type strains were not available for all groups, so the name designations should be considered provisional. It should be noted that group D (hsp60 cluster III) was not treated by Hoffmann as an E. hormaechei subspecies on the basis of hsp60 sequence clustering results but by ANI represents a novel E. hormaechei subspecies. Group A, which includes ST171 strains and the type strain of E. xiangfangensis, also is an E. hormaechei subspecies based on ANI analysis. Groups G and H have a mean between-group ANI of 95.7%, which is consistent with having different hsp60 types and being defined as different E. cloacae subspecies (35).
Relationship of E. aerogenes to other members of the family Enterobacteriaceae.
The group of E. aerogenes genomes was distant from other groups that represent the E. cloacae complex (e.g., E. hormaechei, E. kobei, E. cloacae, and E. asburiae). To better place the E. aerogenes genomes in the context of other members of the family Enterobacteriaceae, genomic sequences of 248 bacterial strains from 48 genera within the Enterobacteriaceae family were downloaded from GenBank and used to construct a phylogenetic tree (Fig. 3). It is apparent from the tree that E. aerogenes is more closely related to K. pneumoniae than to the E. cloacae complex (23). This is consistent with previous findings and proposals to rename the species: K. mobilis (37–39), K. aeromobilis (23), and very recently K. aerogenes (40).
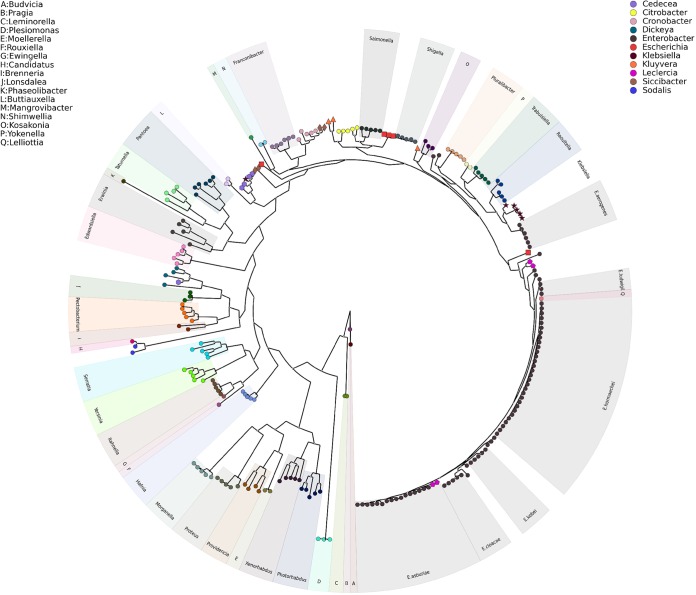
FIG 3 .
Phylogenetic universal marker tree of representative genomes of members of the family Enterobacteriaceae. A maximum-likelihood tree was constructed from a concatenated alignment of 26 conserved universal marker alleles from 248 genomes belonging to 48 different genera. If a genus was represented by one to three genomes, the genus was given a letter designation (A to Q; see key, top left). If a genus was represented by four or more genomes and all members clustered together, the genus was labeled directly on the tree; however, if they did not all cluster together, unique noncircular shapes were used to identify them. Each genus is assigned a unique color (see key, top right).
Core pangenome of E. hormaechei.
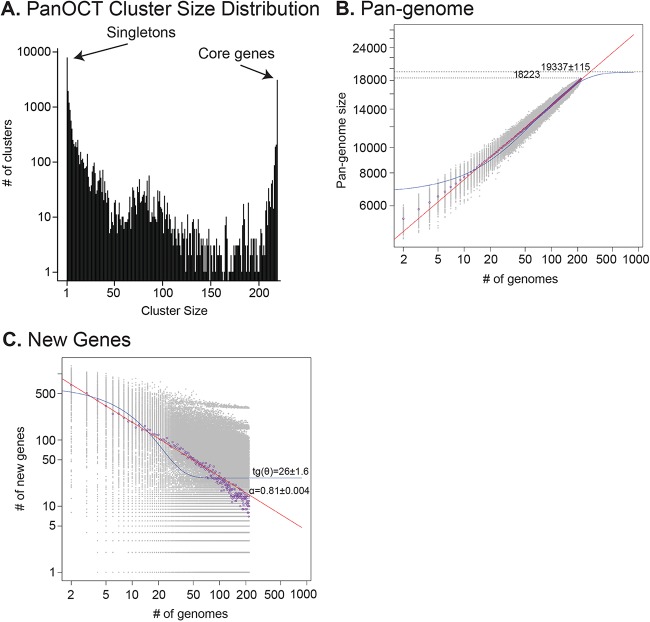
To explore the potential functional significance of distinct E. hormaechei subspecies, the pangenome of all available E. hormaechei genomes was determined (groups A to E, as defined above) with the PanOCT software suite (41, 42). When the core pangenome of all 219 genomes analyzed (100%) was defined, there were 3,048 core/universal protein clusters and 7,794 singleton clusters (i.e., clusters with a single member from a single genome) identified (Fig. 4a). If the core pangenome were instead defined as clusters having protein members from 95% of the genomes analyzed, the core pangenome was 3,718 protein clusters. The pangenome of E. hormaechei appeared to be open (α = 0.8182 ± 0.004; Fig. 4b). The number of new genes found for each genome added to the pangenome was determined from the exponential decay function to be 26 ± 1.6 (Fig. 4c). Details of the analysis of the E. hormaechei pangenome are described in Text S1 in the supplemental material.
FIG 4 .
Analysis of the E. hormaechei pangenome. The distribution of protein cluster sizes (the number of genomes sharing each ortholog) generated from the comparison of 219 E. hormaechei genomes with PanOCT (42) indicates the numbers of singleton and core genes (A). The pangenome size (B) and the number of novel genes discovered with the addition of each new genome (C) were estimated by using a pangenome model as described previously (44). Purple circles are the median of each distribution (gray circles). Power law (red lines) and exponential (blue lines) regressions were plotted to determine α (open/closed status) and tg(θ), the average extrapolated number of strain-specific/novel genes, respectively (45).
fGIs define phenotypic differences between E. hormaechei subspecies.
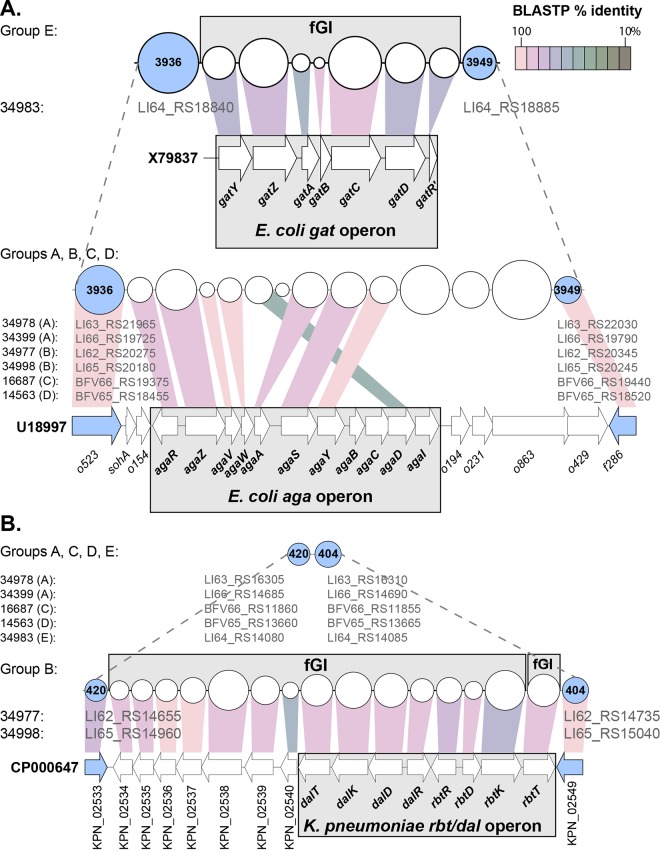
E. hormaechei subsp. hormaechei (hsp60 cluster VII, group E) is distinguishable from E. hormaechei subsp. oharae (hsp60 cluster VI, group C) and E. hormaechei subsp. steigerwaltii (hsp60 cluster VIII, group B) by growing on dulcitol (also called galactitol) as the sole carbon source (33). This phenotype of group E can be explained by the presence of a gat operon (43) located on a flexible genomic island (fGI) between core gene clusters 3936 (d-galactarate dehydratase) and 3949 (16S rRNA methyltransferase) (Fig. 5A), while at the same location, the E. hormaechei subspecies in groups A to D have a related but different operon, encoding N-acetylgalactosamine metabolism (also called the aga locus) (44) (Fig. 5A). Similarly, hsp60 cluster VIII (B) isolates can be distinguished from hsp60 cluster VI and VII isolates (A, C, D, and E) by their ability to grow on adonitol (also called ribitol) and d(+)-arabitol, both five-carbon sugar alcohols known as penitols. An fGI was identified that encodes the rbt and dal operons, which metabolize ribitol and d(+)-arabitol, respectively (45) (Fig. 5B).
FIG 5 .
Historical metabolic differences defining E. hormaechei subspecies explained by fGI content. Variability within the flexible genomic region between core pangenome gene clusters 3936 and 3949 can contain either the gat operon to metabolize galactitol (defining group E) or the aga operon to metabolize N-acetylgalactosamine (defining groups A to D) (A). The presence of an operon between core gene clusters 420 and 404 homologous to the Klebsiella rbt/dal operon (75), for d-arabinitol and ribitiol catabolism, distinguishes group B isolates from A, C, D, and E isolates (B). Circles and arrows represent PanOCT gene clusters and protein coding regions, respectively. The size of a circle is scaled to the gene length of its centroid. The locus identifiers of core genes from applicable PacBio genomes in this study are noted in gray under their respective core clusters. Colored connecting lines represent the levels of conservation between those cluster centroids and genes whose protein sequences match at a BLASTP identity of ≥35% (see key, upper right). Blue circles and arrows denote flanking core clusters and genes, respectively.
Plasmid gene content can influence the topology of the gene content tree.
From the PanOCT output, we can draw trees based on gene content. The gene content tree should be congruent with trees generated from analyses that favor vertical conservation (e.g., kSNP, universal markers, ANI), unless there has been recent acquisition of genes via lateral transfer. A tree generated from the PanOCT ortholog gene clusters representing the presence/absence of genes in each genome (see Fig. S3A in the supplemental material) showed a different clustering of genomes than was present in the kSNP phylogenic tree (Fig. 2). When ortholog clusters corresponding to genes that matched the 34 known plasmids (harbored by the complete genomes used in the PanOCT analysis) were removed, the topology of the tree resembled the kSNP tree more closely (see Fig. S3B).
Plasmid diversity among Enterobacter spp.
The blaKPC gene is reported to be found on plasmids of different Inc groups. In previous studies, certain blaKPC-harboring plasmids were found to be more prevalent among CPE isolates in New York-New Jersey hospitals, including the pBK30683/pBK30661 (IncFIA group) (27), pBK15692 (IncI2 group) (46), and pKpQIL (IncFIIK2 group) (47) plasmids. The plasmid sequences identified from the six closed PacBio genomes suggest that some plasmids found in K. pneumoniae are common in Enterobacter spp. For example, the blaKPC-harboring plasmid in 34978, p34978-F, is identical to IncFIA plasmid pBK30683 in K. pneumoniae. In addition, in four closed genomes from different phylogenetic groups, blaKPC was found to be located on the same pKPC_UVA01-like plasmid. To estimate the occurrence of different blaKPC-harboring plasmids among the 72 KPC-producing Enterobacter strains, we mined the blaKPC-bearing Tn4401 isoforms and their target site duplication (TSD) sequences and examined each blaKPC-harboring contig with BLASTn against completely sequenced plasmids in GenBank. Tn4401 is 10 kb in size, is delimited by two 39-bp imperfect inverted repeat sequences, and is usually associated with 5-bp TSDs at both ends as a result of integration (48). The blaKPC-harboring Tn4401 isoform and its TSD are important genetic markers that track with different plasmids, and consequently, they are useful plasmid genotyping tools (1). In this study, 43 Tn4401b, 2 Tn4401a, 24 Tn4401d, and 3 pKp048-like non-Tn4401 mobile elements (NTMKPC) were identified (see Data Set S1). The two Tn4401a isoforms had the same TSD (ATTGA), which was initially described in plasmid pKpQIL (49). Interestingly, no apparent TSDs were identified upstream or downstream of Tn4401d in the 24 Tn4401d-bearing isolates. The 5-bp upstream (TCTCT) and downstream (GTTCT) adjacent sequences in the Tn4401d-bearing isolates are identical to those in IncFIA plasmids pBK30683 and pBK30661 (27). In contrast, nine different pairs of TSDs were found in the Tn4401b-bearing isolates (see Data Set S1). These data revealed the diverse Tn4401 integration sites on different Enterobacter isolates.
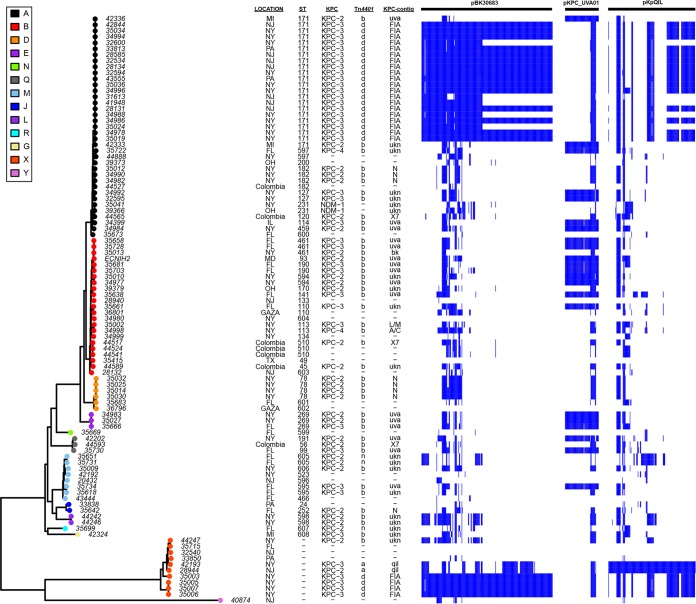
Plasmid classification analysis (see Materials and Methods) successfully assigned the blaKPC-harboring contigs from 55 out of 72 isolates into a known or novel plasmid group (see Data Set S1) (Fig. 6). Among them, blaKPC was found on an IncFIA plasmid (pBK30683- or pBK30661-like) in 24 isolates (24/55), and they all carry blaKPC-3 on the Tn4401d elements, flanked by 5-bp nonmatched adjacent sequences described above (Fig. 6). In addition, blaKPC in 15 isolates was located on Tn4401b on the pKPC_UVA01-like plasmids with identical Tn4401b isoforms and TSDs. The blaKPC genes in the remaining 16 isolates were associated with IncN-like (e.g., pKPC_FCF/3SP-like) (50) (n = 8), pKpQIL-like (n = 2), IncL/M-like (n = 1), IncA/C (p34998-239kb, n = 1), and pBK28610-like (n = 1) plasmids and a novel IncX (X7) plasmid (n = 3) (Fig. 6). Because of the short length of the de novo assembled contigs, we could not link 17 blaKPC-harboring and the 2 blaNDM-harboring contigs with any plasmid group.
FIG 6 .
Representation of major KPC-harboring plasmids among 97 Enterobacter isolates. (Left) Core SNP phylogenetic tree generated by RAxML. Core SNPs were identified by kSNP v 3.0 (see Materials and Methods). (Middle) The metadata, including isolation location, ST, KPC variants, Tn4401 isoforms, and predicated blaKPC-harboring plasmids. (Right) Plasmid composition is illustrated by showing the BLASTn matches to each Enterobacter genome across all of the genes on the three reference plasmids, pBK30683, pKPC_UVA01, and pKpQIL. The blue bar denotes a minimal 95% nucleotide sequence identity to the plasmid genes. Abbreviations: uva, pKPC_UVA01-like plasmid; FIA, pBK30683 or pBK30661-like plasmid; Bk., pBK28610-like plasmid; qil, pKpQIL-like plasmid; ukn, blaKPC-harboring contigs could not be assigned to a known or novel plasmid group; n, pKp048-like non-Tn4401 mobile element (NTMKPC). Blue bars denote a ≥95% nucleotide sequence match to the plasmid genes.
We also examined the distribution of these blaKPC-harboring plasmids regarding their different phylogenetic groups (Fig. 6). The pBK30683-like plasmids were found in either group A or E. aerogenes. In group A, pBK30683-like plasmids were associated exclusively with ST171 isolates, suggesting the clonal spread of carbapenem resistance. In contrast, pKPC_UVA01-like conjugative plasmids appeared to be more promiscuous and have been found in isolates of various groups (A, B, E, M, and Q). Similarly, the newly identified blaKPC-harboring IncX7 plasmids were found in three isolates from three different phylogenetic groups (A, B, and Q). The spread of the same plasmid (e.g., pKPC_UVA01 and X7 plasmids) in unrelated strains suggested that the horizontal plasmid transfer contributed significantly to the dissemination of carbapenem resistance, which in part explains the aforementioned finding that plasmid gene content influences the topology of the gene content tree. Interestingly, pKpQIL-like plasmids, which are among the most common blaKPC-harboring plasmids in multiple K. pneumoniae STs and in other species (e.g., Escherichia coli) (21, 47, 49, 51–55), were identified in only two E. aerogenes isolates in this study, suggesting the likelihood of plasmid restriction between species or subspecies. One of them, plasmid pKpQIL-Ea (113,639 bp in length, from strain 28944), was completely sequenced in a previous study, and the sequence matches the prototype pKpQIL plasmid sequence from Israel, which is 113,637 bp in length (47).
Molecular dissection of carbapenem-resistant E. xiangfangensis ST171.
Currently, the dissemination of KPC in K. pneumoniae has been largely associated with a predominant clone, ST258, and closely related strains (1). It appears that in Enterobacter spp., E. xiangfangensis ST171 strains are an emerging clone that has been increasingly reported among CPE isolates from different hospitals in the United States (15, 56–58). In this study, ST171 is the most common ST among the 97 sequenced genomes, accounting for 22.7% of all genomes. Mining the Enterobacter spp. genome data from the present study and GenBank identified a total of 73 ST171 genomes, collected between 2009 and 2014 in New York, New Jersey, Massachusetts, Minnesota, North Dakota, and Brazil (Fig. 7). Considering all of the genomes analyzed, nearly 100% of the ST171 strains are positive for the blaKPC gene (72/73), whereas only 28% of the non-ST171 strains carry the gene (71/257).
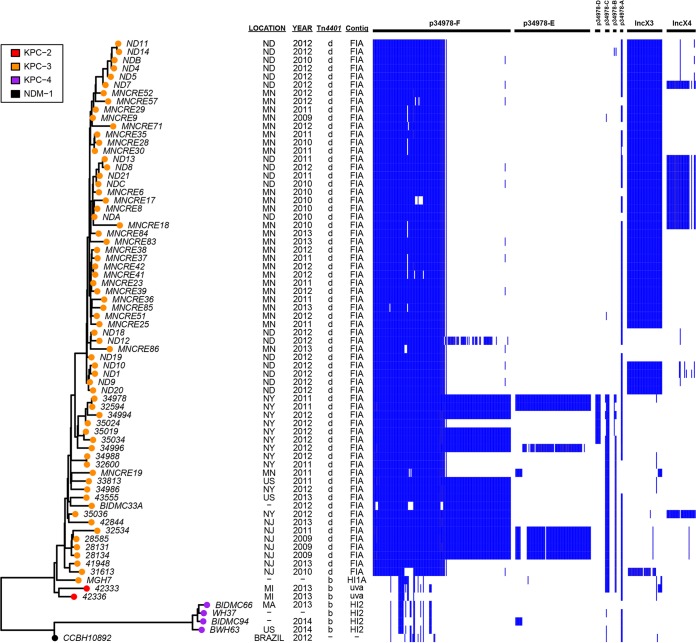
FIG 7 .
Representation of plasmid distribution among ST171 Enterobacter isolates. (Left) Core SNP phylogenetic tree generated by RAxML (see Materials and Methods). (Middle) The metadata, including isolation location, isolation year, Tn4401 isoforms, and predicated blaKPC-harboring plasmids. (Right) Plasmid composition is illustrated by showing the BLASTn matches to each Enterobacter genome across all of the genes on the reference plasmids. Six plasmids from completely sequenced E. xiangfangensis ST171 strain 34978, along with IncX3 plasmid pIncX-SHV (JN247852) and IncX4 plasmid pMNCRE44_4 (CP010880), were used as references. Blue bars denote a ≥95% nucleotide sequence match to the plasmid genes.
Core SNP analysis separated ST171 strains into two distinct clusters, with an average of ~373 core SNP differences and with a phylogeographic profile. The major cluster (cluster I) includes 68 genomes that differ from one another by an average of 42 SNPs. On the basis of their geographic distribution, cluster I can be divided into three subclades: ND/MN, NY/NJ, and MI. Previous genetic studies have indicated that the ND/MN subclade was clonally spreading in the North Dakota-Minnesota region (57). The only outlier isolate, MNCRE19, appears to be a member of the NY/NJ subclade, and it is possible that this isolate originated in the New York-New Jersey area.
Plasmid examination showed that isolates in the ND/MN and NY/NJ subclades all carry blaKPC-3 in a Tn4401d element. Further analysis demonstrated that the blaKPC-3 genes are located on the pBK30683-like IncFIA plasmid. As described above, pBK30683 is a cointegrate plasmid that has an ~70-kb pBK30661 IncFIA backbone and the addition of another tra operon-containing IncF plasmid region (27). Interestingly, the blaKPC-3-harboring IncFIA plasmids from the ND/MN subclade and five from NY/NJ subclade were only half the size of pBK30683, which is similar to pBK30661, and the tra operon-bearing IncFII plasmid region was not present (Fig. 7) (57).
Although the blaKPC-harboring plasmids are similar in ND/MN and NY/NJ isolates, their companion plasmids demonstrated a diverse distribution. NY/NJ isolates contain an additional p34978-70kb-like IncL/M plasmid, as well as ColE plasmids p34978-D and p34978-C, that is not present in ND/MN isolates. In contrast, most ND/MN isolates harbor the blaSHV-12-bearing IncX3 plasmids. Moreover, most isolates from North Dakota carry an additional IncX4 group plasmid, in comparison to Minnesota isolates (Fig. 7).
Cluster II contains four isolates harboring blaKPC-4 on a Tn4401b element on an IncHI2 plasmid that differ from one another by ~26 core SNPs. The outlier strain CCBH10892, harboring blaNDM-1 and isolated from Brazil, is divergent from clusters I and II and has 345 and 350 SNPs, in comparison to isolates from clades I and II, respectively.
DISCUSSION
Identification of species and subspecies within the E. cloacae complex by phenotypic methods is challenging and often presents problems of reproducibility (59). Moreover, the genus Enterobacter is polyphyletic in nature and using 16S rRNA gene sequencing to classify strains into different species is challenging (60). There have been multiple proposals for transfers of species to and from this genus. As an example, E. liquefaciens, a member of the genus Enterobacter, was transferred to the genus Serratia in 1971 on the basis of DNA relatedness studies (60, 61).
We used genome sequences of many diverse Enterobacter species isolates to refine species designations for this genus. Using ANI and SNP analyses, we placed 447 Enterobacter genomes into 19 phylogenomic groups—18 groups (A to R) in the E. cloacae complex and 1 in E. aerogenes. Groups containing type strains were given specific species names; otherwise, just “cloacae complex” was used with the strain name. The distinct phylogenomic grouping of E. aerogenes on the basis of whole-genome sequencing clearly supports the previously proposed argument to rename this species K. aerogenes (40).
Carbapenemase-producing Enterobacter spp. are increasingly being identified in clinical settings; however, knowledge regarding their genomic nature, population structure, and resistance plasmid characteristics remains limited. Carbapenemase-producing strains were found in nearly every group (Fig. 2). The genomic signatures (including resistance determinants, Tn4401 variants, plasmids, and host genomes) strongly suggested that both horizontal gene transfer and clonal expansion have contributed to the dissemination of carbapenem-resistant strains in this genus. We also showed that plasmid gene content can influence the topology of trees drawn from the presence/absence of gene content and therefore should be removed prior to the generation of gene content trees from pangenome studies.
Our study demonstrated that the spread of blaKPC among Enterobacter spp. is due to multiple complex mechanisms. First, we have observed the acquisition of a blaKPC-harboring plasmid by a specific host strain, followed by clonal expansion into different geographic regions. The example is the clonal spread of E. xiangfangensis ST171 harboring blaKPC-3 on an IncFIA plasmid (Fig. 7). IncFIA plasmid pBK30683-like was one of the predominant blaKPC-harboring plasmids in K. pneumoniae and has been circulating in New York-New Jersey hospitals since the early 2000s (27). Subsequently, this plasmid was identified in Enterobacter spp. in 2009 from different hospitals in the New York-New Jersey area (27). We suspected that the pBK30683-like plasmids in Enterobacter spp. may originate through horizontal plasmid transfer from K. pneumoniae. Of note, pBK30683-like plasmids have two different forms, the nonconjugative pBK30661 type and the conjugative/cointegrate pBK30683 type (27). Interestingly, the MN/ND ST171 clade all carry the pBK30661-type plasmid, and in contrast, the majority of NY/NJ isolates (except for 35024, 34988, 32600, 41948, and 31613) harbored the cointegrate pBK30683-type plasmid (Fig. 7). It is hypothesized that the carbapenem-resistant ST171 MN/ND isolates were descendants of an ST171 strain harboring a nonconjugative IncFIA plasmid or originated by the acquisition of an IncFIA cointegrate plasmid by a local ST171 strain, and the IncF region on pBK30683 was subsequently lost. The nonconjugative IncFIA plasmid in MN/ND ST171 strains is not able to conjugate and can only be spread clonally, until it forms novel cointegrates as reported elsewhere (21, 57, 62).
Second, horizontal transfer of a common plasmid across different phylogenomic clades has occurred in Enterobacter spp. One example is the identification of pKPC_UVA01-like plasmids in multiple phylogenomic groups (Fig. 6). pKPC_UVA01, initially described in K. pneumoniae in a Virginia hospital system, has now been frequently found in various species, including K. pneumoniae, E. cloacae, E. aerogenes, E. coli, K. oxytoca, and Citrobacter freundii (25, 63). A recent study indicated that in pKPC_UVA01, Tn4401b was integrated into blaTEM-1-containing transposon Tn2 and the Tn2-Tn4401 nested transposon was translocated into different plasmid backbones (63). The authors of that study warned that the use of reference-based plasmid interpretation may produce misleading results. In this study, only the de novo assembled contigs containing both Tn4401 and additional pKPC_UVA01 core plasmid genes (e.g., replicons) were considered in the analysis (see Materials and Methods). Using this approach, 15 pKPC_UVA01-like plasmids were found. Our results showed that the pKPC_UVA01-like plasmids spread into different groups of Enterobacter spp. and were identified in different geographic regions (New York, Michigan, Maryland, Illinois, and Florida). In addition, six pKPC_UVA01-like plasmids carry blaKPC-2, while nine harbor blaKPC-3, indicating multiple independent acquisitions of different blaKPC variants with Tn4401 integrated into a hot spot on the same plasmid backbone. Another example is the IncX7 plasmid harboring blaKPC-2 from Columbia. We identified nearly identical ~47-kb contigs containing Tn4401b and IncX7 plasmid core operons (encoding replication, stability, and transfer) in 44565 (group A), 44517 (group B), and 44593 (group Q).
Third, multiple discrete acquisition events involving blaKPC-containing elements (e.g., Tn4401) have contributed to the dissemination of carbapenem resistance in Enterobacter spp. We found that Tn4401d and Tn4401a in the 72 blaKPC-bearing isolates were associated with pBK30683- and pKpQIL-like plasmids, respectively. In contrast, Tn4401b showed a high degree of diversity regarding its TSDs and harboring plasmids. Nine unique TSDs were found to be associated with Tn4401b, suggesting the repeated acquisition of Tn4401 on different plasmids or different locations on the same plasmid (e.g., IncN) backbones (see Data Set S1).
Finally, there are common blaKPC-harboring plasmids in K. pneumoniae that are nearly absent in Enterobacter spp. IncFIIK pKpQIL-like plasmids are among the most predominant blaKPC-harboring plasmids in K. pneumoniae and E. coli from the northwestern United States and other geographic regions (21, 47, 49, 51–55), but interestingly, among the 72 blaKPC-harboring isolates, only 2 E. aerogenes isolates have been found to carry pKpQIL. Of note, the time period of the present study overlaps our previous molecular surveillance study that investigated the prevalence of pKpQIL in K. pneumoniae (47). Our previous study found the pKpQIL-like plasmids accounted for as much as 35% of the blaKPC-harboring plasmids in K. pneumoniae (47). In contrast, in the present study, only 3% (2/72) of the blaKPC-harboring plasmids are pKpQIL. In addition, searches of the public Enterobacter genomes did not identify any additional pKpQIL replicons (positive for both IncFIIK and IncFIBqil). This finding may mean that IncFIIK pKpQIL plasmids are not “compatible” or “stable” in the Enterobacter host.
E. xiangfangensis ST171 strains are the most predominant Enterobacter clones reported in our study and in the public databases. However, unlike the epidemic K. pneumoniae ST258 strains, where two distinct subgroups arose because of recombination in the K antigen-encoding capsule polysaccharide biosynthesis gene (cps) region (64), the ST171 isolates likely evolved from a common ancestor and two major clusters each acquired distinct resistance plasmids. Several outbreaks and clonal spread have been linked to the ST171 isolates, suggesting that this genetic background contributes to the epidemiological success of ST171.
Our study has limitations. More than half of the isolates in this study are from the New York-New Jersey area, and consequently, this capture likely does not define the overall molecular epidemiology of carbapenem-resistant Enterobacter spp. in the United States or worldwide. In addition, as KPC is the main carbapenemase in the United States and most of the isolates harbored blaKPC, our study was not able to examine the genomic structures of carbapenem-resistant Enterobacter strains producing other carbapenemases, such as NDM, OXA-48, VIM, and IMP. A further study including Enterobacter isolates producing other carbapenemases will likely reveal additional genomic characteristics contributing to carbapenem resistance. Moreover, only six isolates were characterized by long-read sequencing, which produced closed genomes, and the results of most isolates were interpreted on the basis of de novo assemblies of short-read data. Because of the limitation of short-read data, the genetic structures, particularly repetitive regions, are hard to resolve. This is largely the reason why we were not able to define the structures of blaNDM-harboring plasmids and why no conclusive blaKPC plasmids were identified in 19 out of 72 KPC-producing isolates. Overall, however, our deep genomic analysis across the Enterobacteriaceae family has clearly defined the phylogeny and revealed distinct genomic signatures linked to carbapenem resistance.
The data presented here represent the first comprehensive study of phylogenomic relationships, antibiotic resistance, and plasmid discrimination of carbapenem-resistant Enterobacter spp. Our study suggests that acquisition of specific plasmids, successful host clones, and plasmid-host combinations are driving the molecular evolution of carbapenem resistance in the Enterobacter genus. Carbapenem resistance due to blaKPC has resulted in a pathogen that is difficult to treat, and in many instances, the clinical options are limited to less effective therapy. Improved understanding of the relationships among Enterobacter species and strains and the genetic context of resistance genes that they carry will be of significant value in tracking these organisms in a clinical context and in developing strategies to limit their spread.
MATERIALS AND METHODS
Bacterial isolates.
Ninety-seven Enterobacter sp. clinical isolates were collected from patients at 16 health care institutions in the United States (New York City [n = 43], New Jersey [n = 13], Florida [n = 20], Illinois [n = 1], Michigan [n = 3], Ohio [n = 3], and Pennsylvania [n = 4], Texas [n = 1]), South America (Colombia [n = 7]), and the Mediterranean region (Gaza [n = 2]). We selected six representative KPC-harboring Enterobacter isolates for complete genome sequencing on the basis of the presence of blaKPC gene variants, Tn4401 isoforms, multilocus sequence typing, and geographic and temporal distribution. Enterobacter species isolates were cultured overnight in lysogeny broth at 37°C for subsequent isolation of DNA for genome sequencing (see below). MICs were determined by broth microdilution in cation-adjusted Mueller-Hinton broth according to Clinical and Laboratory Standards Institute methods with Sensititre GNX2F panels (Thermo Fisher Scientific).
Enterobacter sp. de novo DNA sequencing.
Enterobacter sp. DNA was isolated from overnight cultures with a MasterPure Gram Positive DNA Purification kit (Epicentre, United States) as recommended by the manufacturer. Libraries were prepared for sequencing with Illumina NexteraXT kits and sequenced on an Illumina NextSeq with paired 150-base sequence reads. In general, >100-fold coverage was obtained for each genome. Each read set was assembled individually with SPAdes (37) and annotated with NCBI’s PGAAP pipeline (http://www.ncbi.nlm.nih.gov/genome/annotation_prok/). PacBio libraries were constructed and sequenced according to the manufacturer’s recommendations to ~100× coverage.
Characterization of Enterobacter species strains.
In silico multilocus sequence typing of 390 E. cloacae complex strains was performed with the MLST 1.8 online server (24, 38). The antimicrobial resistance genes and plasmid replicons in the sequenced Enterobacter isolates were identified by BLAST searching with the databases of ResFinder 2.1 (39) and PlasmidFinder 1.3 (65). Additional novel plasmid replicons from the present study were included in the analysis as well.
kSNP Enterobacter trees.
A phylogenetic tree was inferred from SNPs identified by kSNP v 3.0 (30) by using a k-mer length of 19 nucleotides and a requirement that at least 80% of the genomes (i.e., 303 genomes) have a nucleotide at a given SNP position in order for the SNP to be considered to be core and included in tree building. A total of 501,576 core SNP positions were identified. These SNPs were used to infer a maximum-likelihood tree with RAxML (31) with 100 bootstrap replicates. The resulting tree was rendered with metadata annotated with GraPhlAn (66).
Universal marker tree.
A total of 248 publically available genomes belonging to 48 Enterobacteriaceae genera were downloaded from GenBank. At least five genomes from each genus were included, prioritizing type strains, closed genomes, high-quality whole-genome sequences (e.g., contig N50 of 20 kbp or greater and ≤500 contigs), and phylogenetic diversity. In cases where there were fewer than five genome representatives of a given genus, all of the genomes meeting minimum assembly quality thresholds were downloaded. Twenty-six different universal marker genes (atpD, rplA, rplB, rplC, rplE, rplF, rplK, rplM, rplN, rplP, rplR, rplV, rpoA, rpsB, rpsC, rpsD, rpsE, rpsG, rpsH, rpsI, rpsK, rpsL, rpsM, rpsO, rpsQ, and secY) were used as seed sequences to identify the corresponding gene locus in each genome. These genes are universally conserved among bacteria and produce monophyletic phylogenies, suggesting that they undergo minimal horizontal transfer (67–69). Individual genes were identified by the J. Craig Venter Institute gene locus-typing program LOCUST (unpublished data), concatenated, and aligned with MUSCLE (70). The resulting alignment was used to generate a maximum-likelihood tree from 100 bootstrapped replicates with RAxML (31). The resulting tree was rendered with metadata annotated with GraPhlAn (66).
Pangenome analysis.
Clusters of orthologous proteins were generated with version 3.24 of PanOCT (41) as previously described (71, 72). Plots and calculations of pangenome size, new genes discovered, and pangenome status (open versus closed) were also determined as described previously (71).
ANI.
ANI of Enterobacter genomes was performed by PanOCT version 3.24, which has been modified to also accept nucleotide sequences as an alternative input to amino acid sequences as in previous versions.
Plasmid classification.
The blaKPC-harboring contigs were extracted from the de novo assemblies, followed by a BLASTn search against publicly available plasmid sequences in GenBank. If the contig coexisted with other plasmid core elements (e.g., the replicon) and showed >99% identity to and >90% query coverage of a known plasmid, the contig was preliminarily classified as the reference-like plasmid (e.g., pUAV01_KPC-like). The contig should have the same Tn4401 variant and TSD as the reference plasmid. The contigs were further aligned to the putative references and visually inspected to confirm the plasmid contents with Geneious (version 8.1; Biomatters Ltd., Auckland, New Zealand). Furthermore, BLASTn comparisons of each isolate’s de novo assembly and the reference plasmid were conducted, and the presence of a reference-like plasmid was defined as ≥99% sequence identity over ≥80% of the length of the reference. The plasmid content diagrams were generated by R with the plotTree.R script (available at https://github.com/katholt/RedDog).
Variant detection.
Single-nucleotide variant (SNV) analysis for ST171 isolates was performed with the RedDog pipeline (https://github.com/katholt/RedDog). In brief, the Illumina reads were mapped to ST171 reference strain 34978 with Bowtie2 (73), and SNVs were called by SAMtools (74) (Phred score, ≥30; read depth, ≥10). Consensus alleles at all SNV sites were then extracted with SAMtools (74). SNV sites present in all ST171 genomes were concatenated to generate a core SNV alignment for phylogenetic analysis. Draft genomes from the public database were each shredded into 1 million 100-bp reads (with SAMtools wgsim) and subjected to the same analysis as the Illumina reads.
Accession number(s).
All of the genomes determined in this study are available at NCBI under BioProject no. PRJNA259658.
SUPPLEMENTAL MATERIAL
Comprehensive table with details about the 97 clinical isolates and six type strains sequenced in this study. Download
Details of 351 Enterobacter genomes from GenBank. Download
Phylogenetic structure, subspecies identification, and pangenome analysis of Enterobacter spp. Download
Comparison of blaKPC-harboring plasmids from four PacBio sequenced strains. Light blue shading denotes shared regions of homology with 99% identities. Light gray shading denotes homologous regions acquired from another plasmid (pENT-c88). Open reading frames are represented by arrows colored on the basis of the predicted gene function (see key, top right). Plasmid scaffold regions are represented by orange arrows. The genes associated with the tra locus are represented by green arrows, and the antimicrobial resistance genes are represented by red arrows. Replication-associated genes are represented by dark blue arrows, while the accessory genes are represented by yellow arrows. Download
Comparison of blaKPC-4-harboring plasmids from BK34998. Light blue shading denotes IncA/C plasmid backbone regions shared among three plasmids, pRA1, p34998-E, and p35734-C. Light gray shading denotes region of homology surrounding blaKPC-4 from IncN plasmid pBK31551. Open reading frames are represented by arrows colored on the basis of the predicted gene function (see key, top right). Download
Plasmid gene content can influence gene content tree topology. An unrooted neighbor-joining tree was constructed by Neighbor by using the PanOCT Jaccard pairwise distance matrix of orthologous gene clusters with (A) and without (B) plasmid genes. Coloring is by assigned E. cloacae complex groupings A to E (see key and Fig. 5). Nodes are labeled by strain name. The asterisks mark those branches of the tree with mixed group assignments prior to the removal of clusters containing known plasmid genes from the complete genomes used in the pangenome analysis. The scale bars indicate distance. Download
Phylogenetic SNP tree of Enterobacter genomes. A whole-genome core SNP tree was constructed for 447 Enterobacter sp. genomes by using kSNP (30) and RAxML (31) (see Materials and Methods). The dendrogram was generated with FigTree v 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/). This data set included genomes within the 379 E. cloacae complex (black) and 68 E. aerogenes (blue) genomes used in this study. The scale bar indicates the number of nucleotide substitutions. Download
Resistance genes and incompatibility groups of plasmids from six PacBio sequenced Enterobacter strains.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants R01AI090155 (to B.N.K.) and R21AI117338 (to L.C.) and Genomic Center for Infectious Diseases grant U19AI110819 from the National Institute of Allergy and Infectious Diseases (NIAID) (to M.D.A.). Research reported in this publication was also supported in part by the NIAID of the NIH under awards R01AI100560, R01AI063517, and R01AI072219 to R.A.B. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This study was supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, award 1I01BX001974 to R.A.B., from the Biomedical Laboratory Research and Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10 to R.A.B..
Funding Statement
This study was also supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, Award Number 1I01BX001974 to RAB from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10 to RAB.
Footnotes
Citation Chavda KD, Chen L, Fouts DE, Sutton G, Brinkac L, Jenkins SG, Bonomo RA, Adams MD, Kreiswirth BN. 2016. Comprehensive genome analysis of carbapenemase-producing Enterobacter spp.: new insights into phylogeny, population structure and resistance mechanisms. mBio 7(6):e02093-16. doi:10.1128/mBio.02093-16.
REFERENCES
- 1.Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. 2014. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol 22:686–696. doi: 10.1016/j.tim.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. 2001. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordmann P, Cuzon G, Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 4.Kitchel B, Rasheed JK, Patel JB, Srinivasan A, Navon-Venezia S, Carmeli Y, Brolund A, Giske CG. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother 53:3365–3370. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitchel B, Sundin DR, Patel JB. 2009. Regional dissemination of KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 53:4511–4513. doi: 10.1128/AAC.00784-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuelsen Ø, Naseer U, Tofteland S, Skutlaberg DH, Onken A, Hjetland R, Sundsfjord A, Giske CG. 2009. Emergence of clonally related Klebsiella pneumoniae isolates of sequence type 258 producing plasmid-mediated KPC carbapenemase in Norway and Sweden. J Antimicrob Chemother 63:654–658. doi: 10.1093/jac/dkp018. [DOI] [PubMed] [Google Scholar]
- 7.Leavitt A, Navon-Venezia S, Chmelnitsky I, Schwaber MJ, Carmeli Y. 2007. Emergence of KPC-2 and KPC-3 in carbapenem-resistant Klebsiella pneumoniae strains in an Israeli hospital. Antimicrob Agents Chemother 51:3026–3029. doi: 10.1128/AAC.00299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazarovitch T, Amity K, Coyle JR, Ackerman B, Tal-Jasper R, Ofer-Friedman H, Hayakawa K, Bogan C, Lephart PR, Kaplansky T, Maskit M, Azouri T, Zaidenstein R, Perez F, Bonomo RA, Kaye KS, Marchaim D. 2015. The complex epidemiology of carbapenem-resistant Enterobacter infections: a multicenter descriptive analysis. Infect Control Hosp Epidemiol 36:1283–1291. doi: 10.1017/ice.2015.186. [DOI] [PubMed] [Google Scholar]
- 9.Hossain A, Ferraro MJ, Pino RM, Dew RB III, Moland ES, Lockhart TJ, Thomson KS, Goering RV, Hanson ND. 2004. Plasmid-mediated carbapenem-hydrolyzing enzyme KPC-2 in an Enterobacter sp. Antimicrob Agents Chemother 48:4438–4440. doi: 10.1128/AAC.48.11.4438-4440.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiedrowski LM, Guerrero DM, Perez F, Viau RA, Rojas LJ, Mojica MF, Rudin SD, Hujer AM, Marshall SH, Bonomo RA. 2014. Carbapenem-resistant Enterobacter cloacae isolates producing KPC-3, North Dakota, USA. Emerg Infect Dis 20:1583–1585. doi: 10.3201/eid2009.140344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haraoui LP, Lévesque S, Lefebvre B, Blanchette R, Tomkinson M, Mataseje L, Mulvey MR, Miller MA. 2013. Polyclonal outbreak of KPC-3-producing Enterobacter cloacae at a single hospital in Montreal, Quebec, Canada. J Clin Microbiol 51:2406–2408. doi: 10.1128/JCM.02480-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez D, Rodulfo HE, Rodriguez L, Caña LE, Medina B, Guzman M, Carreno N, Marcano D, De Donato M. 2014. First report of metallo-beta-lactamases producing Enterobacter spp. strains from Venezuela. Rev Inst Med Trop Sao Paulo 56:67–69. doi: 10.1590/S0036-46652014000100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo Y, Yang J, Ye L, Guo L, Zhao Q, Chen R, Chen Y, Han X, Zhao J, Tian S, Han L. 2014. Characterization of KPC-2-producing Escherichia coli, Citrobacter freundii, Enterobacter cloacae, Enterobacter aerogenes, and Klebsiella oxytoca isolates from a Chinese hospital. Microb Drug Resist 20:264–269. doi: 10.1089/mdr.2013.0150. [DOI] [PubMed] [Google Scholar]
- 14.Bratu S, Landman D, Alam M, Tolentino E, Quale J. 2005. Detection of KPC carbapenem-hydrolyzing enzymes in Enterobacter spp. from Brooklyn, New York. Antimicrob Agents Chemother 49:776–778. doi: 10.1128/AAC.49.2.776-778.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Simmonds A, Hu Y, Sullivan SB, Wang Z, Whittier S, Uhlemann AC. 2016. Evidence from a New York City hospital of rising incidence of genetically diverse carbapenem-resistant Enterobacter cloacae and dominance of ST171, 2007–14. J Antimicrob Chemother 71:2351–2353 doi: 10.1093/jac/dkw132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landman D, Salamera J, Singh M, Quale J. 2011. Accuracy of carbapenem nonsusceptibility for identification of KPC-possessing Enterobacteriaceae by use of the revised CLSI breakpoints. J Clin Microbiol 49:3931–3933. doi: 10.1128/JCM.01176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchaim D, Chopra T, Perez F, Hayakawa K, Lephart PR, Bheemreddy S, Blunden C, Hujer AM, Rudin S, Shango M, Campbell M, Varkey J, Slim J, Ahmad F, Patel D, Chen TY, Pogue JM, Salimnia H, Dhar S, Bonomo RA, Kaye KS. 2011. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit Medical Center. Infect Control Hosp Epidemiol 32:861–871. doi: 10.1086/661597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villa J, Viedma E, Brañas P, Orellana MA, Otero JR, Chaves F. 2014. Multiclonal spread of VIM-1-producing Enterobacter cloacae isolates associated with In624 and In488 integrons located in an IncHI2 plasmid. Int J Antimicrob Agents 43:451–455. doi: 10.1016/j.ijantimicag.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Markovska RD, Stoeva TJ, Bojkova KD, Mitov IG. 2014. Epidemiology and molecular characterization of extended-spectrum beta-lactamase-producing Enterobacter spp., Pantoea agglomerans, and Serratia marcescens isolates from a Bulgarian hospital. Microb Drug Resist 20:131–137. doi: 10.1089/mdr.2013.0102. [DOI] [PubMed] [Google Scholar]
- 20.Liu WY, Wong CF, Chung KM, Jiang JW, Leung FC. 2013. Comparative genome analysis of Enterobacter cloacae. PLoS One 8:e74487. doi: 10.1371/journal.pone.0074487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conlan S, Thomas PJ, Deming C, Park M, Lau AF, Dekker JP, Snitkin ES, Clark TA, Luong K, Song Y, Tsai YC, Boitano M, Dayal J, Brooks SY, Schmidt B, Young AC, Thomas JW, Bouffard GG, Blakesley RW, NISC Comparative Sequencing Program, Mullikin JC, Korlach J, Henderson DK, Frank KM, Palmore TN, Segre JA. 2014. Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae. Sci Transl Med 6:254ra126. doi: 10.1126/scitranslmed.3009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taghavi S, van der Lelie D, Hoffman A, Zhang YB, Walla MD, Vangronsveld J, Newman L, Monchy S. 2010. Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet 6:e1000943. doi: 10.1371/journal.pgen.1000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diene SM, Merhej V, Henry M, El Filali A, Roux V, Robert C, Azza S, Gavory F, Barbe V, La Scola B, Raoult D, Rolain JM. 2013. The rhizome of the multidrug-resistant Enterobacter aerogenes genome reveals how new “killer bugs” are created because of a sympatric lifestyle. Mol Biol Evol 30:369–383. doi: 10.1093/molbev/mss236. [DOI] [PubMed] [Google Scholar]
- 24.Miyoshi-Akiyama T, Hayakawa K, Ohmagari N, Shimojima M, Kirikae T. 2013. Multilocus sequence typing (MLST) for characterization of Enterobacter cloacae. PLoS One 8:e66358. doi: 10.1371/journal.pone.0066358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathers AJ, Stoesser N, Sheppard AE, Pankhurst L, Giess A, Yeh AJ, Didelot X, Turner SD, Sebra R, Kasarskis A, Peto T, Crook D, Sifri CD. 2015. Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae at a single institution: insights into endemicity from whole-genome sequencing. Antimicrob Agents Chemother 59:1656–1663. doi: 10.1128/AAC.04292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Chavda KD, Melano RG, Hong T, Rojtman AD, Jacobs MR, Bonomo RA, Kreiswirth BN. 2014. Molecular survey of the dissemination of two blaKPC-harboring IncFIA plasmids in New Jersey and New York hospitals. Antimicrob Agents Chemother 58:2289–2294. doi: 10.1128/AAC.02749-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fricke WF, Welch TJ, McDermott PF, Mammel MK, LeClerc JE, White DG, Cebula TA, Ravel J. 2009. Comparative genomics of the IncA/C multidrug resistance plasmid family. J Bacteriol 191:4750–4757. doi: 10.1128/JB.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Chavda KD, Fraimow HS, Mediavilla JR, Melano RG, Jacobs MR, Bonomo RA, Kreiswirth BN. 2013. Complete nucleotide sequences of blaKPC-4- and blaKPC-5-harboring IncN and IncX plasmids from Klebsiella pneumoniae strains isolated in New Jersey. Antimicrob Agents Chemother 57:269–276. doi: 10.1128/AAC.01648-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner SN, Slezak T, Hall BG. 2015. kSNP3.0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics 31:2877–2878. doi: 10.1093/bioinformatics/btv271. [DOI] [PubMed] [Google Scholar]
- 31.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann H, Roggenkamp A. 2003. Population genetics of the nomenspecies Enterobacter cloacae. Appl Environ Microbiol 69:5306–5318. doi: 10.1128/AEM.69.9.5306-5318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann H, Stindl S, Ludwig W, Stumpf A, Mehlen A, Monget D, Pierard D, Ziesing S, Heesemann J, Roggenkamp A, Schleifer KH. 2005. Enterobacter hormaechei subsp. oharae subsp. nov., E. hormaechei subsp. hormaechei comb. nov., and E. hormaechei subsp. steigerwaltii subsp. nov., three new subspecies of clinical importance. J Clin Microbiol 43:3297–3303. doi: 10.1128/JCM.43.7.3297-3303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morand PC, Billoet A, Rottman M, Sivadon-Tardy V, Eyrolle L, Jeanne L, Tazi A, Anract P, Courpied JP, Poyart C, Dumaine V. 2009. Specific distribution within the Enterobacter cloacae complex of strains isolated from infected orthopedic implants. J Clin Microbiol 47:2489–2495. doi: 10.1128/JCM.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann H, Stindl S, Ludwig W, Stumpf A, Mehlen A, Heesemann J, Monget D, Schleifer KH, Roggenkamp A. 2005. Reassignment of Enterobacter dissolvens to Enterobacter cloacae as E. cloacae subspecies dissolvens comb. nov. and emended description of Enterobacter asburiae and Enterobacter kobei. Syst Appl Microbiol 28:196–205. doi: 10.1016/j.syapm.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Gu CT, Li CY, Yang LJ, Huo GC. 2014. Enterobacter xiangfangensis sp. nov., isolated from Chinese traditional sourdough, and reclassification of Enterobacter sacchari Zhu et al. 2013 as Kosakonia sacchari comb. nov. Int J Syst Evol Microbiol 64:2650–2656. doi: 10.1099/ijs.0.064709-0. [DOI] [PubMed] [Google Scholar]
- 37.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Pontén T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tindall BJ, Sutton G, Garrity GM. 13 October 2016. Enterobacter aerogenes Hormaeche and Edwards 1960 (Approved Lists 1980) and Klebsiella mobilis Bascomb et al. 1971 (Approved Lists 1980) share the same nomenclatural type (ATCC 13048) on the Approved Lists and are homotypic synonyms, with consequences for the name Klebsiella mobilis Bascomb et al. 1971 (Approved Lists 1980). Int J Syst Evol Microbiol doi: 10.1099/ijsem.0.001572. [DOI] [PubMed] [Google Scholar]
- 41.Fouts DE, Brinkac L, Beck E, Inman J, Sutton G. 2012. PanOCT: automated clustering of orthologs using conserved gene neighborhood for pan-genomic analysis of bacterial strains and closely related species. Nucleic Acids Res 40:e172. doi: 10.1093/nar/gks757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adler A, Hussein O, Ben-David D, Masarwa S, Navon-Venezia S, Schwaber MJ, Carmeli Y, Post-Acute-Care Hospital Carbapenem-Resistant Enterobacteriaceae Working Group . 2015. Persistence of Klebsiella pneumoniae ST258 as the predominant clone of carbapenemase-producing Enterobacteriaceae in post-acute-care hospitals in Israel, 2008–13. J Antimicrob Chemother 70:89–92. doi: 10.1093/jac/dku333. [DOI] [PubMed] [Google Scholar]
- 43.Nobelmann B, Lengeler JW. 1996. Molecular analysis of the gat genes from Escherichia coli and of their roles in galactitol transport and metabolism. J Bacteriol 178:6790–6795. doi: 10.1128/jb.178.23.6790-6795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reizer J, Ramseier TM, Reizer A, Charbit A, Saier MH Jr. 1996. Novel phosphotransferase genes revealed by bacterial genome sequencing: a gene cluster encoding a putative N-acetylgalactosamine metabolic pathway in Escherichia coli. Microbiology 142:231–250. doi: 10.1099/13500872-142-2-231. [DOI] [PubMed] [Google Scholar]
- 45.Wu J, Anderton-Loviny T, Smith CA, Hartley BS. 1985. Structure of wild-type and mutant repressors and of the control region of the rbt operon of Klebsiella aerogenes. EMBO J 4:1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen L, Chavda KD, Al Laham N, Melano RG, Jacobs MR, Bonomo RA, Kreiswirth BN. 2013. Complete nucleotide sequence of a blaKPC-harboring IncI2 plasmid and its dissemination in New Jersey and New York hospitals. Antimicrob Agents Chemother 57:5019–5025. doi: 10.1128/AAC.01397-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Chavda KD, Melano RG, Jacobs MR, Koll B, Hong T, Rojtman AD, Levi MH, Bonomo RA, Kreiswirth BN. 2014. Comparative genomic analysis of KPC-encoding pKpQIL-like plasmids and their distribution in New Jersey and New York hospitals. Antimicrob Agents Chemother 58:2871–2877. doi: 10.1128/AAC.00120-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, Chavda KD, Mediavilla JR, Jacobs MR, Levi MH, Bonomo RA, Kreiswirth BN. 2012. Partial excision of blaKPC from Tn4401 in carbapenem-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother 56:1635–1638. doi: 10.1128/AAC.06182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leavitt A, Chmelnitsky I, Carmeli Y, Navon-Venezia S. 2010. Complete nucleotide sequence of KPC-3-encoding plasmid pKpQIL in the epidemic Klebsiella pneumoniae sequence type 258. Antimicrob Agents Chemother 54:4493–4496. doi: 10.1128/AAC.00175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Chaparro PJ, Cerdeira LT, Queiroz MG, de Lima CP, Levy CE, Pavez M, Lincopan N, Gonçalves EC, Mamizuka EM, Sampaio JL, Nunes MR, McCulloch JA. 2014. Complete nucleotide sequences of two blaKPC-2-bearing IncN plasmids isolated from sequence type 442 Klebsiella pneumoniae clinical strains four years apart. Antimicrob Agents Chemother 58:2958–2960. doi: 10.1128/AAC.02341-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leavitt A, Chmelnitsky I, Ofek I, Carmeli Y, Navon-Venezia S. 2010. Plasmid pKpQIL encoding KPC-3 and TEM-1 confers carbapenem resistance in an extremely drug-resistant epidemic Klebsiella pneumoniae strain. J Antimicrob Chemother 65:243–248. doi: 10.1093/jac/dkp417. [DOI] [PubMed] [Google Scholar]
- 52.García-Fernández A, Villa L, Carta C, Venditti C, Giordano A, Venditti M, Mancini C, Carattoli A. 2012. Klebsiella pneumoniae ST258 producing KPC-3 identified in Italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob Agents Chemother 56:2143–2145. doi: 10.1128/AAC.05308-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright MS, Perez F, Brinkac L, Jacobs MR, Kaye K, Cober E, van Duin D, Marshall SH, Hujer AM, Rudin SD, Hujer KM, Bonomo RA, Adams MD. 2014. Population structure of KPC-producing Klebsiella pneumoniae isolates from midwestern U.S. hospitals. Antimicrob Agents Chemother 58:4961–4965. doi: 10.1128/AAC.00125-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Findlay J, Hopkins KL, Doumith M, Meunier D, Wiuff C, Hill R, Pike R, Loy R, Mustafa N, Livermore DM, Woodford N. 2016. KPC enzymes in the UK: an analysis of the first 160 cases outside the north-west region. J Antimicrob Chemother 71:1199–1206. doi: 10.1093/jac/dkv476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Hara JA, Hu F, Ahn C, Nelson J, Rivera JI, Pasculle AW, Doi Y. 2014. Molecular epidemiology of KPC-producing Escherichia coli: occurrence of ST131-fimH30 subclone harboring pKpQIL-like IncFIIk plasmid. Antimicrob Agents Chemother 58:4234–4237. doi: 10.1128/AAC.02182-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahn C, Syed A, Hu F, O’Hara JA, Rivera JI, Doi Y. 2014. Microbiological features of KPC-producing Enterobacter isolates identified in a U.S. hospital system. Diagn Microbiol Infect Dis 80:154–158. doi: 10.1016/j.diagmicrobio.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hargreaves ML, Shaw KM, Dobbins G, Snippes Vagnone PM, Harper JE, Boxrud D, Lynfield R, Aziz M, Price LB, Silverstein KA, Danzeisen JL, Youmans B, Case K, Sreevatsan S, Johnson TJ. 2015. Clonal dissemination of Enterobacter cloacae harboring blaKPC-3 in the upper midwestern United States. Antimicrob Agents Chemother 59:7723–7734 doi: 10.1128/AAC.01291-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiedrowski LM, Guerrero DM, Perez F, Viau RA, Rojas LJ, Mojica MF, Rudin SD, Hujer AM, Marshall SH, Bonomo RA. 2014. Carbapenem-resistant Enterobacter cloacae isolates producing KPC-3, North Dakota, USA. Emerg Infect Dis 20:1583–1585. doi: 10.3201/eid2009.140344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Navarro F, Miró E, Mirelis B. 2010. Interpretive reading of Enterobacteria antibiograms. Enferm Infecc Microbiol Clin 28:638–645. (In Spanish.) doi: 10.1016/j.eimc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Brady C, Cleenwerck I, Venter S, Coutinho T, De Vos P. 2013. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. gergoviae and E. pyrinus into Pluralibacter gen. nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. oryzae and E. arachidis into Kosakonia gen. nov. as Kosakonia cowanii comb. nov., Kosakonia radicincitans comb. nov., Kosakonia oryzae comb. nov. and Kosakonia arachidis comb. nov., respectively, and E. turicensis, E. helveticus and E. pulveris into Cronobacter as Cronobacter zurichensis nom. nov., Cronobacter helveticus comb. nov. and Cronobacter pulveris comb. nov., respectively, and emended description of the genera Enterobacter and Cronobacter. Syst Appl Microbiol 36:309–319. doi: 10.1016/j.syapm.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Grimont F, Grimont P. 2006. The genus Enterobacter, p 197–214. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes. Springer, New York, NY. [Google Scholar]
- 62.Chavda KD, Chen L, Jacobs MR, Rojtman AD, Bonomo RA, Kreiswirth BN. 2015. Complete sequence of a bla(KPC)-harboring cointegrate plasmid isolated from Escherichia coli. Antimicrob Agents Chemother 59:2956–2959. doi: 10.1128/AAC.00041-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheppard AE, Stoesser N, Wilson DJ, Sebra R, Kasarskis A, Anson LW, Giess A, Pankhurst LJ, Vaughan A, Grim CJ, Cox HL, Yeh AJ, Sifri CD, Walker AS, Peto TE, Crook DW, Mathers AJ. 2016. Nested Russian doll-like genetic mobility drives rapid dissemination of the carbapenem resistance gene blaKPC. Antimicrob Agents Chemother 60:3767–3778 doi: 10.1128/aac.00464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deleo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, Porter AR, Chavda KD, Jacobs MR, Mathema B, Olsen RJ, Bonomo RA, Musser JM, Kreiswirth BN. 2014. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc Natl Acad Sci U S A 111:4988–4993. doi: 10.1073/pnas.1321364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asnicar F, Weingart G, Tickle TL, Huttenhower C, Segata N. 2015. Compact graphical representation of phylogenetic data and metadata with GraPhlAn. PeerJ 3:e1029. doi: 10.7717/peerj.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown JR, Douady CJ, Italia MJ, Marshall WE, Stanhope MJ. 2001. Universal trees based on large combined protein sequence data sets. Nat Genet 28:281–285. doi: 10.1038/90129. [DOI] [PubMed] [Google Scholar]
- 68.Santos SR, Ochman H. 2004. Identification and phylogenetic sorting of bacterial lineages with universally conserved genes and proteins. Environ Microbiol 6:754–759. doi: 10.1111/j.1462-2920.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- 69.Ciccarelli FD, Doerks T, von Mering C, Creevey CJ, Snel B, Bork P. 2006. Toward automatic reconstruction of a highly resolved tree of life. Science 311:1283–1287. doi: 10.1126/science.1123061. [DOI] [PubMed] [Google Scholar]
- 70.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan AP, Sutton G, DePew J, Krishnakumar R, Choi Y, Huang XZ, Beck E, Harkins DM, Kim M, Lesho EP, Nikolich MP, Fouts DE. 2015. A novel method of consensus pan-chromosome assembly and large-scale comparative analysis reveal the highly flexible pan-genome of Acinetobacter baumannii. Genome Biol 16:143. doi: 10.1186/s13059-015-0701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fouts DE, Matthias MA, Adhikarla H, Adler B, Amorim-Santos L, Berg DE, Bulach D, Buschiazzo A, Chang YF, Galloway RL, Haake DA, Haft DH, Hartskeerl R, Ko AI, Levett PN, Matsunaga J, Mechaly AE, Monk JM, Nascimento AL, Nelson KE, Palsson B, Peacock SJ, Picardeau M, Ricaldi JN, Thaipandungpanit J, Wunder EA Jr., Yang XF, Zhang JJ, Vinetz JM. 2016. What makes a bacterial species pathogenic?: comparative genomic analysis of the genus Leptospira. PLOS Negl Trop Dis 10:e0004403. doi: 10.1371/journal.pntd.0004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heuel H, Shakeri-Garakani A, Turgut S, Lengeler JW. 1998. Genes for d-arabinitol and ribitol catabolism from Klebsiella pneumoniae. Microbiology 144:1631–1639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comprehensive table with details about the 97 clinical isolates and six type strains sequenced in this study. Download
Details of 351 Enterobacter genomes from GenBank. Download
Phylogenetic structure, subspecies identification, and pangenome analysis of Enterobacter spp. Download
Comparison of blaKPC-harboring plasmids from four PacBio sequenced strains. Light blue shading denotes shared regions of homology with 99% identities. Light gray shading denotes homologous regions acquired from another plasmid (pENT-c88). Open reading frames are represented by arrows colored on the basis of the predicted gene function (see key, top right). Plasmid scaffold regions are represented by orange arrows. The genes associated with the tra locus are represented by green arrows, and the antimicrobial resistance genes are represented by red arrows. Replication-associated genes are represented by dark blue arrows, while the accessory genes are represented by yellow arrows. Download
Comparison of blaKPC-4-harboring plasmids from BK34998. Light blue shading denotes IncA/C plasmid backbone regions shared among three plasmids, pRA1, p34998-E, and p35734-C. Light gray shading denotes region of homology surrounding blaKPC-4 from IncN plasmid pBK31551. Open reading frames are represented by arrows colored on the basis of the predicted gene function (see key, top right). Download
Plasmid gene content can influence gene content tree topology. An unrooted neighbor-joining tree was constructed by Neighbor by using the PanOCT Jaccard pairwise distance matrix of orthologous gene clusters with (A) and without (B) plasmid genes. Coloring is by assigned E. cloacae complex groupings A to E (see key and Fig. 5). Nodes are labeled by strain name. The asterisks mark those branches of the tree with mixed group assignments prior to the removal of clusters containing known plasmid genes from the complete genomes used in the pangenome analysis. The scale bars indicate distance. Download
Phylogenetic SNP tree of Enterobacter genomes. A whole-genome core SNP tree was constructed for 447 Enterobacter sp. genomes by using kSNP (30) and RAxML (31) (see Materials and Methods). The dendrogram was generated with FigTree v 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/). This data set included genomes within the 379 E. cloacae complex (black) and 68 E. aerogenes (blue) genomes used in this study. The scale bar indicates the number of nucleotide substitutions. Download
Resistance genes and incompatibility groups of plasmids from six PacBio sequenced Enterobacter strains.